Abstract
Background:
Increased inflammation is reported in Major Depressive Disorder (MDD), which may be more pronounced in suicidal subjects. Vitamin D deficiency may drive this pro-inflammatory state due to vitamin D’s anti-inflammatory effects.
Methods:
We quantified plasma 25-hydroxyvitamin D (25(OH)D) and inflammation markers interleukin (IL)-6 and tumor necrosis factor (TNF)-α, and other inflammatory indices, neutrophil-to-lymphocyte ratio (NLR) and white blood cell count (WBC) in 48 un-medicated MDD subjects (n=17 with mild-to-moderate suicidal ideation [SI]) and 54 controls. IL-6 and TNF-α were combined into a composite inflammation score.
Results:
There were no significant differences in 25(OH)D levels between MDD and controls (p=0.24) or between MDD with and without SI (p=0.61). However, 25(OH)D was negatively correlated with all measured inflammation markers; these correlations were stronger in MDD subjects, and particularly in those with SI. MDD status significantly moderated the relationships between 25(OH)D and NLR (p=0.03), and 25(OH)D and WBC (p<0.05), and SI significantly moderated the relationship between 25(OH)D and NLR (p=0.03).
Limitations:
The study was cross-sectional, thereby limiting causal inference, and had a small sample size. Only seventeen of the MDD subjects had SI.
Conclusion:
While 25(OH)D levels did not significantly differ in MDD vs. controls, or in MDD with or without SI, lower 25(OH)D was associated with indices of immune activation in MDD, especially in cases with SI. Although our findings do not address causality, they are consistent with findings that relatively low 25(OH)D levels in MDD are associated with a pro-inflammatory state.
Keywords: Vitamin D, 25(OH)D, Inflammation, Major Depressive Disorder, Suicidal Ideation
1. Introduction
Major Depressive Disorder (MDD) is a significant cause of morbidity and mortality worldwide, yet its pathophysiology and somatic manifestations are not fully understood. Increased blood inflammatory markers have frequently been reported in MDD subjects compared to healthy controls (Dhabhar et al., 2009; Dowlati et al., 2010; Howren et al., 2009; Miller and Raison, 2016; Schiepers et al., 2005), and this immune activation may be even more pronounced in suicidal individuals (Brundin et al., 2017; Brundin et al., 2015; Holmes et al., 2018; Kim et al., 2008; Tonelli et al., 2008). Meta-analyses and reviews indicate that MDD may be associated with decreased vitamin D levels (Anglin et al., 2013; (Humble, 2010), though individual study results have been mixed (Stefanowski et al., 2017). In addition to its well-recognized role in maintaining skeletal calcium balance, vitamin D exerts robust immunomodulatory effects in both the adaptive and the innate immune system by several different mechanisms. These can be summarized as favoring the T-helper 2 (Th-2) cytokine driven humoral immune response and decreasing the pro-inflammatory Th-1 cytokine driven cell-mediated immune response (van Etten and Mathieu, 2005). Several studies have examined plasma vitamin D levels in MDD (Anglin et al., 2013; Milaneschi et al., 2014; Parker et al., 2017; Shaffer et al., 2014; Stefanowski et al., 2017; von Kanel et al., 2015), but very few have investigated the relationship between vitamin D and inflammation in MDD (Accortt et al., 2016; Grudet et al., 2014).
We previously reported that plasma 25-hydroxyvitamin D (25(OH)D) levels are significantly lower in recent suicide attempters compared to controls and compared to depressed subjects without a recent suicide (Grudet et al., 2014). In that study, we reported a significant negative correlation between plasma 25(OH)D and pro-inflammatory markers in psychiatric patients, but not in controls (Grudet et al., 2014). This raises the possibility that a relative deficiency in 25(OH)D may promote a pro-inflammatory state and that the relationship between 25(OH)D and inflammation may be differentially regulated amongst individuals with psychiatric symptoms and healthy subjects. Our previous study included psychiatric subjects with various diagnoses, and the majority had made a recent suicide attempt (Grudet et al., 2014). The suicidal subjects were recruited at the psychiatric emergency unit within one week after a suicide attempt. Furthermore, in that study, most --subjects took psychotropic medications and many had somatic co-morbidities, which could have impacted the results. In the present study, we aimed to replicate these findings of an inverse relationship between 25(OH)D and inflammation in a well characterized, medication-free and somatically healthy MDD sample and healthy controls. Based on our prior results (Grudet et al., 2014), we hypothesized that the negative association between 25(OH)D levels and inflammation would be more robust in MDD subjects compared to controls. Although we did not have the statistical power to directly compare MDD with suicidal ideation (SI) and MDD without SI, we also wanted to test, in exploratory analyses, if the negative correlation between 25(OH)D and inflammatory markers is especially pronounced in those with SI.
2. Subjects and methods
This study was approved by the Committee on Human Research of the University of California, San Francisco (UCFS) (protocol #10–00825). All patients gave their written informed consent before participating in the study and were compensated for their participation.
2.1.1. Study participants
Forty-eight un-medicated MDD subjects and 54 healthy controls were enrolled in the study. Demographic characteristics are described in Table 1. Subjects were recruited by flyers, bulletin board notices, Craigslist postings, newspaper ads, and clinical referrals in the San Francisco Bay Area between 2011–2015.
Table 1.
Demographic characteristics of all subjects and MDD subdivided into Suicidal Ideation/Non-Suicidal Ideation groups. IL-6 and TNF-alpha levels have been previously reported elsewhere (Lindqvist et al., 2017).
