Abstract
Aims
The use of non-steroid anti-inflammatory drugs (NSAIDs) is conventional in management of postoperative pain in cancer patients, and further investigations have reported that some of these drugs correlated with the outcome in cancers. However, the prognostic value of the use of NSAIDs during surgery in non-small cell lung cancer (NSCLC) patients has been less addressed.
Methods
NSCLC patients staged I–III are retrospectively enrolled, and the data of the use of NSAIDs during surgery are collected. Patients are divided into two subgroups according to the use intensity (UI) (low or high) of the NSAIDs, which was calculated by the accumulate dosage of all the NSAIDs divided by the length of hospitalization. The differences of the clinical features among these groups were checked. And the disease-free survival (DFS) and overall survival (OS) differences in these groups were compared by Kaplan–Meier analysis; risk factors for survival were validated by using a Cox proportional hazards model.
Results
The UI was significant in predicting the DFS (AUC = 0.65, 95% CI: 0.57–0.73, P = 0.001) and OS (AUC = 0.70, 95% CI: 0.59–0.81, P = 0.001). Clinical features including type of resection (P = 0.001), N stages (P < 0.001), and TNM stages (P = 0.004) were significantly different in UI low (< 74.55 mg/day) or high (≥ 74.55 mg/day) subgroups. Patients in UI-high subgroups displayed significant superior DFS (log rank = 11.46, P = 0.001) and OS (log rank = 7.63, P = 0.006) than the UI-low ones. At last, the UI was found to be an independent risk factor for DFS (HR: 0.52, 95% CI: 0.28–0.95, P = 0.034).
Conclusions
The use of NSAIDs during radical resection in NSCLC patients correlated with the outcome and patients with a relative high UI has better outcome.
Keywords: Lung cancer, Non-steroid anti-inflammatory drugs, Surgery, Disease-free survival, Overall survival
Introduction
Lung cancer is still a great health threat worldwide [1] with non-small cell lung cancer (NSCLC) which accounts as the predominant pattern [2]. In recent years, with the success of neoadjuvant, adjuvant immunotherapies [3, 4], and target therapies [5, 6], the overall survival (OS) of NSCLC patients was greatly improved even in those locally advanced cases. Nonetheless, radical resection was still the most important treatment for the majority of the patients; however, some complications like pain are still a serious problem to harm the quality of life for the patients after surgery.
It was reported the prevalence of clinically relevant postoperative pain for lung cancer patients could be up to 63% for those received thoracotomy [7] and could persist to 36 months (m) in 17.4% patients [8]. Clinically, opioids and non-steroid anti-inflammatory drugs (NSAIDs) are the most used agents to alleviate the postoperative pain in these patients [9], the latter of which include those unselected COX inhibitors like aspirin, diclofenac, and ibuprofen and those selected COX-2 inhibitors like rofecoxib and celecoxib [10]. Interestingly, a great number of previous studies have indicated some of these NSAIDs may have additional role in regulating lung cancer cells besides its function in anti-inflammation and alleviate pain. For example, aspirin could improve cisplatin resistance by inhibiting cancer cell stemness [11] or reduce the metastasis of the cancer cells to regional lymph nodes [12]; ibuprofen could enhance the effect of cisplatin by suppressing the heat shock protein 70 in cancer cells [13], which indicated a positive role of these agents in anti-cancer. On the contrary, celecoxib could induce the epithelial-mesenchymal transition and increase the risks of cancer metastasis [14]. Based on these facts, some clinical observations have conducted to explore the role of the NSAIDs in lung cancer patients as it was found that the combined use of aspirin with osimertinib [15] or immunotherapies [16] contributes to the good survival in patients; however, no such role was detected for the combination with celecoxib [17, 18]. Of note, all these trials were performed in advanced or metastatic settings. Up to date, only one study has explored the use of the NSAIDs in postoperative stages I–III NSCLC patients [19], but it only includes the use of indomethacin and ibuprofen which aimed to alleviate the postoperative fever; other types of NSAIDs were not included. Nonetheless, none of the study has explored the prognostic value of the use of NSAIDs during surgery in these patients.
In this study, we sought to explore the prognostic value of the use of NSAIDs during radical resection in stages I–III NSCLC.
Methods
Data collection
NSCLC patients who experienced radical resection in Hainan Hospital of PLA General Hospital from December 2012 to May 2020 were retrospectively enrolled. Clinic data including age, sex, type of resection, pathology, smoking or alcohol status, and comorbidity (hypertension or diabetes mellitus) were collected. Patients met any of the following criteria which were ruled out: (1) receive any kind or duration of neoadjuvant therapies; (2) with suspected remote lesions by examinations before surgery; (3) with previous secondary malignant disease; (4) with a use history of any kind of NSAIDs due to cardiovascular or cerebrovascular disease and others, or those with oxycodone/acetaminophen; and (5) follow up problems (refuse or lost). The study followed the principles stated in the Declaration of Helsinki and was approved by the Ethics Committee of Hainan Hospital of PLA General Hospital (ID: S2023-12). Written informed consent was not required because of its retrospective nature.
NSAIDs data collection and patient assignment
The categories of NSAIDs used in this study include aspirin/aspirin DL-lysine, loxoprofen, ibuprofen, diclofenac sodium, and ketorolac tromethamine, and no records of celecoxib were registered. The accumulated dosage (AD) of these agents is figured out individually according to the package insert and was summed up with a convert ratio at 1 to each other for the convenience of subsequent calculation. All the NSAIDs were used in these patients aimed to alleviate postoperative pain except five patients who experienced fever. Patients were then assigned into two subgroups based on the use intensity (UI), which was calculated by the sum of all the dosage of the NSAIDs (mg) divided by the length of hospitalization (days, d). In addition, patients were also divided into use status (US, no/yes) of which the ones with any kind and any dosage of NSAIDs prescription were assigned into the US yes groups.
Set the study endpoints
The follow-up was started after the resection with an interval of 3–6 months in the first 2 years (y) and then annually. DFS and OS were selected as the primary endpoints for the study as described previously [20], and the last follow-up point was stopped in May 2023.
