Abstract
Stress is inevitable in humans and stress changes our physical and mental states. Stress has been studied epidemiologically, biologically, and psychologically. First defined in 1990, emotional intelligence (EI) affects psychological stress management. In contrast, the prefrontal cortex (PFC) is suggested to play a vital role in stress management. Human PFC activity can be inferred from the balance of oxygenated and deoxygenated hemoglobin in cerebral blood flow, which can be measured and calculated using functional near-infrared spectroscopy (fNIRS). An important cognitive activation task to activate the PFC is the verbal fluency task (VFT). Therefore, if the PFC is activated by the VFT and monitored by fNIRS, and the activity correlates with EI, fNIRS can be used to measure EI. In this study, Psychological tests using the self-rating depression scale, state-trait anxiety inventory (STAI), and trait emotional intelligence questionnaire-short form (TEIQue-SF) were conducted to evaluate the correlation with VFT performance. Relative oxygenated and deoxygenated hemoglobin concentrations were measured using an fNIRS device, and their correlation with VFT performance was tested. Spearman correlation coefficient was used to determine correlations. Results were as follows. Although VFT performance did not correlate with the oxygenated hemoglobin concentration ([Oxy-Hb]) changes, [Oxy-Hb] was elevated in all channels. VFT performance was significantly negatively correlated with the Zung self-rating depression scale (ρ = 0.063, P = .759), trait anxiety or anxiety level as a personal characteristic of STAI (ρ = 0.243, P = .232), and state anxiety or anxiety about an event of STAI (ρ = −0.138, P = .500), whereas no correlation was found with the TEIQue-SF (ρ = 0.303, P = .132). Healthy individuals PFC activity is not severely affected by their mental state and cognitive activation successfully activates the PFC, supporting the hypothesis that EI is correlated with frontal cortical activation during the VFT in a nonclinical population. EI may play a vital role in reducing stress associated with depression and anxiety in our social lives. Although we failed to show a statistical correlation between TEIQue-SF and [Oxy-Hb] due to a sample size shortage, our preliminary study was the first to attempt to show the PFC activity of EI through a hemodynamic response. Future research may elucidate the role of EI in reducing psychological stress in social life.
Keywords: anxiety, depressive symptoms, functional near-infrared spectroscopy, trait emotional intelligence, verbal fluency task
1. Introduction
Stress is one of the main factors that change our physical and mental states. Because stress can lead to homeostatic and metabolic regulation dysfunction, it is inevitable for human survival. Traditionally, stress has been researched using 3 approaches: epidemiological, psychological, and biological.[1] However, these approaches cannot be delineated. Psychological stress can trigger negative emotions, which perturb biological homeostasis, such as the hypothalamic–pituitary–adrenal axis.[1] Moreover, psychological stress is often associated with the social environment. During the coronavirus disease pandemic, it was suggested that stress, anxiety, and depression levels were significantly higher in individuals aged 21 to 40 years.[2]
Emotional intelligence (EI) affects stress management. In 1990, Salovey and Mayer defined EI as “the ability to monitor one’s own and others’ feelings and emotions, to discriminate among them and use this information to guide one’s thinking and actions”.[3] EI can be divided into 3 categories; ability EI, trait EI, and mixed EI. While ability EI measures the ability to understand emotions and their functions through objective tests rather than participant self-reports, trait EI is assessed by the participants subjective self-reported items,[4] because emotionally intelligent individuals can understand what brought about some of their emotions (e.g., happiness, angriness, sadness) and are able to manage such emotions. Previous research has shown that high trait EI is associated with individual job satisfaction and productivity.[4] There are several measures to assess EI, and Bru-Luna et al[5] recently conducted a systematic review of EI measures and concluded that the trait emotional intelligence questionnaire (TEIQue) is the leading measure of trait EI. The TEIQue has a variety of translated versions with good internal consistency. As mentioned earlier, psychological stress and its management can be partly predicted by EI, using measures such as the TEIQue.