| MDD (n=48) | Control (n=54) | P-value | MDD with Suicidal Ideation (n=17) | MDD without Suicidal Ideation (n=31) | P-value | |
|---|---|---|---|---|---|---|
| Age (years. mean ± SD) | 39.3 ± 14.9 | 37.9 ± 13.9 | 0.71 | 42.1 ± 14.3 | 37.7 ± 15.2 | 0.27 |
| Sex (% female) | 56% | 61% | 0.62 | 53% | 58% | 0.73 |
| BMI (mean ± SD) | 26.0 ± 4.5 | 24.5 ± 4.9 | 0.08 | 25.5 ± 4.2 | 26.3 ± 4.7 | 0.61 |
|
Ethniciy (%) Caucasian Black/African-American All other |
52% 8% 40% |
57% 6% 37% |
0.80 |
53 0% 47% |
51.5% 13% 35.5% |
0.28 |
| Smoking (% Smokers) | 27% | 6% | <0.01** | 24% | 29% | 0.68 |
| Sampling season (% summertime) | 48% | 50% | 0.83 | 29% | 58% | 0.06 |
| HDRS total score (mean ± SD) | 20.3 ± 33.1 | N/A | N/A | 22.1 ± 4.25 | 19.3 ± 2.18 | <0.01** |
| 25(OH)D, nmol/L (mean ± SD) a) | 51.1 ± 20.0 | 54.9 ± 21.0 | 0.24 | 48.4 ± 19.0 | 52.6 ± 20.7 | 0.61 |
| NLR (mean ± SD) a) | 2.2 ± 0.9 | 2.3 ± 1.2 | 0.98 | 2.3 ± 1.0 | 2.2 ± 0.8 | 0.81 |
| WBC (mean ± SD) a) | 5.5 ± 1.4 | 5.6 ± 1.5 | 0.58 | 5.5 ± 1.8 | 5.4 ± 1.2 | 0.96 |
Correlation is significant at the 0.05 level (2-tailed)
Correlation is significant at the 0.01 level (2-tailed)
25(OH)D, NLR and WBC were normalized using 90% winsorization prior to parametric analyses. P-values are based on these calculations but raw, non-winsorized, means are given here.
The Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID) (First, 1997) was used to determine all diagnoses, which were confirmed by a separate clinical interview with a board-certified psychiatrist. Control subjects had no history of any DSM-IV-TR Axis I disorder. Depressed subjects were diagnosed with current MDD, without psychotic features, and scored ≥17 on the 17-item version of the Hamilton Depression Rating Scale (HDRS) (Hamilton, 1960). Exclusion criteria for MDD subjects were: a suicide attempt or current suicidal intent within the past week, psychotic symptoms during their current major depressive episode, any history of psychosis that did not occur within the context of a past depressive episode, history of mania or hypomania, post-traumatic stress disorder (PTSD) or any eating disorder within one month of study participation, and substance/alcohol abuse or dependence within six months of study entry. Individuals who were clinically determined to have current suicide intent were excluded from the present study and referred to more appropriate resources for treatment. Aside from PTSD, the presence of co-morbid anxiety disorders was not exclusionary if MDD was considered the primary diagnosis.
All study participants underwent routine physical examination, medical history assessment, and routine blood screening (complete chemistry panel, including electrolytes, kidney and liver function, protein and albumin, complete blood count with differential count, lipid panel, folate, fasting glucose, HbA1c, and thyroid function tests) to rule out the presence of any acute or chronic inflammatory disorders, neurological disorders, or other major medical conditions considered to be potentially confounding. None of the subjects had any vaccinations for at least six weeks prior to enrollment in the study. Further, all subjects were free of hormonal or psychotropic medications (e.g., antidepressants, steroids, hormonal birth control, etc.) or any other potentially interfering medications. None of the subjects were taking any vitamin supplements (including 25(OH)D) above the U.S. recommended daily allowances (25(OH)D, 15 μg/day) for six weeks prior to study start and all but one MDD participant stopped taking 25(OH)D entirely for two weeks before study start. If needed, MDD subjects were allowed to take short-acting sedative-hypnotics for sleep up to a maximum of three times per week, but none within one week of participation. On the day of each study visit, all subjects had to pass a urine toxicology screen (marijuana, cocaine, amphetamines, phencyclidine, opiates, methamphetamine, tricyclic antidepressants, and barbiturates) and a urine test for pregnancy in women of child-bearing potential.
2.1.2. Ratings
Depression severity was rated using the 17-item version of the Hamilton Depression Rating Scale (HDRS). Each HDRS rating session was conducted with two raters present who scored within one point of each other; if this was not achieved, a consensus rating was determined. For exploratory analyses, MDD subjects were categorized based on their HDRS suicidality item scores, which have a possible range of 0–4. Those with scores of 0 (indicating absence of suicidal ideation within the past week) were categorized as the “non-Suicidal Ideation group” (NSI; n=31). Those with scores of 1–3 (indicating responses ranging from “feelings that life is not worth living” to suicidal “ideas or gestures” in the past week) were categorized as the “Suicidal Ideation group” (SI; n=17). Subjects scoring 4 on the HDRS suicidality item, indicating a suicide attempt or current suicidal intent within the past week, were excluded from the study, as explained above and in (Khan et al., 2019). Physical activity was measured with the Yale Physical Activity Survey (YPAS) Vigorous Activity Index Score (Dipietro et al., 1993).
2.2. Study procedures
Subjects were admitted as outpatients to the UCSF Clinical and Translational Science Institute between 8:00 a.m. and 11:00 a.m., having fasted (except water) since 10:00 p.m. the night before. Subjects were instructed to sit quietly and relax for 25 – 45 minutes before blood samples were obtained. Following this blood draw, subjects underwent depression severity rating using the 17-item version of Hamilton Depression Rating Scale (HDRS).
2.3. Vitamin D assays, 25(OH)D2 and 25(OH)D3
Serum was collected into serum separator tubes and stored at −80° Celsius until assay. Stored serum samples were thawed once before the 25(OH)D2 (vitamin D2) and 25(OH)D3 (vitamin D3) analysis. The time of storage (4–7 years) is not likely to have had an impact on the quality of the analysis due to the relatively stable 25(OH)D molecule (Wielders and Wijnberg, 2009).
Analyses of 25(OH)D2 and 25(OH)D3 were done by liquid-chromatography-mass-spectrometry, model Sciex API 4000 LC/MS/MS (MA, USA). Coefficient of variation (CV) values were as follows: for 25(OH)D2, 6,0% at 40 nmol/L and 5% at 120 nmol/L and for 25(OH)D3 6% at 40 nmol/L and 4% at 120 nmol/L. The lowest detection limit is 6 nmol/L for both for 25(OH)D2 and 25(OH)D3. The analyzes were conducted by The department of Clinical Chemistry at Scania University Hospital, which is accredited by SWEDAC (the Swedish Board for Accreditation and Conformity Assessment) and participates in the external assurance program of DEQAS (Vitamin D External Quality Assessment Scheme, UK). In line with clinical guidelines (Phinney et al., 2017), in cases of a 25(OH)D2 level >10 nmol/L, the 25(OH)D2 levels were added to the 25(OH)D3 levels in the statistical analysis, i.e. while describing vitamin D (25(OH)D) in the article, the sum of 25(OH)D2 and 25(OH)D3 is referred to as 25(OH)D. Only two MDD subjects had 25(OH)D2 levels >10 nmol/L; (11 nmol/L and 37 nmol/L, respectively. Blood sampling was performed across the year. Sampling season was divided into “Summertime”, which equals April-September and “Wintertime”, which equals October-March.