Statistical analysis
The significance of UI, US, and AD in predicting DFS and OS was checked by the receiver operating characteristic curve (ROC) analysis, after comparing the area under the curve (AUC) of these indexes; patients were then assigned into UI low or high subgroups according to the optimal cut-off point (taken DFS as the endpoint). Clinical features in these subgroups were tested by chi-square test. The DFS and OS differences among UI low or high and US no or yes subgroups were analyzed by Kaplan–Meier analysis and subsequent log-rank tests. A Cox proportional hazards model was used to validate the risk factors for the outcome with the iterative forward LR method. Double side P < 0.050 was deemed as statistically significant. All the data were processed by using SPSS 20.0 (SPSS Inc., Chicago, IL, USA).
Results
General features of the patients
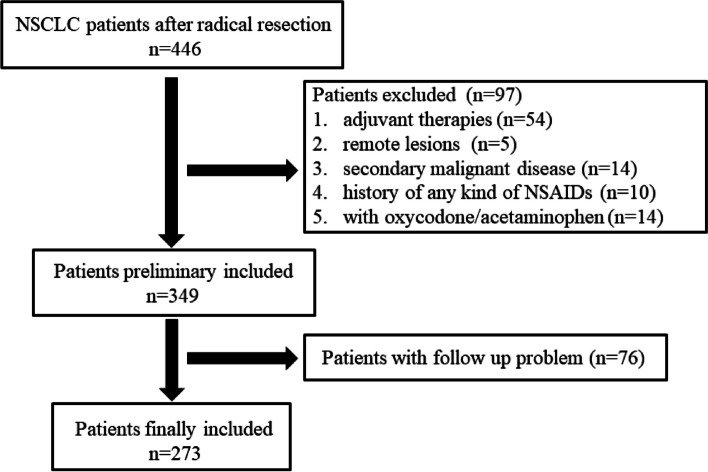
A total of 273 patients were enrolled in the last analytic set (Fig. 1) with 136 males and 137 females, the median age of the patients was 58 years (range: 23–84 years), and the median follow-up was 52 months (range: 2–128 months). At the end of the follow-up, 10 patients in stage I, 7 patients in stage II, and 8 patients in stage III died. The median of the AD was 1260 mg (range: 0–8260 mg), and the median of the length of hospitalization was 17 days (range: 6–54 days). The distribution of the categories of NSAIDs was as follows: none (n = 77); single agents: aspirin/aspirin-dl-lysine (n = 4, 2 for each), loxoprofen (n = 57), and diclofenac sodium (n = 67); and two agents: loxoprofen + diclofenac sodium (n = 40), loxoprofen + ketorolac tromethamine (n = 1), loxoprofen + ibuprofen (n = 1), ibuprofen + diclofenac sodium (n = 2), aspirin + diclofenac sodium (n = 1), and ketorolac tromethamine + diclofenac sodium (n = 19), and the rest of the patients (n = 4) used 3 categories.
Fig. 1.
A flowchart of patient enrollment
UI, US, and AD in predicting the DFS and OS
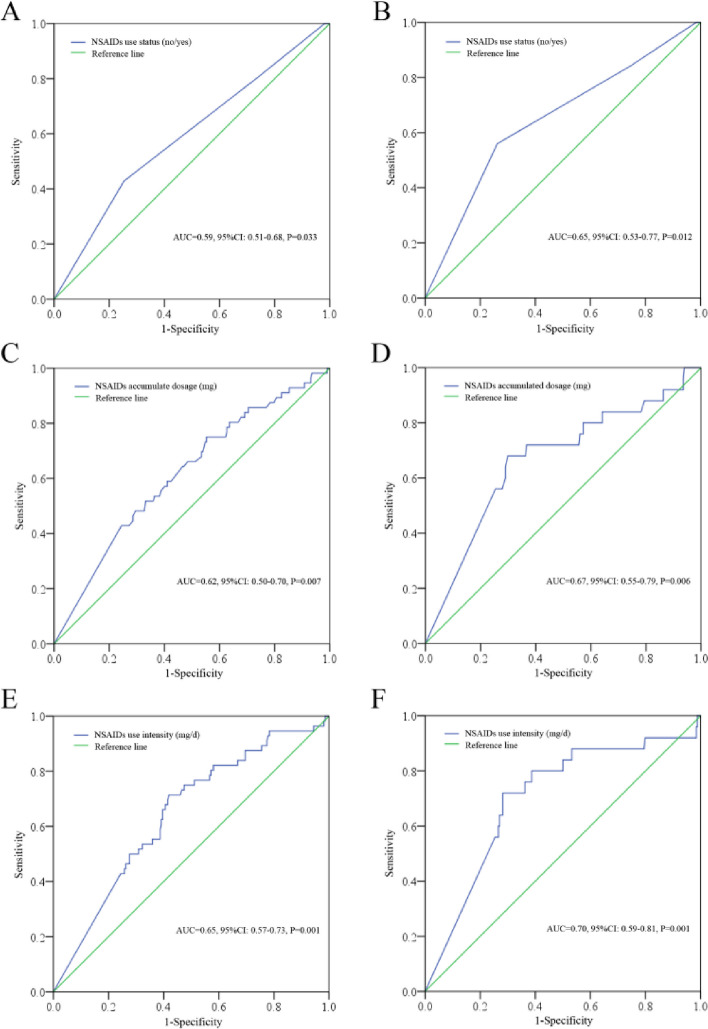
By ROC analysis, the UI was significant in predicting the DFS (AUC = 0.65, 95% CI: 0.57–0.73, P = 0.001) with a sensitivity and specificity at 71.40% and 58.10%, respectively, and OS (AUC = 0.70, 95% CI: 0.59–0.81, P = 0.001) with a sensitivity and specificity at 72.00% and 71.80%, respectively. In addition, the US was also significant in predicting DFS (AUC = 0.59, 95% CI: 0.51–0.68, P = 0.033) and OS (AUC = 0.65, 95% CI: 0.53–0.77, P = 0.012). We also checked the AD in predicting the survival, and the results suggested that it was also significant in predicting the DFS (AUC = 0.62, 95% CI: 0.50–0.70, P = 0.007) and OS (AUC = 0.67, 95% CI: 0.55–0.79, P = 0.006) (Fig. 2). The UI displayed the highest AUC among these indexes; we then divided the patients into UI low (< 74.55 mg/d, n = 131) or high (≥ 74.55 mg/d, n = 142) subgroups according to the optimal cut-off point for DFS. Further comparison for clinical features indicated that type of resection (P = 0.001), N stages (P < 0.001), and TNM stages (P = 0.004) were significantly different in UI low or high subgroups (Table 1).
