Abstract
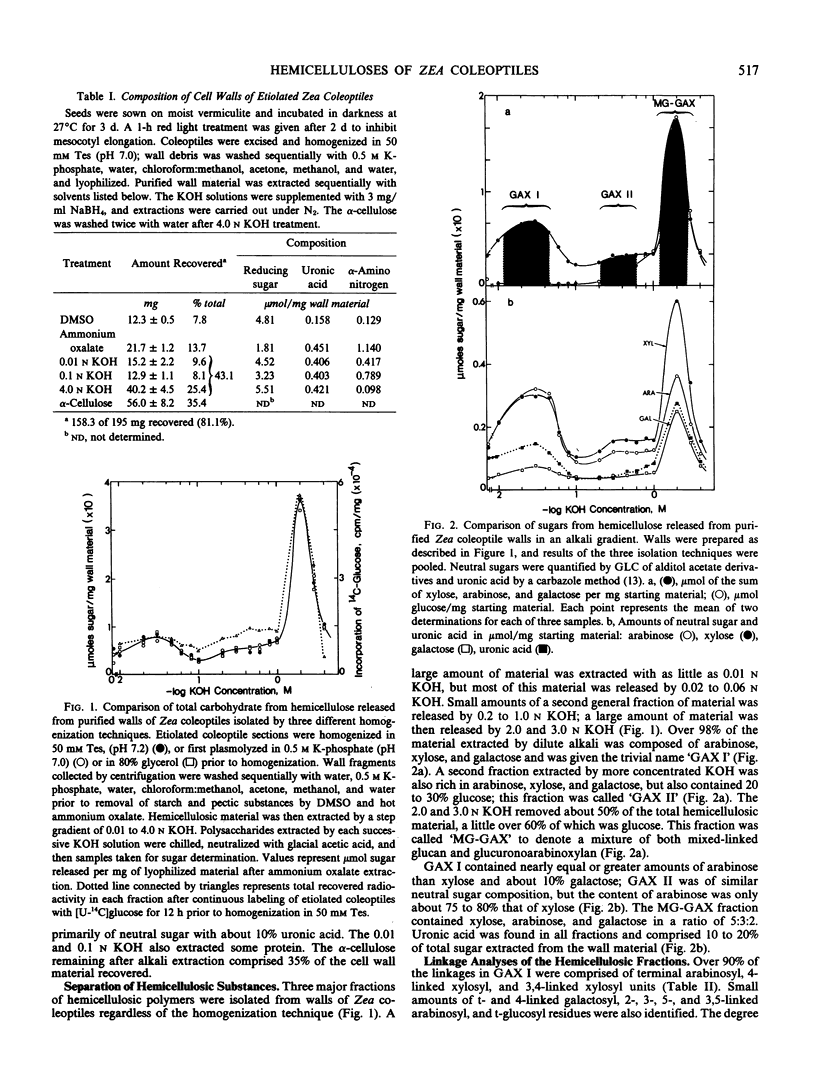
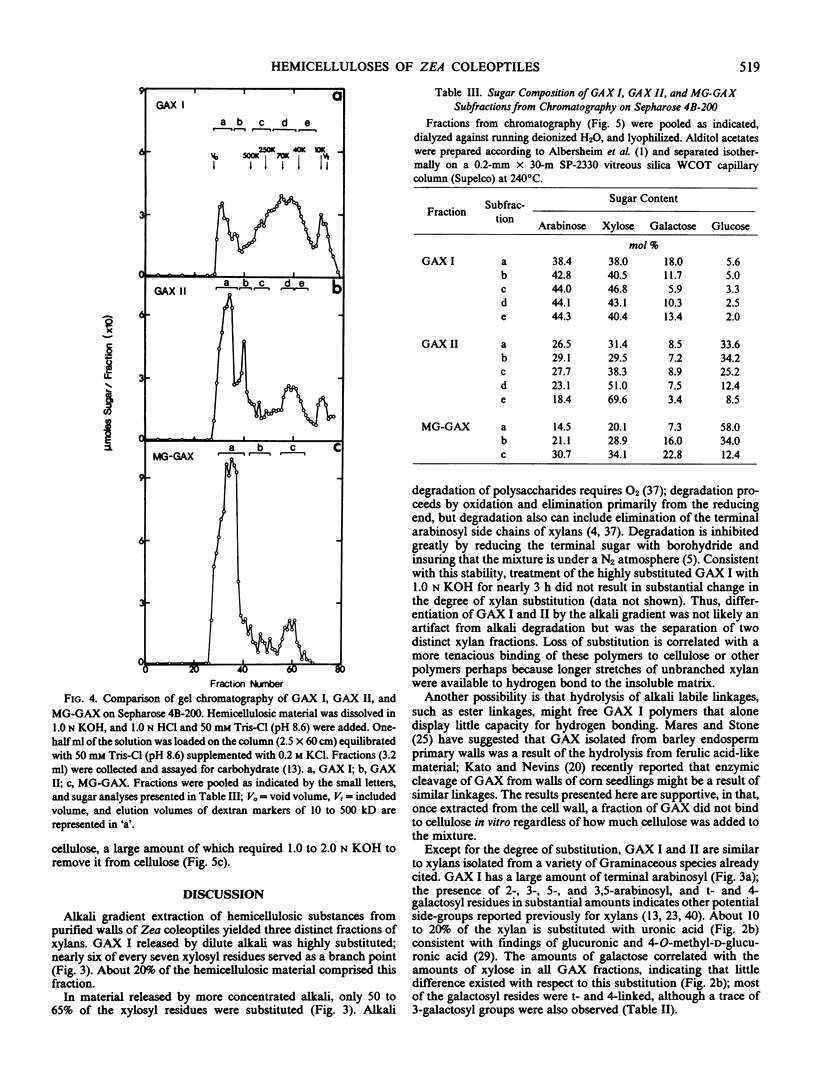
Hemicellulosic polymers comprised about 43% of the primary walls of Zea mays L. cv WF9 × Bear 38 coleoptiles; these polymers were separated by an alkali-gradient into three major fractions. Fraction 1 (GAX I) was solubilized from walls with 0.01 to 0.045 n KOH and consisted of novel glucuronoarabino(galacto)xylans. Nearly six of every seven residues of these xylans were substituted predominantly with single arabinosyl sidegroups. Fraction 2 (GAX II), material released by 0.45 to 0.8 n KOH, was also enriched with glucuronoarabinoxylan, but only two of every three xylose residues was substituted. This xylan was similar to those found in Zea and other Graminaceous species. Both of these xylan fractions contained uronic acid, terminal- and 4-linked galactosyl, and small amounts of 2-, 3-, 5-, and 3,5-linked arabinosyl units. Fraction 3 (MG-GAX) was released by 2.0 to 3.0 n KOH and consisted of about 60% mixed-linked glucan and about 40% glucuronoarabinoxylan. This fraction represented about half of the total hemicellulosic material of the primary walls of these coleoptiles.
The molecular weight of the highly substituted GAX I was approximately 21 kilodaltons as determined by the ratio of reducing sugar to total sugar, but ultracentrifugation studies and gel chromatography on Sepharose 4B-200 indicated that GAX I formed larger aggregates of primarily 50 to 90 kilodaltons, whereas most of the GAX II and virtually all of the MG-GAX materials were excluded by Sepharose 4B; exclusion from the Sepharose was correlated with the presence of mixed-linked glucan. Only GAX II and MG-GAX material demonstrated any appreciable binding to cellulose in vitro.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bretthauer R. K., Wu S. Synthesis of the mannosyl-O-serine (threonine) linkage of glycoproteins from polyisoprenylphosphate mannose in yeast (Hansenula holstii). Arch Biochem Biophys. 1975 Mar;167(1):151–160. doi: 10.1016/0003-9861(75)90451-8. [DOI] [PubMed] [Google Scholar]