In this study, we used a cutoff of <50 nmol/L to indicate “lower vitamin D”. Levels between 50–74 nmol/L were considered “suboptimal/insufficient” and levels ≥75 nmol/L as “sufficient” (Holick, 2007).
2.4. Inflammatory markers assays
Plasma was collected into lavender EDTA vacutainer tubes and stored at −80° Celsius until assay. We assayed plasma cytokines IL-6 and TNF-α, two well established markers of inflammation that we previously reported to be elevated in MDD subjects in the current sample (Lindqvist et al., 2017). Absolute values of IL-6 and TNF-α are not reported here in order to avoid duplicate publication, but these can be found in our previous report (Lindqvist et al., 2017). In addition, we assessed the neutrophile-to-lymphocyte ratio (NLR), as well as white blood cell count (WBC), which are considered to be inflammatory markers of systemic, low-grade inflammation and which have recently been related to vitamin D (Akbas et al., 2016). Previous literature has suggested NLR to be an independent prognostic factor of morbidity and useful in stratification of mortality in several conditions, for example in certain cancers and cardiovascular diseases (Forget et al., 2017).
Assey methodology is described in Lindqvist et al (2017).
2.5. Statistics
All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS, version 25). All tests were 2-tailed with alpha = 0.05. 25(OH)D, NLR and WBC data were skewed; therefore we did a 90% winsorization to normalize the data. In order to limit the number of statistical comparisons, we decided to calculate a composite inflammation score by summarizing the z-scores of IL-6 and TNF-α. The neutrophil-to-lymphocyte ratio was based on the absolute neutrophil and lymphocyte count. For unadjusted group-wise comparisons, we used Studentʼs T-test and Pearsonʼs Chi-square test, ANCOVAs were used in order to adjust for potential confounders. For correlation analysis we used Pearsonʼs correlation and we used the PROCESS tool for SPSS in moderation analysis.
Based on their known association with vitamin D and/or inflammation we adjusted for the following variables in the analyses: age, sex, tobacco use, BMI and sampling season. In exploratory analyses, we subdivided MDD subjects into a “suicidal ideation group” (SI) and a “non-suicidal ideation group” (NSI), as defined in the “Ratings” section.
3. Results
3.1. Demographics and group comparisons
Demographic characteristics are summarized in Table 1. MDD subjects were more likely to be smokers (χ2=8.9; p<0.01) and tended to have higher BMI (t=1.8; p=0.08), and lower YPAS score (t=1.8, p=0.07) than controls. There were no significant differences between MDD subjects and controls regarding age, sex, ethnicity, blood sampling season or physical activity (all p>0.60). 25(OH)D correlated negatively with BMI (r=−0.34; p<0.001) but not with YPAS score (r=0.07, p=0.48). Ethnicity was significantly associated with 25(OH)D levels (Caucasian mean 25(OH)D=59±20 nmol/L, Black/African-American mean 25(OH)D=35±10 nmol/L and all other mean 25(OH)D=45±19 nmol/L)(p<0.01). This pattern is consistent with known differences in 25(OH)D levels between ethnic groups due to differences in skin pigmentation (Weishaar et al., 2016). Women had significantly higher 25(OH)D levels compared to men (t=2.7; p<0.01) and current tobacco users tended to have lower 25(OH)D levels compared to tobacco non-users (t=1.9; p=0.07).
3.2. 25(OH)D and inflammatory markers in MDD and controls
Absolute levels of 25(OH)D and inflammation markers are summarized in Table 1. There were no significant differences in 25(OH)D, NLR or WBC between MDD subjects and controls (all p>0.6), or between MDD subjects with and without SI (all p>0.8). These results did not change after adjusting for age, sex, BMI, smoking and sampling season (all p>0.44).
Fifty-four percent of MDD subjects and 39% of controls had lower 25(OH)D (<50 nmol/L). Eight percent of MDD subjects and 17% of controls had sufficient 25(OH)D levels (>75 nmol/L) and 39 % of MDD subjects and 35 % of the controls had suboptimal 25(OH)D levels (50 – 74 nmol/L). None of these differences were significant (χ2 = 2.97; p=0.28). There were no significant differences in categorical 25(OH)D levels between SI and NSI (χ2 = 0.94; p=0.63).