Fig. 2.
The significance of US, AD, and UI in predicting the survival. A US no or yes in predicting the DFS. B US no or yes in predicting the OS. C AD in predicting the DFS. D AD in predicting the OS. E UI in predicting the DFS. D UI in predicting the OS. US, use status; AD, accumulated dosage; UI, use intensity; DFS, disease-free survival; OS, overall survival
Table 1.
Differences of the parameters among UI low or high subgroups
| NSAIDs UI | ||||
|---|---|---|---|---|
| No. of the patients | Low | High | P | |
| Age (y) | 0.463 | |||
| < 60 | 157 | 72 | 85 | |
| ≥ 60 | 116 | 59 | 57 | |
| Sex | 0.070 | |||
| Male | 136 | 73 | 63 | |
| Female | 137 | 58 | 79 | |
| Type of resection | 0.001* | |||
| Lobectomy | 208 | 112 | 96 | |
| Segmentectomy | 65 | 19 | 46 | |
| Pathology | 0.181 | |||
| ADC | 252 | 117 | 135 | |
| SCC | 14 | 10 | 4 | |
| Others | 7 | 4 | 3 | |
| Smoking status | 0.143 | |||
| Never | 195 | 88 | 107 | |
| Current + former | 78 | 43 | 35 | |
| Alcohol status | 0.608 | |||
| Never | 182 | 85 | 97 | |
| Current + former | 91 | 46 | 45 | |
| Comorbidity | 0.580 | |||
| With | 69 | 31 | 38 | |
| Without | 204 | 100 | 104 | |
| T stages | 0.686 | |||
| T1 + T2 | 267 | 129 | 138 | |
| T3 + T4 | 6 | 2 | 4 | |
| N stages | < 0.001* | |||
| N0 | 241 | 106 | 135 | |
| N1 + N2 | 32 | 25 | 7 | |
| TNM stages | 0.004* | |||
| I | 237 | 105 | 132 | |
| II | 22 | 17 | 5 | |
| III | 14 | 9 | 5 | |
UI use intensity, NSAIDs non-steroid anti-inflammatory drugs, ADC adenocarcinoma, SCC squamous carcinoma
*With significant difference
The survival differences in US yes or no and UI low or high subgroups
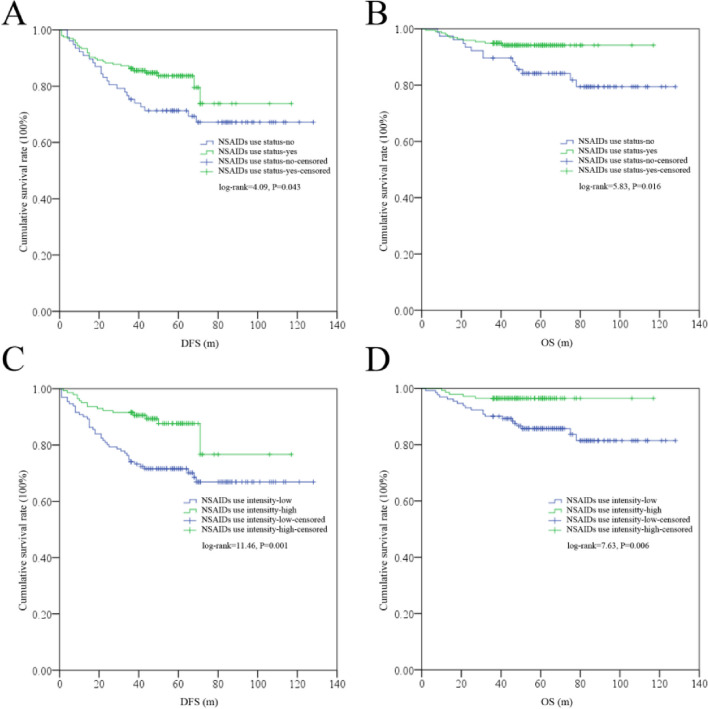
By Kaplan–Meier tests, significant differences for DFS (log rank = 11.46, P = 0.001) and OS (log rank = 7.63, P = 0.006) were found in UI low or high subgroups; similarly, significant difference was also found for DFS (log rank = 4.09, P = 0.043) and OS (log rank = 5.83, P = 0.016) in US no or yes subgroups (Fig. 3).
Fig. 3.
The survival differences among US no or yes and UI low or high subgroups. A DFS differences among US no or yes subgroups. B OS differences among US no or yes subgroups. C DFS differences among UI low or high subgroups. D OS differences among UI low or high subgroup. US, use status; UI, use intensity; DFS, disease-free survival; OS, overall survival
Univariate and multivariate tests to validate the risk factors for survival
By using the Cox hazard model, it was found that age, sex, pathology, smoking status, N stages, TNM stages, and UI were shared risk factors both for DFS and OS, whereas T stages were identified as additional risk factors for DFS and alcohol status were identified as an additional risk factor for OS (Table 2). Subsequently, these factors were entered into the multivariate tests for DFS and OS, respectively; the results indicated that UI was one of the independent factors for DFS (HR: 0.50, 95% CI: 0.29–0.87, P = 0.014) but not OS (Table 3).
Table 2.