On the contrary, the brain’s prefrontal cortex (PFC) is a region of higher-order functionality, and ventromedial PFC controls emotional responses. When an individual experiences psychological stress, PFC regulation is affected by activating stress pathways through catecholamine release.[6] Recently, anxiety induced by chronic stress has been shown to dysregulate the medial PFC’s control over the basolateral amygdala in rodents, suggesting that the PFC plays a vital role in stress management.[7]
In humans, PFC activity can be inferred from the cerebral blood flow (hemodynamics). Cerebral hemodynamic activity was measured and calculated using functional near-infrared spectroscopy (fNIRS). Briefly, the calculated balance between oxygenated and deoxygenated hemoglobin on fNIRS reflects the hemodynamics of the region of interest, such as the PFC. fNIRS has been widely applied in emotion recognition because of its noninvasiveness[8]; therefore, it can be inferred that EI can correlate with the PFC’s hemodynamic activity. Moreover, the forehead region, including the PFC, is accessible using fNIRS. Our previous review suggested that cognitive activation during the verbal fluency task (VFT) can be used to assess PFC activity.[8]
VFT assesses cognitive ability. Periáñez et al[9] recently compared the VFT and other tests, including the Stroop test, and suggested that the VFT only significantly correlated with the Stroop color–word (SCWT; Pearson coefficients of r = −0.21) and digit symbol substitution tests in the Wechsler adult intelligence scale (4th edition; Pearson coefficients of R = 0.22). Moreover, Tran et al[10] recently compared the VFT with the SCWT for the detection of schizophrenia by the change in hemoglobin concentration obtained from fNIRS and showed that the area under the receiver operating characteristic curve of the VFT was higher than that of the SCWT in all regions of interest (ventrolateral PFC, dorsolateral PFC, frontopolar PFC, and orbitofrontal cortex), suggesting that the VFT has higher sensitivity and specificity than the SCWT.
In summary, if EI assessed by the TEIQue reflects the ability to manage psychological stress, and psychological stress intensity can be inferred by measuring the PFC’s hemodynamic response using fNIRS, then EI can be monitored using fNIRS, which enables noninvasive real-time monitoring. To the best of our knowledge, there have been no reports on the real-time monitoring of EI. To achieve noninvasive monitoring of psychological stress, anxiety, and depression, we studied the correlation between psychological and neurophysiological measurements.
2. Methods
2.1. Participants
This study was conducted at the Department of Psychosomatic Internal Medicine, Kagoshima University Graduate School of Medical and Dental Sciences, Kagoshima, Japan after approval from the Institutional Review Board of Kagoshima University Hospital (approval number:170172). Written informed consent was obtained from all participants before their participation in the study. The inclusion criteria were as follows: Male sex; Age between 20 and 39 years, and; Absence of psychiatric symptoms. Participants with psychiatric and physical symptoms were excluded from the study after evaluation by experienced psychosomatic internal medicine doctors. Therefore, at the time of enrollment, none of the participants had a medical history of major psychiatric disorders, including major depressive disorder, anxiety disorder, neurological disorder, drug dependence, major head injury, major physical illness, or a history of psychotropic medication use. Recruitment was performed using posters, and all candidates who met the inclusion criteria were enrolled. Because this was a preliminary study, we recruited 30 healthy men (mean age 28 ± 4.71 years) without sample size estimation.
2.2. Evaluation of depressive symptoms, anxiety, and trait emotional intelligence
Subjective depressive symptoms were evaluated using the Zung self-rating depression scale (SDS), a self-reported questionnaire comprising 20 items. The SDS was developed by Zung in 1965.[11] The scores ranged from 20 to 80, with higher scores indicating a greater degree of depressed mood; the score is a quantitative measure of depression.