3.3. Associations between 25(OH)D, inflammatory markers and symptom severity
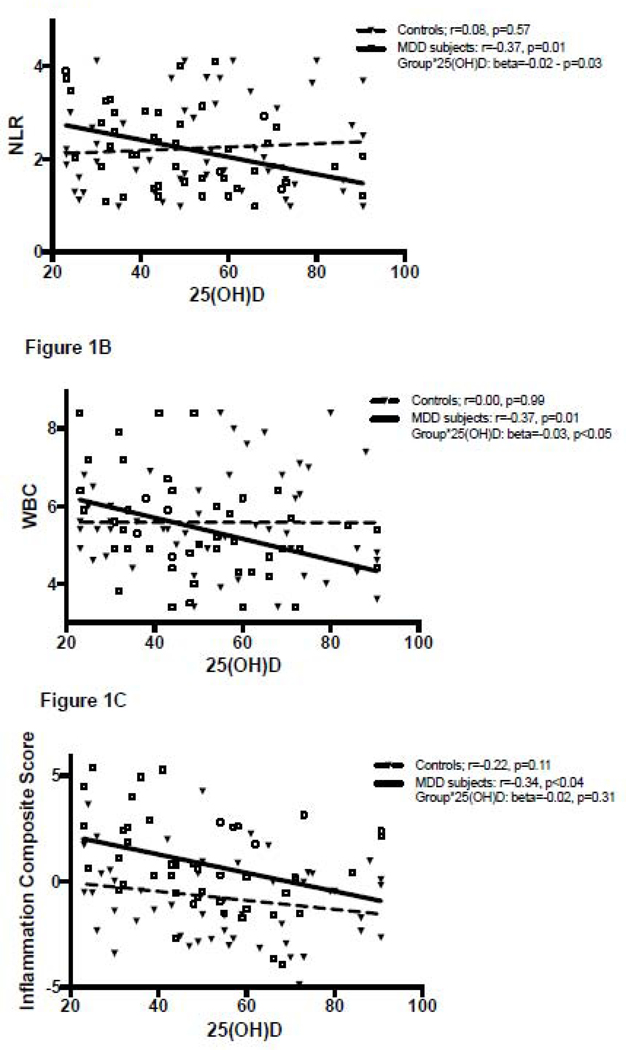
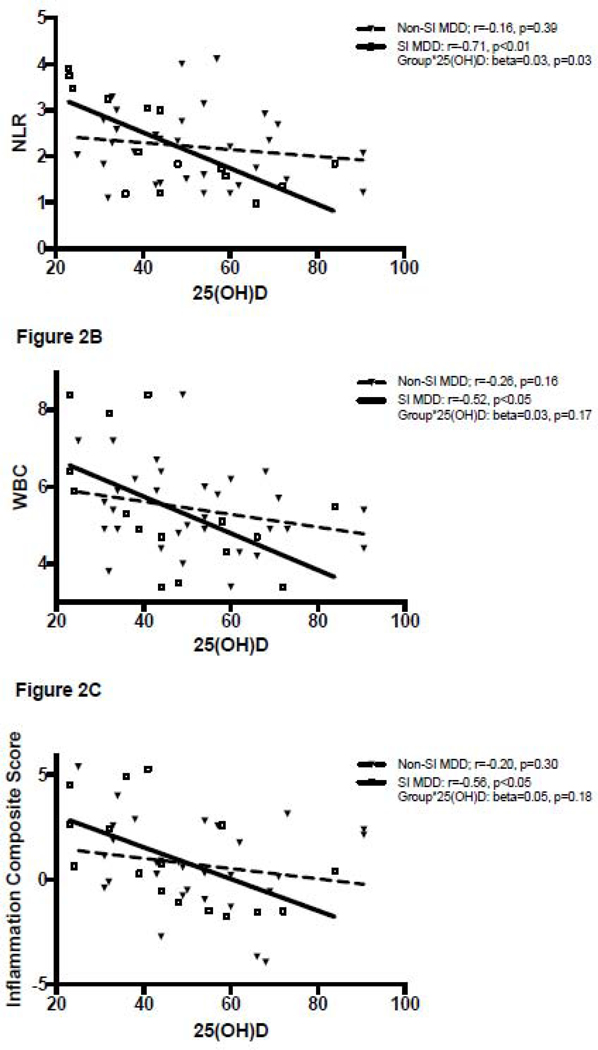
Correlations between 25(OH)D and inflammatory markers are summarized in Table 2 and shown in Figures 1 and 2. 25(OH)D levels were significantly negatively correlated with the inflammation composite score across all subjects (r=−0.30, p<0.01) and within the MDD group alone (r=−0.34, p<0.05), but this relationship was not statistically significant within the healthy control group (r=−0.22, p=0.11). A significant negative correlation between 25(OH)D levels and the inflammation composite score was seen amongst MDD subjects with SI (r=−0.56, p<0.05), but not MDD NSI subjects (r=−0.20, p=0.30). A similar pattern was seen with the NLR and the WBC, which were both significantly negatively correlated with 25(OH)D levels in the MDD group (25(OH)D correlation with NLR r=−0.37 p=0.01, 25(OH)D correlation with WBC r=−0.37 p=0.01) but not in the healthy controls (25(OH)D correlation with NLR r=0.08 p=0.57, 25(OH)D correlation with WBC r=0.00, p=0.99). Within the MDD group, this relationship was significant in those with SI (25(OH)D correlation with NLR r=−0.71 p<0.01, 25(OH)D correlation with WBC r=−0.52 p<0.05) but not in the NSI group (25(OH)D correlation with NLR r=−0.16 p=0.39, 25(OH)D correlation with WBC r=−0.26 p=0.16).
Table 2.
Correlations between 25(OH)D and different variables in all subjects and MDD divided into Suicidal Ideation/Non-Suicidal Ideation groups, Pearson’s r.
| Variable | All subjects (n = 102) | MDD (n = 48) | Control (n = 54) | Suicidal Ideation MDD(n = 17) | Non-Suicidal Ideation MDD (n = 31) |
|---|---|---|---|---|---|
| Inflammation Composite Score | r=−0.30 (p<0.01)** | r=−0.34 (p<0.05)* | r=−0.22 (p=0.11) | r=−0.56 (p<0.05)* | r=−0.20 (p=0.30) |
| NLR a | r=−0.10 (p=0.34) | r=−0.37 (p=0.01)** | r=0.08 (p=0.57) | r=−0.71 (p<0.01)** | r=−0.16 (p=0.39) |
| WBC b | r=−0.15 (p=0.14) | r=−0.37 (p=0.01)** | r=0.00 (p=0.99) | r=−0.52 (p<0.05)* | r=−0.26 (p=0.16) |
Correlation is significant at the 0.05 level (2-tailed)
Correlation is significant at the 0.01 level (2-tailed)
Missing data from one MDD and two controls
Missing data from one control
Figure 1.
A-C. Inflammation markers plotted against 25(OH)D in MDD subjects and healthy controls. Data were normalized using 90% winsorization.
Figure 2.
A-C. Inflammation markers plotted against 25(OH)D in MDD subjects with suicidal ideation (SI) and MDD subjects without suicidal ideation (NSI). Data was normalized using 90% winsorization.
MDD status was a significant moderator of the relationship between 25(OH)D and NLR (p=0.03; Fig. 1a) and between 25(OH)D and WBC (p<0.05; Fig. 1b), but not between 25(OH)D and the inflammation composite score (p=0.31; Fig. 1c). Adjusting for age, sex, BMI, sampling season and smoking did not significantly change the results of these moderation analyses.