Determination for risk factors for DFS or OS by univariate tests
| DFS | OS | |||
|---|---|---|---|---|
| P | HR (95% CI) | P | HR (95% CI) | |
| Age (y) | ||||
| < 60 | 1 | 1 | ||
| ≥ 60 | 0.033* | 1.78 (1.05–3.01) | 0.072 | 2.09 (0.94–4.64) |
| Sex | ||||
| Male | 1 | 1 | ||
| Female | 0.001* | 0.37 (0.21–0.66) | 0.002* | 0.19 (0.06–0.55) |
| Type of resection | ||||
| Lobectomy | 1 | 1 | ||
| Segmentectomy | 0.105 | 1.86 (0.88–3.95) | 0.100 | 3.37 (0.79–14.32) |
| Pathology | ||||
| ADC | 1 | 1 | ||
| SCC | 0.196 | 1.84 (0.73–4.67) | 0.023* | 3.52 (1.19–10.44) |
| Others | 0.006* | 4.28 (1.53–12.02) | 0.091 | 3.54 (0.82–15.34) |
| Smoking status | ||||
| Never | 1 | 1 | ||
| Current + former | < 0.001* | 3.19 (1.88–5.40) | < 0.001* | 4.45 (1.96–10.08) |
| Alcohol status | ||||
| Never | 1 | 1 | ||
| Current + former | 0.126 | 1.51 (0.89–2.57) | 0.024* | 2.49 (1.13–5.49) |
| Comorbidity | ||||
| With | 1 | 1 | ||
| Without | 0.880 | 0.95 (0.52–1.75) | 0.295 | 0.57 (0.19–1.65) |
| T stages | ||||
| T1 + T2 | 1 | 1 | ||
| T3 + T4 | 0.002* | 5.16 (1.86–14.31) | 0.058 | 4.07 (0.95–17.37) |
| N stages | ||||
| N0 | 1 | 1 | ||
| N1 + N2 | < 0.001* | 9.70 (5.63–16.71) | < 0.001* | 14.47 (6.26–33.46) |
| TNM stages | ||||
| I | 1 | 1 | ||
| II | < 0.001* | 7.06 (3.66–13.64) | < 0.001* | 9.34 (3.50–24.92) |
| III | < 0.001* | 20.36 (12.74–50.49) | < 0.001* | 24.23 (9.23–63.60) |
| NSAIDs UI | ||||
| Low | 1 | 1 | ||
| High | 0.001* | 0.38 (0.21–0.68) | 0.010* | 0.27 (0.10–0.73) |
UI use intensity, ADC adenocarcinoma, SCC squamous carcinoma, DFS disease-free survival, OS overall survival
*With significant difference
Table 3.
Determination for risk factors for DFS or OS by multivariate tests
| DFS | OS | |||
|---|---|---|---|---|
| P | HR (95% CI) | P | HR (95% CI) | |
| Smoking status | ||||
| Never | 1 | |||
| Current + former | 0.007* | 2.10 (1.22–3.62) | ||
| Alcohol status | ||||
| Never | 1 | |||
| Current + former | 0.014* | 2.75 (1.23–6.16) | ||
| TNM stages | ||||
| I | 1 | 1 | ||
| II | < 0.001* | 4.72 (2.35–9.50) | < 0.001* | 8.90 (3.36–23.59) |
| III | < 0.001* | 19.18 (9.49–38.78) | < 0.001* | 26.78 (10.13–70.83) |
| NSAIDs UI | ||||
| Low | 1 | |||
| High | 0.034* | 0.52 (0.28–0.95) | ||
UI use intensity, DFS disease-free survival, OS overall survival
*With significant difference
Discussion
In this study, the use of NSAIDs during radical resection was considered to be correlated with the outcome in NSCLC patients. UI was found to be the most robust prognostic indicator for the use of NSAIDs in these patients, and UI high patients would have the superior outcome in contrast to the low ones. Moreover, UI was recognized as an independent risk factor for DFS.
Previously, a great number of epidemiological investigations have indicated the use of NSAIDs like aspirin and ibuprofen can reduce the incidence of cancers including lung cancer [21–24]. As in cancer patients, these agents also maintained a positive role in the patients’ outcome. For example, Giampieri et al. in a study with 66 previously heavily treated metastatic colorectal cancer found that aspirin could obviously improve the survival [25]. With regard to lung cancer, Chuang et al. in a large retrospective study with 38,842 inoperable NSCLC patients found the use of aspirin correlated with improved OS [26]; Kanda et al. in a study with 217 stages IIIB and IV NSCLC patients also found the use of loxoprofen sodium that could extend the survival in older NSCLC patients [27]. In recent years, with the popular target and immunotherapies in NSCLC, the value of the use of NSAIDs was also explored with these therapies. For example, Liu et al. in a study with 365 metastatic EGFR-mutant NSCLC patients received osimertinib ± aspirin and found that a combination of aspirin with osimertinib could improve the progression-free survival (PFS) and OS [15]; similarly, Aiad et al. in a study with 500 stages I–IV patients received immunotherapies ± aspirin and also found that the combination of aspirin could extend the outcome [16]. However, it was also notable that some studies suggested that use of celecoxib could have less positive role in combination with gefitinib [17], docetaxel, or other platinum-based chemotherapy [18, 28]. Nonetheless, all these studies were conducted in advance or metastatic staged settings. Up to date, only one study has explored the use of the NSAIDs in postoperative stages I–III NSCLC patients, and the results indicated the use of the NSAIDs correlated with good PFS and OS [19]. In addition, the use or not (corresponding to the US in our study) was found to be an independent risk factor both for PFS and OS [19]. However, this study only includes the use of indomethacin and ibuprofen which aimed to treat the postoperative fever; other types of NSAIDs or other intentions of the use of these agents were not involved. Indeed, some previous studies in colorectal cancer and breast cancer have validated the protective role of aspirin in adjuvant settings [29, 30]. Our study for the first time supported the protective role of the use of NSAIDs in addition to indomethacin and ibuprofen in NSCLC patients in postoperative scenario, which was partly in line with previous studies in adjuvant settings [19, 29, 30]. In addition, none of the patients received celecoxib, which is also in line with previous results with these agents in aforementioned advanced or metastatic cases.
