Abstract
The objectives of this study were to select and initially characterize mutants of soybean (Glycine max L. Merr. cv Williams) with decreased ability to reduce nitrate. Selection involved a chlorate screen of approximately 12,000 seedlings (progeny of mutagenized seed) and subsequent analyses for low nitrate reductase (LNR) activity. Three lines, designated LNR-2, LNR-3, and LNR-4, were selected by this procedure.
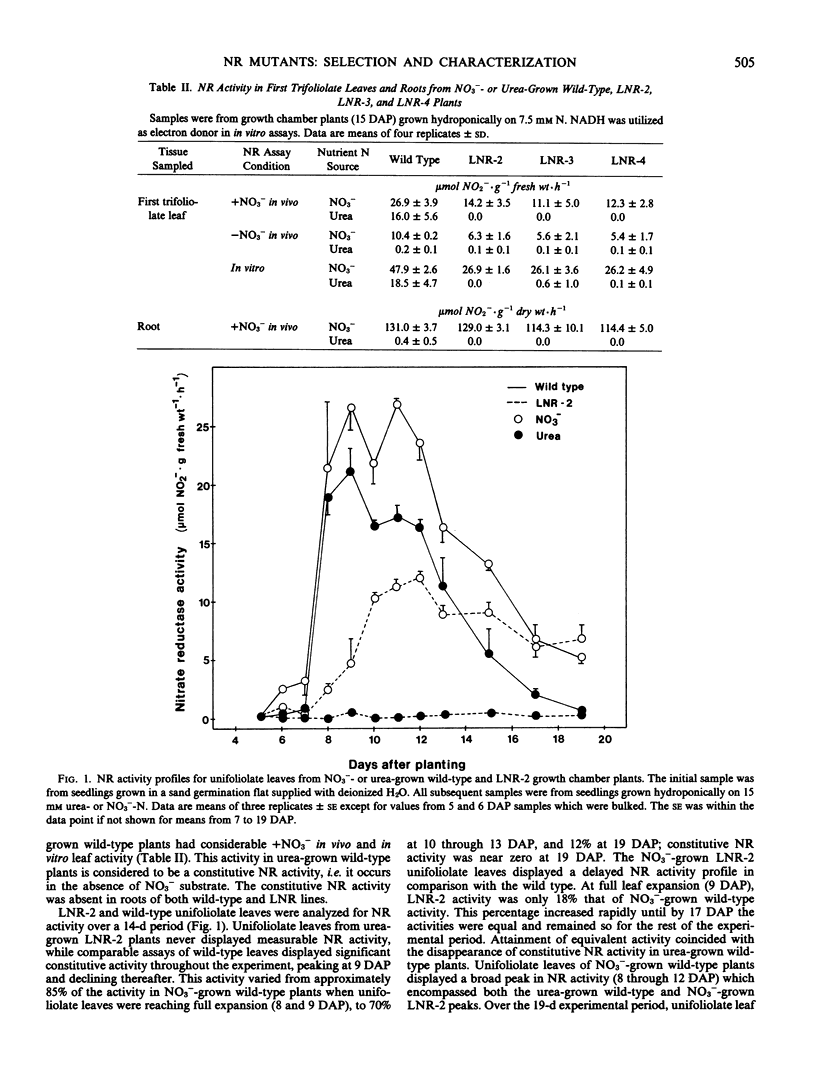
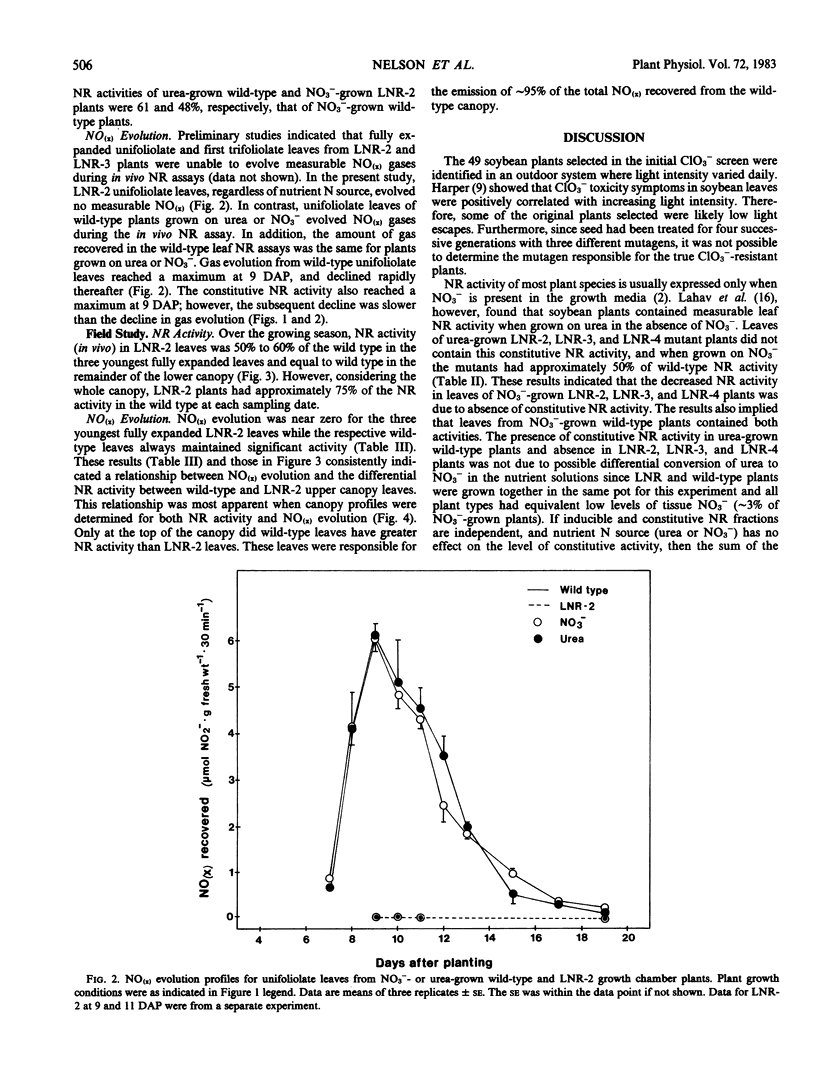
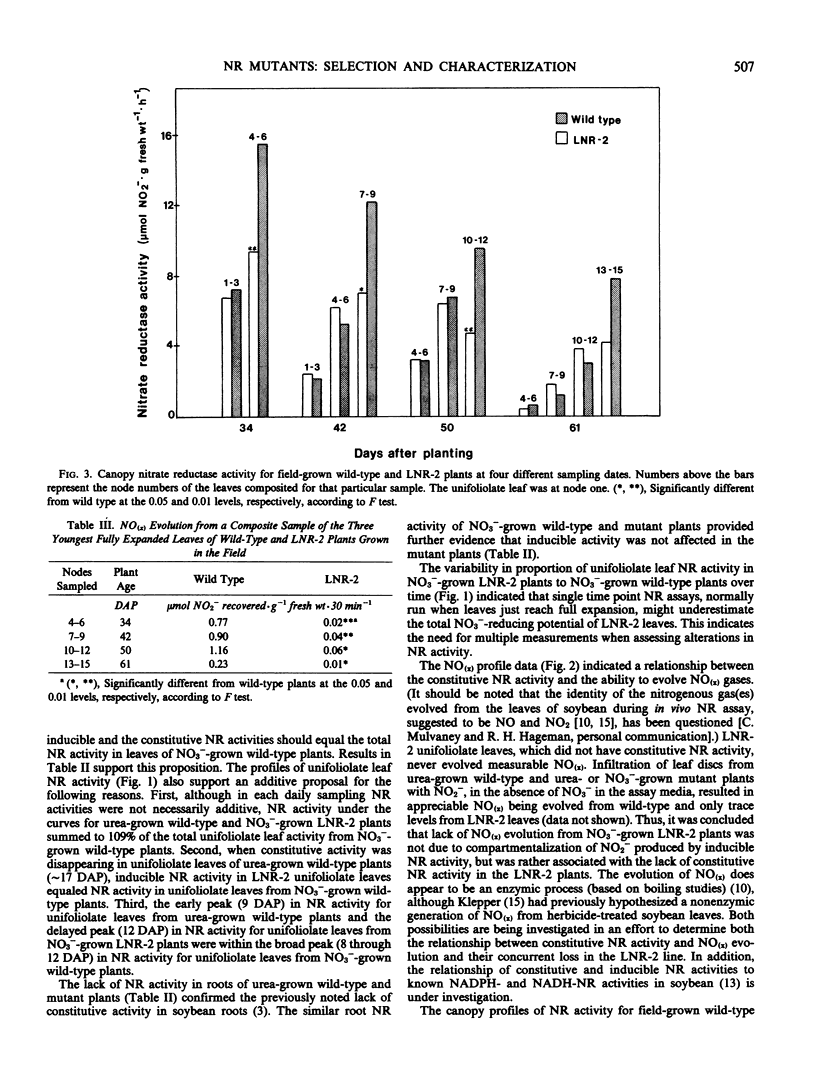
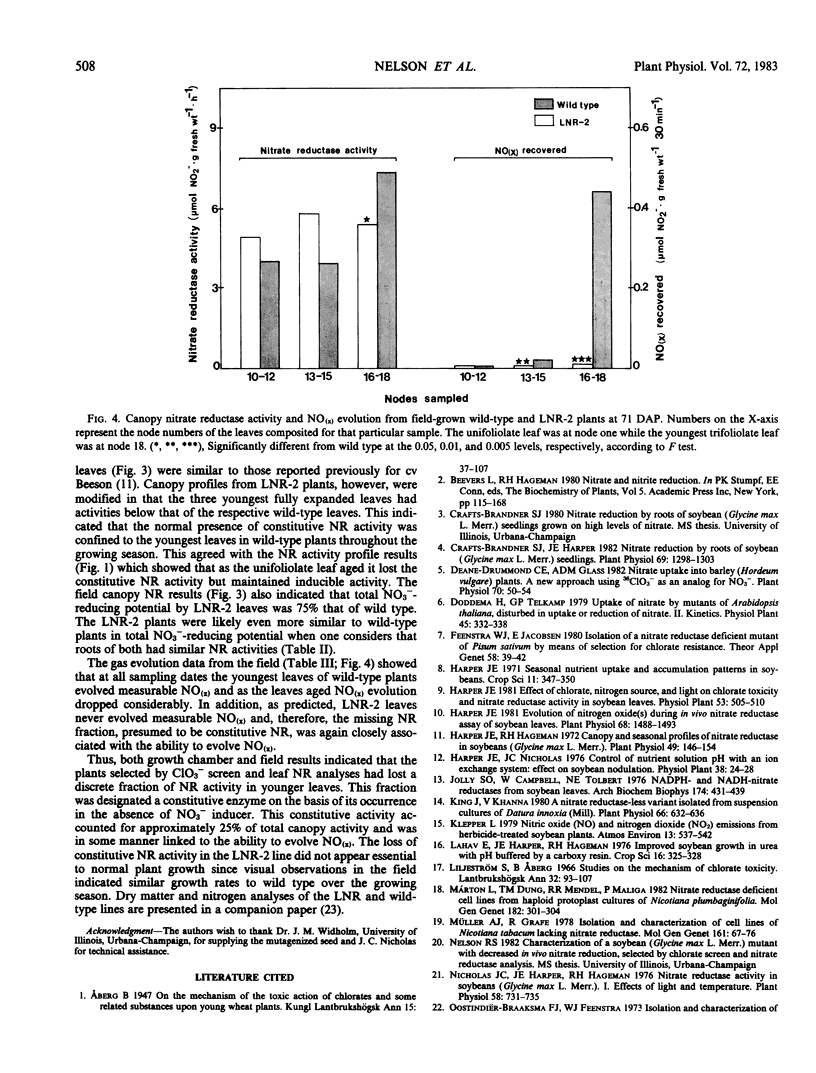
In growth chamber studies, the fully expanded first trifoliolate leaf from NO3−-grown LNR-2, LNR-3, and LNR-4 plants had approximately 50% of the wild-type NR activity. Leaves from urea-grown LNR-2, LNR-3, and LNR-4 plants had no NR activity while leaves from comparable wild-type plants had considerable activity; the latter activity does not require the presence of NO3− in the nutrient solution for induction and on this basis is tentatively considered as a constitutive enzyme. Summation of constitutive (urea-grown wild-type plants) and inducible (NO3−-grown LNR-2, LNR-3, or LNR-4 plants) leaf NR activities approximated activity in leaves of NO3−-grown wild-type