In MDD subjects only, presence of SI was a significant moderator of the relationship between 25(OH)D and NLR (p=0.03), but not WBC (p=0.17) or the inflammation composite score (p=0.18). Adjusting for age, sex, BMI, sampling season and smoking did not significantly change the results of these analyses.
Total HDRS scores were not significantly correlated with 25(OH)D, NLR, WBC or inflammation composite score (all p>0.15).
4. Discussion
Though we did not detect any significant group differences in 25(OH)D levels between MDD subjects and healthy controls, nor any significant association between 25(OH)D and depression severity, we did find that the relationship between 25(OH)D and inflammation differed between MDD and control subjects. Specifically, 25(OH)D levels were significantly and negatively correlated with inflammatory markers in MDD, but not in healthy controls. Exploratory analyses found no significant group differences in 25(OH)D levels between MDD subjects with and without SI, though the relationship between 25(OH)D and inflammation also differed between these two groups. Specifically, 25(OH)D levels were significantly negatively correlated with inflammatory markers in MDD subjects with SI, but not in those without SI. Adjusting for for age, sex, BMI, sampling season and tobacco use did not change these results.
In the present study, we did not find a significant difference in 25(OH)D levels between MDD subjects and healthy controls, nor did we find a correlation with depressive symptom severity, which is in line with some previous reports (Dana-Alamdari et al., 2015; Grudet et al., 2014), but not others (Hoogendijk et al., 2008). There are several potential explanations for these inconsistent findings across studies, including differences in various sample characteristics such as sample size, age, gender, BMI, ethnicity, depression severity, degree of comorbidity, lack of data about UVB-radiation exposure, seasonality and latitude, medication status (especially antidepressants medication and vitamin D or multi-vitamin intake), smoking habits and dietary habits. Results from several previous studies suggest that vitamin D levels are the lowest among subjects with severe-to-very severe depression symptoms. For example, in the largest cross-sectional study to date (the Netherlands Study of Depression and Anxiety, NESDA, comprising > 2000 subjects) participants with current depressive disorder had significantly lower vitamin D levels compared to controls (Milaneschi et al., 2014). The vitamin D levels declined progressively in a “dose-response” manner in relation to depression severity. Thus, the difference in vitamin D levels between MDD subjects and healthy controls was consequently more pronounced in those with the most severe symptoms. In the present study, most of the MDD subjects had only moderate depression severity. Moreover, in the study by Milaneschi et al., a large proportion of the subjects used antidepressants and had somatic co-morbidities, while all the subjects in the present study were un-medicated and somatically healthy. There are several additional factors that may explain why we did not find any group difference in 25(OH)D levels between MDD subjects and controls. Although we did adjust for several known vitamin D-related factors such as sex, age, BMI, smoking and seasonality, we cannot rule out the possibility of residual confounding due to factors that we were not able to take into account, such as dietary habits and actual UVB-radiation exposure. Lastly, low vitamin D may be more closely associated with certain clinical features of MDD than others. For instance, we and others have previously shown that low 25(OH)D may be associated with attempted and completed suicide (Grudet et al., 2014; Umhau et al., 2013), suggesting that vitamin D may be more closely linked suicidal depression rather than a MDD diagnosis per se (Tariq et al., 2011). In the current study we were not able to confirm (or refute) this hypothesis since a suicide attempt or current suicidal intent within the past week were exclusion criteria. Consequently, the lack of difference in 25(OH)D levels between MDD with and without suicidal ideation does not contradict our previous results where recent suicide attempters had significantly lower 25(OH)D levels than MDD without a recent suicide attempt, and healthy controls (Grudet et al., 2014).
In the present study, we found a significant and negative correlation between 25(OH)D and the inflammatory composite score, based on IL-6 and TNF-α levels, in MDD subjects but not in the controls. Additional markers of systemic and low-grade inflammation, NLR and WBC, were also significantly negatively correlated with 25(OH)D in MDD subjects, but not in controls. We cannot rule out that the lack of significant findings in the controls is a result of insufficient power, although the sample size of the MDD group was smaller than that of the control group. While dividing MDD subjects according to presence/absence of suicidal ideation (SI or NSI), these correlations lost significance in the NSI group but remained significant in the SI group. These results should be interpreted with caution, however, due to the small subgroups of MDD subjects with (n=17) and without SI (n=31). These data are largely consistent with results from our previous study, where a significant negative association between 25(OH)D and inflammatory markers was found in MDD outpatients without a recent suicide attempt and in inpatients with mixed psychiatric diagnoses who had a recent suicide attempt, but not in healthy controls (Grudet et al., 2014). Only a few studies to date have investigated the relationship between vitamin D and inflammation in psychiatric cohorts (Accortt et al., 2016; Antai-Otong, 2014; Grudet et al., 2014). However, one prospective study also provides evidence of an inverse relationship between vitamin D and inflammation in depression. This study reported an inverse correlation between vitamin D levels and post-partum depression (PPD), and this effect was significantly moderated by inflammatory markers, i.e. low vitamin D was associated with PPD, but only in those women with higher inflammation (Accortt et al., 2016). It is not known whether the association between vitamin D and inflammation in subjects with MDD speaks to a causal relationship. Whereas it is usually presumed that low vitamin D levels may promote an inflammatory state (as described in Introduction), it is also possible that higher inflammation can lead to lower vitamin D levels. In fact, some studies suggest that an inflammatory process in the body per se is causing a decline in vitamin D levels (Autier et al., 2014; Mangin et al., 2014). This theory proposes that the circulating, inactive form of vitamin D decreases in response to an immunological challenge, i.e. the vitamin D that we measure is being consumed, which would then reflect the negative association between 25(OH)D and inflammatory markers observed in our sample (Albert et al., 2009). As such, it is possible that low vitamin D may promote low-grade inflammation, inflammation may decrease vitamin D, or that this relationship may be bidirectional.