It continued to be lacking of well-acknowledged definition concerning the use and use intensity of NSAIDs in cancer patients particularly in postoperative background. Previously, some studies have defined the aspirin or other NSAIDs exposure in cancer patients. For example, Liao et al. defined the use of aspirin as one or more prescriptions recorded before and after the diagnosis with any dosage [31], whereas in Giampieri et al.’s study, the definition of exposure was the ones taken for at least 12 months at a dose of at least 100 mg/day [25]; other studies defined various criteria of exposure of these agents [30, 32]. As in lung cancer, Chuang et al. defined the aspirin users as those who used it for > 28 defined daily doses after diagnosis [26]; Jiang et al. defined the use or not of NSAIDs (only indomethacin and ibuprofen) as the standardized dosage in clinic [19], whereas in reports about the combination of it with osimertinib or immunotherapies, the definition of exposure was obscure [15, 16]. Interestingly, all these studies are conducted retrospectively. However, in prospective clinical trials about celecoxib, its exposure was rigorously defined with fixed dosage concurrent with gefitinib [17] or chemotherapy [18]. In our study, all the patients received daily dose of the NSAIDs irrespective of the categories, and we thus refer to Jiang et al.’s and Liao et al.’s study [19, 31] to define the exposure as US; in addition, we also explored the prognostic value of AD and UI in these patients. The results indicated UI displayed the highest AUC among these indexes and was found to be an independent risk factor for survival. Our results for the first time indicated that the UI may a reasonable index for the use of NSAIDs in these patients; however, more studies are still required to validate our speculation in future.
Mechanically, the role of NSAIDs in regulating lung cancer cells has been under extensive study. For example, aspirin could manipulate the miR-98/WNT1 axis to inhibit cancer progression [33]; it could also suppress the growth of cancer cells via targeting the TAZ/PD-L1 axis [34]; in addition, other NSAIDs like ibuprofen could enhance the effect of cisplatin by suppressing the heat shock protein 70 [13], and acetaminophen could promote ferroptosis by regulating Nrf2/heme oxygenase-1 signaling pathway [35], and loxoprofen sodium could inhibit tumor growth by suppressing vascular endothelial growth factor in a mouse model [36]. In recent years, the key role of circulating tumor cells (CTCs) has been identified in tumor recurrence and metastasis [37, 38]; in particular, some of these cells presented features that are similar to the so-called cancer stem cells (CSCs) in many cancers [39, 40] including lung cancer [41, 42], the latter of which are characterized by high potential of self-renew and multiple treatment resistance, and complete removal of these cells was regarded as the ultimate approach to cure the disease [43–45]. In lung cancer, it was notable that the CTCs could be found in up to 51.8% stages I–III radically resected patients before surgery [38], and these cells could be even found in 96.5% patients during surgery in pulmonary vein [46]. Notably, the use of some NSAIDs like aspirin could decrease the CTCs numbers in colorectal and breast cancer patients [47]; moreover, aspirin could have a broad spectrum of inhibition for many CSCs (including lung cancer) by complex mechanisms [48, 49]. Based on these facts, it was notable that the use of NSAIDs after surgery could potentially reduce the quantity of the CTCs and in particular eliminate a cluster of these cells featured like CSCs, which could then improve the patients’ outcome. Except these, it was well established that inflammation plays an important role in cancer initiation, recurrence, and metastasis [50]. Some cytokines, like IL-6, could be remarkably elevated in lung cancer patients [51] in particular for those who underwent surgery [52]. It is noteworthy that IL-6 could not only promote the cell proliferation [53], induce treatment resistance [54, 55], and promote metastasis [56] in lung cancer but also promote the expansion of the CSCs [57]. Interestingly, some NSAIDs like aspirin could not only cancel the pro-tumorigenic effects of IL-6 [58] but also downregulate the IL-6-STAT3 signaling pathway to induce cancer cell apoptosis, which have been validated in colorectal cancer [59] and glioblastoma A172 cells [60]. Based on these facts, it was also plausible that the use of NSAIDs may also have a role in interrupting the correlation of inflammation and cancer in NSCLC, which may result in a sound outcome for these patients.
Our study could have some clinic implications. First, taking into consideration the evolution of lung cancer cells during its development [61] and the role of NSAIDs in NSCLC, it would be reasonable to take these agents routinely as a tertiary chemoprevention of the disease immediately after surgery; second, since low-dose aspirin (< 75 mg/day) rarely contribute to improve cancer-specific mortality in lung cancer [62] and could even promote the cell growth [63], it was recommend that these patients should take the NSAIDs with a relatively high dose (like UI ≥ 74.55 mg/day in our study); however, adverse effects like gastric erosions and ulcers should also be balanced in clinic [64]. There are also some limitations for present study. First, it was a retrospective study with limited sample, the cases in US no groups were only 77, and potential bias cannot be satisfactorily ruled out; second, the pharmacokinetics and pharmacodynamics of different NSAIDs were not identical, we still lack strong evidence to support the convert ratio as 1 for each other in our study, and there are still lack of relevant studies to full support our explanations for the role of these agents in NSCLC except aspirin; and third, although UI was found to be a robust prognostic index compared to US and AD, the question for the duration of these agents cannot be answered at present. We advocated more studies; in particular, those prospective clinical trials should be carried out to validate our results in future.
Conclusion
As a summary, we found that the use of NSAIDs during radical resection in NSCLC patients correlated with the outcome, and patients with a relative high UI have better outcome.
Acknowledgements
None.
Authors’ contributions
MC and SF were responsible for the conception of the work. JX and QY obtained the data. RC, XL, and GL analyzed the data. RC and XL wrote the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by Hainan Provincial Natural Science Foundation of China (No. 821MS0822 and No. 823MS140).
Availability of data and materials
The datasets generated or analyzed during the current study are available from the corresponding author (Minbiao Chen) on reasonable request.
Declarations
Ethics approval and consent to participate
The study followed the principles stated in the Declaration of Helsinki and was approved by the Ethics Committee of Hainan Hospital of PLA General Hospital (ID: S2023-12). Written informed consent was not required because of its retrospective nature.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Renzhong Cai, Xuqiang Liao, Gao Li, Minbiao Chen, and Shouhan Feng contributed equally.
Contributor Information
Minbiao Chen, Email: Chenminbiaokeyan@163.com.