Anxiety levels were assessed using the state-trait anxiety inventory Form X.[12] The STAI-X is a self-administered questionnaire that measures the following types of anxiety: State anxiety or anxiety about an event (STAI-S) and; Trait anxiety or anxiety level as a personal characteristic (STAI-T). It consists of 40 questions (20 each for state and trait anxiety). The scale is rated on a 4-point Likert scale. Higher scores indicate greater anxiety.[12]
To assess individual subjective EI, we measured trait EI using the Trait Emotional Intelligence Questionnaire-Short Form (TEIQue-SF). We used the validated Japanese version of the TEIQue-SF.[13] The TEIQue-SF comprises 30 items selected from 153 items in the original TEIQue. The global trait EI score was first calculated by averaging the score for each item on a 7-point Likert scale ranging from 1 (completely disagree) to 7 (completely agree). Subscale scores of the 4 factors (Well-Being, Self-Control, Emotionality, and Sociability) were derived from 6, 6, 8, and 6 of the 30 items, respectively.[14]
2.3. Cognitive activation
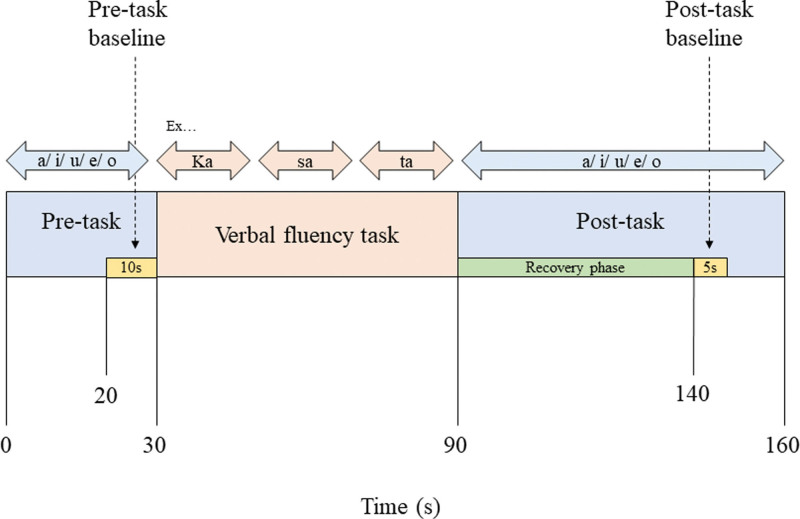
As mentioned previously, we measured the hemodynamic response of the PFC during cognitive activation using the VFT. Our protocol was based on that of Takizawa et al[15] The protocol was specially designed for neuropsychiatric assessment, including the fNIRS.[16] Figure 1 shows the timeline of the cognitive activation task. During the task, each participant was seated on a comfortable chair in a quiet room without speaking, and was instructed to fix the head position as best as possible. Following 30 seconds of a pre-task period, 60 seconds of the VFT was performed before a 70-second post-task period. The first 50 seconds of the post-task period was defined as the recovery phase. The participants initially repeated the Japanese vowels/a/, i/, u/, e/, and o/, followed by the generation of as many words as they could using the initial designated syllables/ka/, sa/, etc. The 3 initial syllables were changed every 20 seconds during the 60 seconds of the VFT. The total number of correct words was recorded as the task performance for each individual. During the pre- and post-task periods, participants were instructed to repeat the vowels/a/, i/, u/, e/, and o/ at the conclusion of the task (Fig. 1).
Figure 1.
Verbal fluency task protocol.
