Abstract
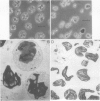
A method for cellular fractionation of Chlamydomonas reinhardii, SAG 11-32/b, and isolation of intact chloroplasts from synchronized cells of the alga is described. The procedure for cell fractionation comprises essentially four steps: (1) protoplast production with autolysine; (2) lysis of the protoplasts with digitonin; (3) aggregation of broken protoplasts; and (4) separation of organelles by differential centrifugations.
Replacing the differential centrifugations (step 4) by Percoll cushion centrifugations yields intact chloroplasts. Starting with 100 milliliters of an algal culture containing 3000 micrograms chlorophyll, intact chloroplasts with 100 to 200 micrograms of chlorophyll can be isolated. Envelope integrity is about 90% (ferricyanide assay). Examination of the chloroplasts by electron microscopy and marker enzyme activities indicated some mitochondrial and cytoplasmic contamination.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger E. S., King D., Erbes D. L., Gibbs M. H(2) and CO(2) Evolution by Anaerobically Adapted Chlamydomonas reinhardtii F-60. Plant Physiol. 1982 Jun;69(6):1268–1273. doi: 10.1104/pp.69.6.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap W. R., Togasaki R. K. Chlamydomonas reinhardtii cell preparation with altered permeability toward substrates of organellar reactions. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2310–2314. doi: 10.1073/pnas.78.4.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Amotz A., Gibbs M. H2 metabolism in photosynthetic organisms. II. Light-dependent H2 evolution by preparations from Chlamydomonas, Scenedesmus and spinach. Biochem Biophys Res Commun. 1975 May 5;64(1):355–359. doi: 10.1016/0006-291x(75)90261-2. [DOI] [PubMed] [Google Scholar]
- Eley M. H., Namihira G., Hamilton L., Munk P., Reed L. J. -Keto acid dehydrogenase complexes. 18. Subunit composition of the Escherichia coli pyruvate dehydrogenase complex. Arch Biochem Biophys. 1972 Oct;152(2):655–669. doi: 10.1016/0003-9861(72)90262-7. [DOI] [PubMed] [Google Scholar]
- Klein U., Chen C., Gibbs M. Photosynthetic Properties of Chloroplasts from Chlamydomonas reinhardii. Plant Physiol. 1983 Jun;72(2):488–491. doi: 10.1104/pp.72.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latzko E., Gibbs M. Enzyme activities of the carbon reduction cycle in some photosynthetic organisms. Plant Physiol. 1969 Feb;44(2):295–300. doi: 10.1104/pp.44.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlösser U. G., Sachs H., Robinson D. G. Isolation of protoplasts by means of a "species-specific" autolysine in Chlamydomonas. Protoplasma. 1976;88(1):51–64. doi: 10.1007/BF01280359. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]