Shouhan Feng, Email: fengshouhan@sina.com.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Chen P, Liu Y, Wen Y, Zhou C. Non-small cell lung cancer in China. Cancer Commun. 2022;42(10):937–970. doi: 10.1002/cac2.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973–1985. doi: 10.1056/NEJMoa2202170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Brien M, Paz-Ares L, Marreaud S, Dafni U, Oselin K, Havel L, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022;23(10):1274–1286. doi: 10.1016/S1470-2045(22)00518-6. [DOI] [PubMed] [Google Scholar]
- 5.Lv C, Fang W, Wu N, Jiao W, Xu S, Ma H, et al. Osimertinib as neoadjuvant therapy in patients with EGFR-mutant resectable stage II-IIIB lung adenocarcinoma (NEOS): a multicenter, single-arm, open-label phase 2b trial. Lung Cancer. 2023;178:151–156. doi: 10.1016/j.lungcan.2023.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2020;383(18):1711–1723. doi: 10.1056/NEJMoa2027071. [DOI] [PubMed] [Google Scholar]
- 7.Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. 2016;17(6):836–844. doi: 10.1016/S1470-2045(16)00173-X. [DOI] [PubMed] [Google Scholar]
- 8.Yoon S, Hong WP, Joo H, Kim H, Park S, Bahk JH, et al. Long-term incidence of chronic postsurgical pain after thoracic surgery for lung cancer: a 10-year single-center retrospective study. Reg Anesth Pain Med. 2020;45(5):331–336. doi: 10.1136/rapm-2020-101292. [DOI] [PubMed] [Google Scholar]
- 9.Oh TK, Jeon JH, Lee JM, Kim MS, Kim JH, Cho H, et al. Investigation of opioid use and long-term oncologic outcomes for non-small cell lung cancer patients treated with surgery. PLoS One. 2017;12(7):e0181672. doi: 10.1371/journal.pone.0181672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawkey CJ. COX-1 and COX-2 inhibitors. Best Pract Res Clin Gastroenterol. 2001;15(5):801–820. doi: 10.1053/bega.2001.0236. [DOI] [PubMed] [Google Scholar]
- 11.Zhao M, Wang T, Hui Z. Aspirin overcomes cisplatin resistance in lung cancer by inhibiting cancer cell stemness. Thoracic Cancer. 2020;11(11):3117–3125. doi: 10.1111/1759-7714.13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogawa F, Amano H, Ito Y, Matsui Y, Hosono K, Kitasato H, et al. Aspirin reduces lung cancer metastasis to regional lymph nodes. Biomed Pharmacother. 2014;68(1):79–86. doi: 10.1016/j.biopha.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Endo H, Yano M, Okumura Y, Kido H. Ibuprofen enhances the anticancer activity of cisplatin in lung cancer cells by inhibiting the heat shock protein 70. Cell Death Dis. 2014;5(1):e1027. doi: 10.1038/cddis.2013.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang ZL, Fan ZQ, Jiang HD, Qu JM. Selective Cox-2 inhibitor celecoxib induces epithelial-mesenchymal transition in human lung cancer cells via activating MEK-ERK signaling. Carcinogenesis. 2013;34(3):638–646. doi: 10.1093/carcin/bgs367. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Hong L, Nilsson M, Hubert SM, Wu S, Rinsurongkawong W, et al. Concurrent use of aspirin with osimertinib is associated with improved survival in advanced EGFR-mutant non-small cell lung cancer. Lung Cancer. 2020;149:33–40. doi: 10.1016/j.lungcan.2020.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aiad M, Tahir A, Fresco K, Prenatt Z, Ramos-Feliciano K, Walia J, et al. Does the combined use of aspirin and immunotherapy result in better outcomes in non-small cell lung cancer than immunotherapy alone? Cureus. 2022;14(6):e25891. doi: 10.7759/cureus.25891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gadgeel SM, Ruckdeschel JC, Heath EI, Heilbrun LK, Venkatramanamoorthy R, Wozniak A. Phase II study of gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), and celecoxib, a cyclooxygenase-2 (COX-2) inhibitor, in patients with platinum refractory non-small cell lung cancer (NSCLC) J Thorac Oncol. 2007;2(4):299–305. doi: 10.1097/01.JTO.0000263712.61697.69. [DOI] [PubMed] [Google Scholar]
- 18.Groen HJ, Sietsma H, Vincent A, Hochstenbag MM, van Putten JW, van den Berg A, et al. Randomized, placebo-controlled phase III study of docetaxel plus carboplatin with celecoxib and cyclooxygenase-2 expression as a biomarker for patients with advanced non-small-cell lung cancer: the NVALT-4 study. J Clin Oncol. 2011;29(32):4320–4326. doi: 10.1200/JCO.2011.35.5214. [DOI] [PubMed] [Google Scholar]
- 19.Jiang W, Wang L, Zhang J, Shen H, Dong W, Zhang T, et al. Effects of postoperative non-steroidal anti-inflammatory drugs on long-term survival and recurrence of patients with non-small cell lung cancer. Medicine. 2018;97(39):e12442. doi: 10.1097/MD.0000000000012442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H, Zhao G, Lin J, Ye Q, Xiang J, Yan B. A combined preoperative red cell distribution width and carcinoembryonic antigen score contribute to prognosis prediction in stage I lung adenocarcinoma. World J Surg Oncol. 2023;21(1):56. doi: 10.1186/s12957-023-02945-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chudy-Onwugaje K, Huang WY, Su LJ, Purdue MP, Johnson CC, Wang L, et al. Aspirin, ibuprofen, and reduced risk of advanced colorectal adenoma incidence and recurrence and colorectal cancer in the PLCO Cancer Screening Trial. Cancer. 2021;127(17):3145–3155. doi: 10.1002/cncr.33623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim WY, Chuah KL, Eng P, Leong SS, Lim E, Lim TK, et al. Aspirin and non-aspirin non-steroidal anti-inflammatory drug use and risk of lung cancer. Lung Cancer. 2012;77(2):246–251. doi: 10.1016/j.lungcan.