2.4. fNIRS evaluation of verbal fluency
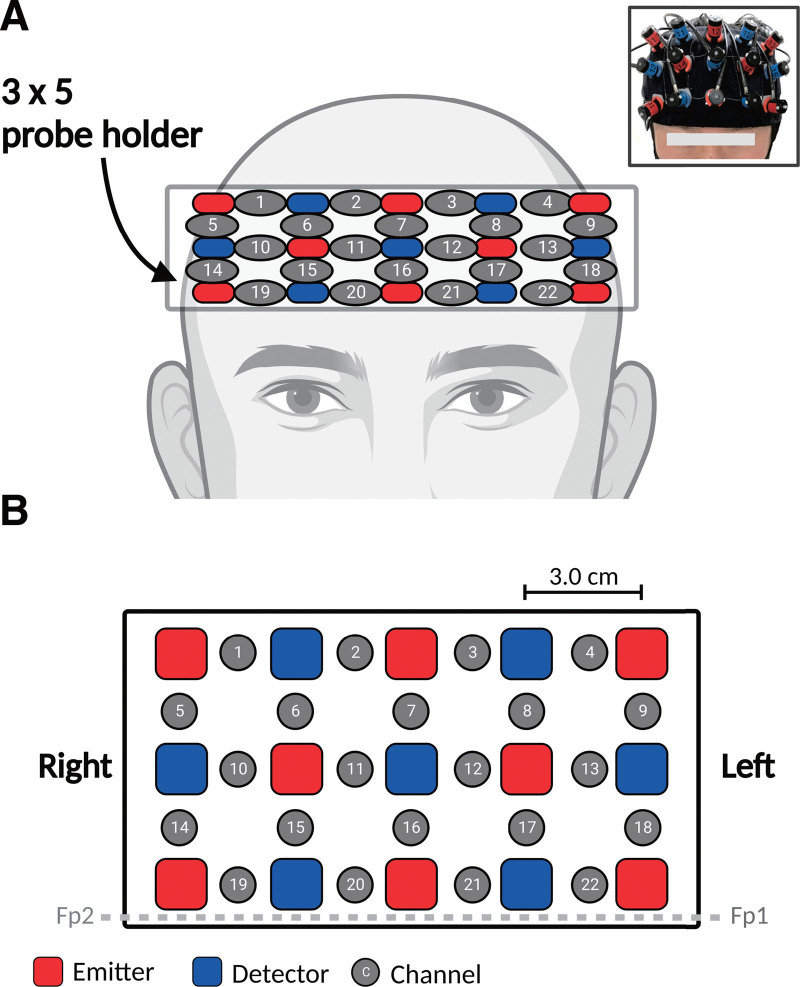
We used the fNIRS system ETG-4100 (FUJIFILM Corporation, Japan) with near-infrared light at 2 wavelengths (695 nm and 830 nm). Figure 2 shows the probe placement. The 3 × 5 probe holder had 15 electrodes comprising 8 emitters and 7 detectors placed every 3 cm, equivalent to the skull to the surface of the cerebral cortex.[17,18] A channel was defined as the area between 2 electrodes. Therefore, the probe holder had 22 channels, indicating that the hemodynamic responses of 22 different brain regions of interest could be monitored noninvasively. In the brain, the points Fp1 and Fp2 were determined according to the international 10/20 system used in electroencephalography, and the probe holder was positioned such that its bottom line matched the Fp1 to Fp2 line.
Figure 2.
Probe settings and measurement points used by the 22-channel fNIRS device. The probes are placed over the participant’s frontal region bilaterally (A). Precise locations of the probes (B). fNIRS = functional near-infrared spectroscopy.
The relative changes in oxygenated and deoxygenated hemoglobin concentrations were calculated 10 times per second. The entire measurement time was 160 seconds, which is equivalent to 1600 samples. The pre-task baseline was determined as the mean over a 10-second period just prior to the task period, and the post-task baseline was determined as the mean over the last 5 seconds of the post-task period.[19] Because the baseline fluctuates, we were required to calculate the theoretical baseline per sample. To calculate the theoretical baseline, the fNIRS system builds a linear regression model from the pre- and post-task samples to minimize the root mean squared error. The concentration of oxygenated hemoglobin ([Oxy-Hb]) was measured considering the theoretical baseline as the origin. We averaged 600 samples of [Oxy-Hb] per participant per channel during VFT stimulation. The average obtained was considered as the [Oxy-Hb] elevation induced by VFT stimulation. If the obtained [Oxy-Hb] data were produced from motion artifacts, those data were excluded from the analysis. The entire process was performed automatically using an ETG-4100 instrument (FUJIFILM Corporation, Japan).