plants. Root NR activities were comparable in wild-type and mutant plants grown on NO3−, and roots of both plant types lacked constitutive NR activity when grown on urea. In both growth chamber- and field-grown plants, oxides of nitrogen [NO(x)] were evolved from young leaves of wild-type plants, but not from leaves of LNR-2 plants, during in vivo NR assays. Analysis of leaves from different canopy locations showed that constitutive NR activity was confined to the youngest three fully expanded leaves of the wild-type plant and, therefore, on a total plant canopy basis, the NR activity of LNR-2 plants was approximately 75% that of wild-type plants. It is concluded that: (a) the NR activity in leaves of NO3−-grown wild-type plants includes both constitutive and inducible activity; (b) the missing NR activity in LNR-2, LNR-3, and LNR-4 leaves is the constitutive component; and (c) the constitutive NR activity is associated with NO(x) evolution and occurs only in physiologically young leaves.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crafts-Brandner S. J., Harper J. E. Nitrate Reduction by Roots of Soybean (Glycine max [L.] Merr.) Seedlings. Plant Physiol. 1982 Jun;69(6):1298–1303. doi: 10.1104/pp.69.6.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane-Drummond C. E., Glass A. D. Nitrate Uptake into Barley (Hordeum vulgare) Plants : A New Approach Using ClO(3) as an Analog for NO(3). Plant Physiol. 1982 Jul;70(1):50–54. doi: 10.1104/pp.70.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. E. Canopy and Seasonal Profiles of Nitrate Reductase in Soybeans (Glycine max L. Merr.). Plant Physiol. 1972 Feb;49(2):146–154. doi: 10.1104/pp.49.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. E. Evolution of Nitrogen Oxide(s) during In Vivo Nitrate Reductase Assay of Soybean Leaves. Plant Physiol. 1981 Dec;68(6):1488–1493. doi: 10.1104/pp.68.6.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly S. O., Campbell W., Tolbert N. E. NADPH- and NADH-nitrate reductases from soybean leaves. Arch Biochem Biophys. 1976 Jun;174(2):431–439. doi: 10.1016/0003-9861(76)90371-4. [DOI] [PubMed] [Google Scholar]
- King J., Khanna V. A Nitrate Reductase-less Variant Isolated from Suspension Cultures of Datura innoxia (Mill.). Plant Physiol. 1980 Oct;66(4):632–636. doi: 10.1104/pp.66.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas J. C., Harper J. E., Hageman R. H. Nitrate Reductase Activity in Soybeans (Glycine max [L.] Merr.): I. Effects of Light and Temperature. Plant Physiol. 1976 Dec;58(6):731–735. doi: 10.1104/pp.58.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan S. A., Nelson R. S., Harper J. E. Soybean Mutants Lacking Constitutive Nitrate Reductase Activity : II. Nitrogen Assimilation, Chlorate Resistance, and Inheritance. Plant Physiol. 1983 Jun;72(2):510–514. doi: 10.1104/pp.72.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl R. L., Harper J. E., Hageman R. H. Improvements of the nitrite color development in assays of nitrate reductase by phenazine methosulfate and zinc acetate. Plant Physiol. 1974 Jun;53(6):825–828. doi: 10.1104/pp.53.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomonson L. P., Vennesland B. Nitrate Reductase and Chlorate Toxicity in Chlorella vulgaris Beijerinck. Plant Physiol. 1972 Oct;50(4):421–424. doi: 10.1104/pp.50.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner R. L. Nitrate Utilization by Nitrate Reductase-deficient Barley Mutants. Plant Physiol. 1981 Apr;67(4):740–743. doi: 10.1104/pp.67.4.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. J. SOME RESPONSES OF THE BEAN PLANT TO CHLORATE AND PERCHLORATE IONS. Plant Physiol. 1942 Jan;17(1):123–128. doi: 10.1104/pp.17.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]