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 23.McCormack VA, Hung RJ, Brenner DR, Bickeböller H, Rosenberger A, Muscat JE, et al. Aspirin and NSAID use and lung cancer risk: a pooled analysis in the International Lung Cancer Consortium (ILCCO) Cancer Causes Control. 2011;22(12):1709–1720. doi: 10.1007/s10552-011-9847-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye S, Lee M, Lee D, Ha EH, Chun EM. Association of long-term use of low-dose aspirin as chemoprevention with risk of lung cancer. JAMA Netw Open. 2019;2(3):e190185. doi: 10.1001/jamanetworkopen.2019.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giampieri R, Restivo A, Pusceddu V, Del Prete M, Maccaroni E, Bittoni AF, et al. The role of aspirin as antitumoral agent for heavily pretreated patients with metastatic colorectal cancer receiving capecitabine monotherapy. Clin Colorectal Cancer. 2017;16(1):38–43. doi: 10.1016/j.clcc.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Chuang MC, Yang YH, Hsieh MJ, Lin YC, Yang TM, Chen PC, et al. The association of aspirin use with overall survival of patients with inoperable non-small cell lung cancer: a retrospective study. BMC Cancer. 2021;21(1):1257. doi: 10.1186/s12885-021-08999-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanda A, Ebihara S, Okazaki T, Yasuda H, Sasaki H. Loxoprofen sodium and survival in older people with advanced non-small cell lung cancer. J Am Geriatr Soc. 2004;52(3):471–472. doi: 10.1111/j.1532-5415.2004.52125_6.x. [DOI] [PubMed] [Google Scholar]
- 28.Gulyas M, Mattsson JSM, Lindgren A, Ek L, Lamberg Lundström K, Behndig A, et al. COX-2 expression and effects of celecoxib in addition to standard chemotherapy in advanced non-small cell lung cancer. Acta Oncol. 2018;57(2):244–250. doi: 10.1080/0284186X.2017.1400685. [DOI] [PubMed] [Google Scholar]
- 29.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302(6):649–658. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmes MD, Chen WY, Li L, Hertzmark E, Spiegelman D, Hankinson SE. Aspirin intake and survival after breast cancer. J Clin Oncol. 2010;28(9):1467–1472. doi: 10.1200/JCO.2009.22.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao SF, Koshiol J, Huang YH, Jackson SS, Huang YH, Chan C, et al. Postdiagnosis aspirin use associated with decreased biliary tract cancer-specific mortality in a large nationwide cohort. Hepatology. 2021;74(4):1994–2006. doi: 10.1002/hep.31879. [DOI] [PubMed] [Google Scholar]
- 32.Walker AJ, Grainge MJ, Card TR. Aspirin and other non-steroidal anti-inflammatory drug use and colorectal cancer survival: a cohort study. Br J Cancer. 2012;107(9):1602–1607. doi: 10.1038/bjc.2012.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gan H, Lin L, Hu N, Yang Y, Gao Y, Pei Y, et al. Aspirin ameliorates lung cancer by targeting the miR-98/WNT1 axis. Thorac Cancer. 2019;10(4):744–750. doi: 10.1111/1759-7714.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Lv C, Dong Y, Yang Q. Aspirin-targeted PD-L1 in lung cancer growth inhibition. Thorac Cancer. 2020;11(6):1587–1593. doi: 10.1111/1759-7714.13433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gai C, Yu M, Li Z, Wang Y, Ding D, Zheng J, et al. Acetaminophen sensitizing erastin-induced ferroptosis via modulation of Nrf2/heme oxygenase-1 signaling pathway in non-small-cell lung cancer. J Cell Physiol. 2020;235(4):3329–3339. doi: 10.1002/jcp.29221. [DOI] [PubMed] [Google Scholar]
- 36.Kanda A, Ebihara S, Takahashi H, Sasaki H. Loxoprofen sodium suppresses mouse tumor growth by inhibiting vascular endothelial growth factor. Acta Oncol. 2003;42(1):62–70. doi: 10.1080/0891060310002258. [DOI] [PubMed] [Google Scholar]
- 37.O'Flaherty JD, Gray S, Richard D, Fennell D, O'Leary JJ, Blackhall FH, et al. Circulating tumour cells, their role in metastasis and their clinical utility in lung cancer. Lung Cancer. 2012;76(1):19–25. doi: 10.1016/j.lungcan.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Bayarri-Lara C, Ortega FG, de CuetoLadrónGuevara A, Puche JL, Ruiz Zafra J, de Miguel-Pérez D, et al. Circulating tumor cells identify early recurrence in patients with non-small cell lung cancer undergoing radical resection. PLoS One. 2016;11(2):e0148659. doi: 10.1371/journal.pone.0148659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grillet F, Bayet E, Villeronce O, Zappia L, Lagerqvist EL, Lunke S, et al. Circulating tumour cells from patients with colorectal cancer have cancer stem cell hallmarks in ex vivo culture. Gut. 2017;66(10):1802–1810. doi: 10.1136/gutjnl-2016-311447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe T, Okumura T, Hirano K, Yamaguchi T, Sekine S, Nagata T, et al. Circulating tumor cells expressing cancer stem cell marker CD44 as a diagnostic biomarker in patients with gastric cancer. Oncol Lett. 2017;13(1):281–288. doi: 10.3892/ol.2016.5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Obermayr E, Koppensteiner N, Heinzl N, Schuster E, Holzer B, Fabikan H, et al. Cancer stem cell-like circulating tumor cells are prognostic in non-small cell lung cancer. J Pers Med. 2021;11(11):1225. doi: 10.3390/jpm11111225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pantazaka E, Vardas V, Roumeliotou A, Kakavogiannis S, Kallergi G. Clinical relevance of mesenchymal- and stem-associated phenotypes in circulating tumor cells isolated from lung cancer patients. Cancers. 2021;13(9):2158. doi: 10.3390/cancers13092158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Makena MR, Ranjan A, Thirumala V, Reddy AP. Cancer stem cells: road to therapeutic resistance and strategies to overcome resistance. Biochim Biophys Acta Mol Basis Dis. 2020;1866(4):165339. doi: 10.1016/j.bbadis.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 44.Chen SY, Huang YC, Liu SP, Tsai FJ, Shyu WC, Lin SZ. An overview of concepts for cancer stem cells. Cell Transplant. 2011;20(1):113–120. doi: 10.3727/096368910X532837. [DOI] [PubMed] [Google Scholar]