Vitamin D’s regulation of systemic immune functions involves key regulatory mechanisms of both innate and adaptive immune responses. These processes are mainly mediated by influencing downstream effects of activated Toll-like receptors (TLRs), expressed on antigen-presenting cells such as macrophages and dendritic cells (DC). TLRs are key players in both the innate and the adaptive immune systems and excessive activation of these induce chronic low-grade inflammation (Hemmi and Akira, 2005), associated with various pathological conditions such as depression and suicide (Brundin et al., 2017; Schiepers et al., 2005). There is an interplay between TLRs and vitamin D and many studies have demonstrated a negative correlation between the expression of TLRs and 25(OH)D, especially in diseases with an inflammatory component (Adamczak, 2017). By influencing the DC phenotype and by inhibiting the maturation of DCs, vitamin D has modulatory effects on the nature of the T cell response upon TLR activation (Chambers and Hawrylowicz, 2011). Vitamin D inhibits or suppresses, directly or indirectly, the proliferation and differentiation of T helper cells into pro-inflammatory Th-1 and Th-17 immune cells. Vitamin D also promotes the production of anti-inflammatory Th-2 and regulatory T immune cells (Tregs), the latter being an important inhibitor of many immune responses and a maintainer of the immunologic tolerance in the periphery (Chambers and Hawrylowicz, 2011; Do et al., 2008; Sassi et al., 2018). The suggested net result of the effects of Vitamin D is a skew towards a Th-2/Treg immune response with increased production of anti-inflammatory cytokines such as IL-10, IL-4, IL-5 and transforming growth factor (TGF)-β, and decreased pro-inflammatory cytokines such as IL-1β, IL-2, IL-6, interferon (INF)-γ, TNF-α and IL-12 (Aranow, 2011; Baeke et al., 2010; Umar et al., 2018; Zhang et al., 2012).
In addition to its influence on systemic inflammation, Vitamin D may also directly influence immune functions of the brain. The 25(OH)D activating enzyme, 1α-hydroxylase, and vitamin D receptors (VDRs) are present is numerous cell types and tissues in the body, including most cells of the immune system (Skrobot et al., 2018) as well as in neurons and glial cells, thus making it possible for vitamin D to act locally in the brain via autocrine, paracrine and intracrine mechanisms (Borges et al., 2011; Cannell et al., 2014). In animal studies, activated microglia have been demonstrated to increase the expression of VDR and 1α-hydroxylase, thus enhancing their sensitivity to vitamin D. (Hanisch, 2002). Notably, activated microglia exposed to 25(OH)D and 1,25(OH)2D had reduced expression of pro-inflammatory cytokines IL-6, IL-12, and TNF-α and increased expression of the anti-inflammatory cytokine IL-10 (Boontanrart et al., 2016; Singhal and Baune, 2017). Activated microglia cells exposed to vitamin D may therefore promote a switch from the pro-inflammatory Th-1 cell immune response to the less inflammatory Th-2 cell immune response in the brain. Thus, by altering the immune response by microglia, vitamin D is suggested to be a control mechanism for avoiding a prolonged inflammatory state in the CNS (Singhal and Baune, 2017).
Some studies have reported that MDD subjects are at a higher risk of vitamin D deficiency and that vitamin D supplementation may reduce depressive symptoms, particularly among those with severe deficiency and clinically significant depression (Anglin et al., 2013; Bahrami et al., 2018; Ju et al., 2013; Kjaergaard et al., 2011; McCue et al., 2012; Milaneschi et al., 2014; Parker et al., 2017; Shaffer et al., 2014; Stefanowski et al., 2017). However, randomized controlled studies investigating vitamin D treatment in depression have yielded inconsistent results. In a meta-analysis, Gowda et al. (2015) did not find any significant reduction in depression after vitamin D treatment. Most previous studies, however, focused on individuals with low levels of depression and with sufficient vitamin D levels at baseline, which may be two reasons why any beneficial effects of vitamin D treatment could not been detected. (Gowda et al., 2015; Kjaergaard et al., 2012).
A strength of the present study is the inclusion of medication-free, well phenotyped, somatically healthy MDD subjects as well as healthy controls, where none had taken any vitamin D supplements above the recommended daily dose for at least six weeks before study start. All subjects were free of any psychotropic medications (including antidepressants) and other potentially interfering medications for a minimum of six weeks. A relevant limitation of the study is the cross-sectional study design, which makes it impossible to investigate any causality in the material. Additionally, due to the limited sample size, we were unable to perform subgroup analyses in 25(OH)D deficient/sub-optimal/sufficient subgroups. Further, the sample sizes of the SI and NSI MDD subgroups were too small for meaningful conclusions to be drawn, but they do suggest hypotheses to be tested in future studies.
5. Conclusions
In summary, we here report a significant negative relationship between 25(OH)D and inflammatory markers in MDD subjects but not in controls, and this association was strongest in those MDD subjects with SI. Our findings suggest that low 25(OH)D is associated with a pro-inflammatory state, that is frequently observed in depressed and suicidal individuals, and that this relationship between 25(OH)D and inflammation may be differentially regulated in MDD subjects (especially in those with SI) and healthy controls. Although speculative and a question for future research; there is a possibility that vitamin D supplementation might attenuate inflammation in MDD in individuals with low vitamin D levels, which would be of importance since inflammation may underlie certain medical comorbidities in MDD and may foster antidepressant resistance.
Highlights.
Vitamin D was negatively correlated with inflammation in MDD but not in controls
These associations were stronger in those MDD subjects with suicidal ideation
Relatively low vitamin D may promote inflammation in depressed and suicidal subjects
Acknowledgments
This study was funded by grants from the National Institute of Mental Health (NIMH) (Grant Number R01- MH083784), the O’Shaughnessy Foundation, the Tinberg family, and grants from the UCSF Academic Senate, the UCSF Research Evaluation and Allocation Committee (REAC). This project was also supported by the National Institute of Mental Health (grant number R01 MH083784) and by the National Institutes of Health/National Center for Research Resources (NIH/NCRR) and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Christina Hough is supported by the National Science Foundation Graduate Research Fellowship Program (NSF Grant Number DGE-1650604). Daniel Lindqvist was supported by the Swedish Research Council (registration number 2015-00387), Marie Sklodowska Curie Actions, Cofund (Project INCA 600398), the Swedish Society of Medicine, the Söderström-Königska Foundation, the Sjöbring Foundation, OM Persson Foundation and the province of Scania (Sweden) state grants (ALF). None of the granting or funding agencies had a role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review, or approval of the manuscript. The Co-Principal Investigators, Owen Wolkowitz, MD and Synthia Mellon, PhD, as well as the senior author, Daniel Lindqvist, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. We also acknowledge the statistical advice of Kevin Delucchi, PhD.