- Carpita N. C., Brown R. A., Weller K. M. Uptake and Metabolic Fate of Glucose, Arabinose, and Xylose by Zea mays Coleoptiles in Relation to Cell Wall Synthesis. Plant Physiol. 1982 May;69(5):1173–1180. doi: 10.1104/pp.69.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. E. The control of cell enlargement. Symp Soc Exp Biol. 1977;31:101–115. [PubMed] [Google Scholar]
- Huber D. J., Nevins D. J. beta-d-Glucan Hydrolase Activity in Zea Coleoptile Cell Walls. Plant Physiol. 1980 May;65(5):768–773. doi: 10.1104/pp.65.5.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson C. A., Jeanes A. A new modification of the carbazole analysis: application to heteropolysaccharides. Anal Biochem. 1968 Sep;24(3):470–481. doi: 10.1016/0003-2697(68)90154-1. [DOI] [PubMed] [Google Scholar]
- Loescher W., Nevins D. J. Auxin-induced Changes in Avena Coleoptile Cell Wall Composition. Plant Physiol. 1972 Nov;50(5):556–563. doi: 10.1104/pp.50.5.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Rottenberg D. A. Uronic acid constituents of oat-coleoptile cell walls. Biochem J. 1964 Mar;90(3):646–655. doi: 10.1042/bj0900646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Sugar composition of oat-coleoptile cell walls. Biochem J. 1963 Oct;89(1):144–150. doi: 10.1042/bj0890144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandford P. A., Conrad H. E. The structure of the Aerobacter aerogenes A3(S1) polysaccharide. I. A reexamination using improved procedures for methylation analysis. Biochemistry. 1966 May;5(5):1508–1517. doi: 10.1021/bi00869a009. [DOI] [PubMed] [Google Scholar]
- WHISTLER R. L., BEMILLER J. N. Alkaline degradation of polysaccharides. Adv Carbohydr Chem. 1958;13:289–329. doi: 10.1016/s0096-5332(08)60359-8. [DOI] [PubMed] [Google Scholar]