- 45.Clara JA, Monge C, Yang Y, Takebe N. Targeting signalling pathways and the immune microenvironment of cancer stem cells-a clinical update. Nat Rev Clin Oncol. 2020;17(4):204–232. doi: 10.1038/s41571-019-0293-2. [DOI] [PubMed] [Google Scholar]
- 46.Dong J, Zhu D, Tang X, Qiu X, Lu D, Li B, et al. Detection of circulating tumor cell molecular subtype in pulmonary vein predicting prognosis of stage I-III non-small cell lung cancer patients. Front Oncol. 2019;9:1139. doi: 10.3389/fonc.2019.01139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang L, Lv Z, Xia W, Zhang W, Xin Y, Yuan H, et al. The effect of aspirin on circulating tumor cells in metastatic colorectal and breast cancer patients: a phase II trial study. Clin Transl Oncol. 2018;20(7):912–921. doi: 10.1007/s12094-017-1806-z. [DOI] [PubMed] [Google Scholar]
- 48.Zou Z, Zheng W, Fan H, Deng G, Lu SH, Jiang W, et al. Aspirin enhances the therapeutic efficacy of cisplatin in oesophageal squamous cell carcinoma by inhibition of putative cancer stem cells. Br J Cancer. 2021;125(6):826–838. doi: 10.1038/s41416-021-01499-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen J, Xu R, Xia J, Huang J, Su B, Wang S. Aspirin inhibits hypoxia-mediated lung cancer cell stemness and exosome function. Pathol Res Prac. 2019;215(6):152379. doi: 10.1016/j.prp.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Gao Y, Lin T. Expression of interleukin-1 (IL-1), IL-6, and tumor necrosis factor-α (TNF-α) in non-small cell lung cancer and its relationship with the occurrence and prognosis of cancer pain. Ann Palliat Med. 2021;10(12):12759–12766. doi: 10.21037/apm-21-3471. [DOI] [PubMed] [Google Scholar]
- 52.Jawa RS, Anillo S, Huntoon K, Baumann H, Kulaylat M. Interleukin-6 in surgery, trauma, and critical care part II: clinical implications. J Intensive Care Med. 2011;26(2):73–87. doi: 10.1177/0885066610384188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang KT, Tsai CM, Chiou YC, Chiu CH, Jeng KS, Huang CY. IL-6 induces neuroendocrine dedifferentiation and cell proliferation in non-small cell lung cancer cells. Am J Physiol Lung Cell Mol Physiol. 2005;289(3):L446–453. doi: 10.1152/ajplung.00089.2005. [DOI] [PubMed] [Google Scholar]
- 54.Kim SM, Kwon OJ, Hong YK, Kim JH, Solca F, Ha SJ, et al. Activation of IL-6R/JAK1/STAT3 signaling induces de novo resistance to irreversible EGFR inhibitors in non-small cell lung cancer with T790M resistance mutation. Mol Cancer Ther. 2012;11(10):2254–2264. doi: 10.1158/1535-7163.MCT-12-0311. [DOI] [PubMed] [Google Scholar]
- 55.Duan S, Tsai Y, Keng P, Chen Y, Lee SO. IL-6 signaling contributes to cisplatin resistance in non-small cell lung cancer via the up-regulation of anti-apoptotic and DNA repair associated molecules. Oncotarget. 2015;6(29):27651–27660. doi: 10.18632/oncotarget.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shang GS, Liu L, Qin YW. IL-6 and TNF-α promote metastasis of lung cancer by inducing epithelial-mesenchymal transition. Oncol Lett. 2017;13(6):4657–4660. doi: 10.3892/ol.2017.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu CC, Lin JH, Hsu TW, Su K, Li AF, Hsu HS, et al. IL-6 enriched lung cancer stem-like cell population by inhibition of cell cycle regulators via DNMT1 upregulation. Int J Cancer. 2015;136(3):547–559. doi: 10.1002/ijc.29033. [DOI] [PubMed] [Google Scholar]
- 58.Brighenti E, Giannone FA, Fornari F, Onofrillo C, Govoni M, Montanaro L, et al. Therapeutic dosages of aspirin counteract the IL-6 induced pro-tumorigenic effects by slowing down the ribosome biogenesis rate. Oncotarget. 2016;7(39):63226–63241. doi: 10.18632/oncotarget.11441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tian Y, Ye Y, Gao W, Chen H, Song T, Wang D, et al. Aspirin promotes apoptosis in a murine model of colorectal cancer by mechanisms involving downregulation of IL-6-STAT3 signaling pathway. Int J Colorectal Dis. 2011;26(1):13–22. doi: 10.1007/s00384-010-1060-0. [DOI] [PubMed] [Google Scholar]
- 60.Kim SR, Bae MK, Kim JY, Wee HJ, Yoo MA, Bae SK. Aspirin induces apoptosis through the blockade of IL-6-STAT3 signaling pathway in human glioblastoma A172 cells. Biochem Biophys Res Commun. 2009;387(2):342–347. doi: 10.1016/j.bbrc.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 61.Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S, et al. Tracking the evolution of non-small-cell lung cancer. N Engl J Med. 2017;376(22):2109–2121. doi: 10.1056/NEJMoa1616288. [DOI] [PubMed] [Google Scholar]
- 62.Mc Menamin ÚC, Cardwell CR, Hughes CM, Murray LM. Low-dose aspirin and survival from lung cancer: a population-based cohort study. BMC Cancer. 2015;15:911. doi: 10.1186/s12885-015-1910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qian Y, Dai H, Li H. Low-doses of aspirin promote the growth of human PC-9 lung cancer cells through activation of the MAPK family. Exp Ther Med. 2021;22(6):1440. doi: 10.3892/etm.2021.10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bjarnason I, Scarpignato C, Holmgren E, Olszewski M, Rainsford KD, Lanas A. Mechanisms of damage to the gastrointestinal tract from nonsteroidal anti-inflammatory drugs. Gastroenterology. 2018;154(3):500–514. doi: 10.1053/j.gastro.2017.10.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the current study are available from the corresponding author (Minbiao Chen) on reasonable request.