Role of the Funding Source
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
The authors declare no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accortt EE, Schetter CD, Peters RM, Cassidy-Bushrow AE, 2016. Lower prenatal vitamin D status and postpartum depressive symptomatology in African American women: Preliminary evidence for moderation by inflammatory cytokines. Archives of women’s mental health 19, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamczak DM, 2017. The Role of Toll-Like Receptors and Vitamin D in Cardiovascular Diseases-A Review. International journal of molecular sciences 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbas EM, Gungor A, Ozcicek A, Akbas N, Askin S, Polat M, 2016. Vitamin D and inflammation: evaluation with neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio. Archives of medical science : AMS 12, 721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert PJ, Proal AD, Marshall TG, 2009. Vitamin D: the alternative hypothesis. Autoimmunity reviews 8, 639–644. [DOI] [PubMed] [Google Scholar]
- Anglin RE, Samaan Z, Walter SD, McDonald SD, 2013. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry 202, 100–107. [DOI] [PubMed] [Google Scholar]
- Antai-Otong D, 2014. Vitamin D: an anti-inflammatory treatment option for depression? Issues in mental health nursing 35, 227–234. [DOI] [PubMed] [Google Scholar]
- Aranow C, 2011. Vitamin D and the immune system. Journal of investigative medicine : the official publication of the American Federation for Clinical Research 59, 881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autier P, Boniol M, Pizot C, Mullie P, 2014. Vitamin D status and ill health: a systematic review. The lancet. Diabetes & endocrinology 2, 76–89. [DOI] [PubMed] [Google Scholar]
- Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C, 2010. Vitamin D: modulator of the immune system. Current opinion in pharmacology 10, 482–496. [DOI] [PubMed] [Google Scholar]
- Bahrami A, Mazloum SR, Maghsoudi S, Soleimani D, Khayyatzadeh SS, Arekhi S, Arya A, Mirmoosavi SJ, Ferns GA, Bahrami-Taghanaki H, Ghayour-Mobarhan M, 2018. High Dose Vitamin D Supplementation Is Associated With a Reduction in Depression Score Among Adolescent Girls: A Nine-Week Follow-Up Study. Journal of dietary supplements 15, 173–182. [DOI] [PubMed] [Google Scholar]
- Boontanrart M, Hall SD, Spanier JA, Hayes CE, Olson JK, 2016. Vitamin D3 alters microglia immune activation by an IL-10 dependent SOCS3 mechanism. Journal of neuroimmunology 292, 126–136. [DOI] [PubMed] [Google Scholar]
- Borges MC, Martini LA, Rogero MM, 2011. Current perspectives on vitamin D, immune system, and chronic diseases. Nutrition (Burbank, Los Angeles County, Calif.) 27, 399–404. [DOI] [PubMed] [Google Scholar]
- Brundin L, Bryleva EY, Thirtamara Rajamani K, 2017. Role of Inflammation in Suicide: From Mechanisms to Treatment. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 42, 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin L, Erhardt S, Bryleva EY, Achtyes ED, Postolache TT, 2015. The role of inflammation in suicidal behaviour. Acta Psychiatr Scand 132, 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell JJ, Grant WB, Holick MF, 2014. Vitamin D and inflammation. Dermato-endocrinology 6, e983401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers ES, Hawrylowicz CM, 2011. The impact of vitamin D on regulatory T cells. Current allergy and asthma reports 11, 29–36. [DOI] [PubMed] [Google Scholar]
- Dana-Alamdari L, Kheirouri S, Noorazar SG, 2015. Serum 25-Hydroxyvitamin D in Patients with Major Depressive Disorder. Iranian journal of public health 44, 690–697. [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, Burke HM, Epel ES, Mellon SH, Rosser R, Reus VI, Wolkowitz OM, 2009. Low serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depression. J Psychiatr Res 43, 962–969. [DOI] [PubMed] [Google Scholar]
- Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER, 1993. A survey for assessing physical activity among older adults. Med Sci Sports Exerc 25, 628–642. [PubMed] [Google Scholar]
- Do JE, Kwon SY, Park S, Lee ES, 2008. Effects of vitamin D on expression of Toll-like receptors of monocytes from patients with Behcet’s disease. Rheumatology (Oxford, England) 47, 840–848. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL, 2010. A meta-analysis of cytokines in major depression. Biol Psychiatry 67, 446–457. [DOI] [PubMed] [Google Scholar]
- First MB, 1997. Structured Clinical Interview for DSM-IV axis I disorders. American Psychiatric Press; Washington, DC. [Google Scholar]
- Forget P, Khalifa C, Defour JP, Latinne D, Van Pel MC, De Kock M, 2017. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC research notes 10, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda U, Mutowo MP, Smith BJ, Wluka AE, Renzaho AM, 2015. Vitamin D supplementation to reduce depression in adults: meta-analysis of randomized controlled trials. Nutrition (Burbank, Los Angeles County, Calif.) 31, 421–429. [DOI] [PubMed] [Google Scholar]
- Grudet C, Malm J, Westrin A, Brundin L, 2014. Suicidal patients are deficient in vitamin D, associated with a pro-inflammatory status in the blood. Psychoneuroendocrinology 50, 210–219. [DOI] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK, 2002. Microglia as a source and target of cytokines. Glia 40, 140–155. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Akira S, 2005. TLR signalling and the function of dendritic cells. Chemical immunology and allergy 86, 120–135. [DOI] [PubMed] [Google Scholar]
- Holick MF, 2007. Vitamin D deficiency. The New England journal of medicine 357, 266–281. [DOI] [PubMed] [Google Scholar]
- Holmes SE, Hinz R, Conen S, Gregory CJ, Matthews JC, Anton-Rodriguez JM, Gerhard A, Talbot PS, 2018. Elevated Translocator Protein in Anterior Cingulate in Major Depression and a Role for Inflammation in Suicidal Thinking: A Positron Emission Tomography Study. Biological psychiatry 83, 61–69. [DOI] [PubMed] [Google Scholar]
- Hoogendijk WJ, Lips P, Dik MG, Deeg DJ, Beekman AT, Penninx BW, 2008. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Archives of general psychiatry 65, 508–512. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J, 2009. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosomatic medicine 71, 171–186. [DOI] [PubMed] [Google Scholar]
- Humble MB, 2010. Vitamin D, light and mental health. J Photochem Photobiol B 101, 142–149. [DOI] [PubMed] [Google Scholar]
- Ju SY, Lee YJ, Jeong SN, 2013. Serum 25-hydroxyvitamin D levels and the risk of depression: a systematic review and meta-analysis. The journal of nutrition, health & aging 17, 447–455. [DOI] [PubMed] [Google Scholar]
- Khan MS, Wu GWY, Reus VI, Hough CM, Lindqvist D, Westrin A, Nier BM, Wolkowitz OM, Mellon SH, 2019. Low serum brain-derived neurotrophic factor is associated with suicidal ideation in major depressive disorder. Psychiatry research 273, 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Lee SW, Kim SH, Shim SH, Han SW, Choi SH, Lee BH, 2008. Differences in cytokines between non-suicidal patients and suicidal patients in major depression. Progress in neuro-psychopharmacology & biological psychiatry 32, 356–361. [DOI] [PubMed] [Google Scholar]
- Kjaergaard M, Joakimsen R, Jorde R, 2011. Low serum 25-hydroxyvitamin D levels are associated with depression in an adult Norwegian population. Psychiatry research 190, 221–225. [DOI] [PubMed] [Google Scholar]
- Kjaergaard M, Waterloo K, Wang CE, Almas B, Figenschau Y, Hutchinson MS, Svartberg J, Jorde R, 2012. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: nested case-control study and randomised clinical trial. Br J Psychiatry 201, 360–368. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Dhabhar FS, James SJ, Hough CM, Jain FA, Bersani FS, Reus VI, Verhoeven JE, Epel ES, Mahan L, Rosser R, Wolkowitz OM, Mellon SH, 2017. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 76, 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangin M, Sinha R, Fincher K, 2014. Inflammation and vitamin D: the infection connection. Inflammation research : official journal of the European Histamine Research Society... [et al. ] 63, 803–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue RE, Charles RA, Orendain GC, Joseph MD, Abanishe JO, 2012. Vitamin d deficiency among psychiatric inpatients. The primary care companion for CNS disorders 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milaneschi Y, Hoogendijk W, Lips P, Heijboer AC, Schoevers R, van Hemert AM, Beekman AT, Smit JH, Penninx BW, 2014. The association between low vitamin D and depressive disorders. Mol Psychiatry 19, 444–451. [DOI] [PubMed] [Google Scholar]
- Miller AH, Raison CL, 2016. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature reviews. Immunology 16, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker GB, Brotchie H, Graham RK, 2017. Vitamin D and depression. Journal of affective disorders 208, 56–61. [DOI] [PubMed] [Google Scholar]
- Phinney KW, Tai SS, Bedner M, Camara JE, Chia RRC, Sander LC, Sharpless KE, Wise SA, Yen JH, Schleicher RL, Chaudhary-Webb M, Maw KL, Rahmani Y, Betz JM, Merkel J, Sempos CT, Coates PM, Durazo-Arvizu RA, Sarafin K, Brooks SPJ, 2017. Development of an Improved Standard Reference Material for Vitamin D Metabolites in Human Serum. Analytical chemistry 89, 4907–4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassi F, Tamone C, D’Amelio P, 2018. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiepers OJ, Wichers MC, Maes M, 2005. Cytokines and major depression. Progress in neuro-psychopharmacology & biological psychiatry 29, 201–217. [DOI] [PubMed] [Google Scholar]
- Shaffer JA, Edmondson D, Wasson LT, Falzon L, Homma K, Ezeokoli N, Li P, Davidson KW, 2014. Vitamin D supplementation for depressive symptoms: a systematic review and meta-analysis of randomized controlled trials. Psychosom Med 76, 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal G, Baune BT, 2017. Microglia: An Interface between the Loss of Neuroplasticity and Depression. Frontiers in cellular neuroscience 11, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrobot A, Demkow U, Wachowska M, 2018. Immunomodulatory Role of Vitamin D: A Review. Adv Exp Med Biol 1108, 13–23. [DOI] [PubMed] [Google Scholar]
- Stefanowski B, Antosik-Wojcinska AZ, Swiecicki L, 2017. The effect of vitamin D3 deficiency on the severity of depressive symptoms. Overview of current research. Psychiatria polska 51, 437–454. [DOI] [PubMed] [Google Scholar]
- Tariq MM, Streeten EA, Smith HA, Sleemi A, Khabazghazvini B, Vaswani D, Postolache TT, 2011. Vitamin D: a potential role in reducing suicide risk? Int J Adolesc Med Health 23, 157–165. [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Stiller J, Rujescu D, Giegling I, Schneider B, Maurer K, Schnabel A, Moller HJ, Chen HH, Postolache TT, 2008. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr Scand 117, 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umar M, Sastry KS, Chouchane AI, 2018. Role of Vitamin D Beyond the Skeletal Function: A Review of the Molecular and Clinical Studies. International journal of molecular sciences 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umhau JC, George DT, Heaney RP, Lewis MD, Ursano RJ, Heilig M, Hibbeln JR, Schwandt ML, 2013. Low vitamin D status and suicide: a case-control study of active duty military service members. PloS one 8, e51543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Etten E, Mathieu C, 2005. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. The Journal of steroid biochemistry and molecular biology 97, 93–101. [DOI] [PubMed] [Google Scholar]
- von Kanel R, Fardad N, Steurer N, Horak N, Hindermann E, Fischer F, Gessler K, 2015. Vitamin D Deficiency and Depressive Symptomatology in Psychiatric Patients Hospitalized with a Current Depressive Episode: A Factor Analytic Study. PloS one 10, e0138550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weishaar T, Rajan S, Keller B, 2016. Probability of Vitamin D Deficiency by Body Weight and Race/Ethnicity. Journal of the American Board of Family Medicine : JABFM 29, 226–232. [DOI] [PubMed] [Google Scholar]
- Wielders JP, Wijnberg FA, 2009. Preanalytical stability of 25(OH)-vitamin D3 in human blood or serum at room temperature: solid as a rock. Clinical chemistry 55, 1584–1585. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, Goleva E, 2012. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. Journal of immunology (Baltimore, Md. : 1950) 188, 2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]