2.5. Data analyses
Spearman correlation coefficient was used to determine the correlation between [Oxy-Hb] changes in the psychological tests (SDS, STAI-X, and TEIQue-SF) and VFT performance. The significance level was set at P < .05. If the calculated P value was below the threshold, the calculated Spearman coefficient ρ were considered statistically significant. All statistical analyses were performed using the IBM SPSS Statistics version 25 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.).
3. Results
3.1. Psychological tests in healthy individuals
Owing to an experimental setting error, 4 participants data were excluded from the analysis. Therefore, a total of 26 participants (aged 28.4 ± 4.9 years, mean ± standard deviation) data were analyzed. Table 1 shows individual psychological test scores. The mean and standard deviation of each psychological test and VFT performance were as follows: SDS, 34.4 ± 6.3; STAI-S, 33.7 ± 7.9; STAI-T, 40.5 ± 8.5; TEIQue-SF, 4.6 ± 0.6; and VFT performance, 16.6 ± 4.2.
Table 1.
Individual psychological tests and VFT performance.
| Age | Psychological tests | VFT performance | |||
|---|---|---|---|---|---|
| SDS | STAI-S | STAI-T | TEIQue-SF | ||
| 31 | 42 | 37 | 45 | 4.5 | 9 |
| 35 | 34 | 45 | 39 | 5.1 | 14 |
| 31 | 49 | 59 | 61 | 3.9 | 20 |
| 31 | 45 | 42 | 50 | 4.3 | 16 |
| 31 | 32 | 35 | 44 | 4.9 | 15 |
| 37 | 33 | 27 | 41 | 5.4 | 21 |
| 26 | 44 | 25 | 46 | 3.7 | 22 |
| 25 | 25 | 21 | 22 | 6.1 | 18 |
| 25 | 38 | 38 | 43 | 4.5 | 15 |
| 24 | 34 | 31 | 55 | 4.3 | 24 |
| 23 | 33 | 36 | 46 | 4.2 | 18 |
| 24 | 31 | 27 | 30 | 5.0 | 24 |
| 25 | 35 | 39 | 44 | 3.9 | 20 |
| 35 | 37 | 32 | 41 | 3.9 | 8 |
| 31 | 35 | 36 | 39 | 4.7 | 13 |
| 33 | 33 | 31 | 40 | 4.5 | 18 |
| 30 | 28 | 29 | 28 | 5.2 | 16 |
| 35 | 29 | 24 | 36 | 4.3 | 13 |
| 23 | 27 | 31 | 39 | 4.6 | 11 |
| 26 | 39 | 29 | 44 | 3.1 | 15 |
| 25 | 30 | 41 | 33 | 5.1 | 15 |
| 25 | 37 | 29 | 38 | 4.4 | 14 |
| 38 | 36 | 38 | 37 | 5.5 | 17 |
| 23 | 27 | 32 | 38 | 4.7 | 14 |
| 24 | 23 | 26 | 27 | 5.1 | 18 |
| 22 | 39 | 37 | 48 | 4.9 | 23 |
SDS = Zung Self-rating Depression Scale, STAI-S = State-Trait Anxiety Inventory Scale Trait anxiety, STAI-T = State-Trait Anxiety Inventory Scale State anxiety, TEIQue-SF = Trait Emotional Intelligence Questionnaire-Short Form, VFT = verbal fluency task.
3.2. Correlation between psychological tests and task performance
VFT performance was not significantly correlated with the SDS (ρ = 0.063, P = .759), STAI-T (ρ = 0.243, P = .232), STAI-S (ρ = −0.138, P = .500), or TEIQue-SF (ρ = 0.303, P = .132) scores.
3.3. Changes in [oxy-Hb] changes during cognitive activation
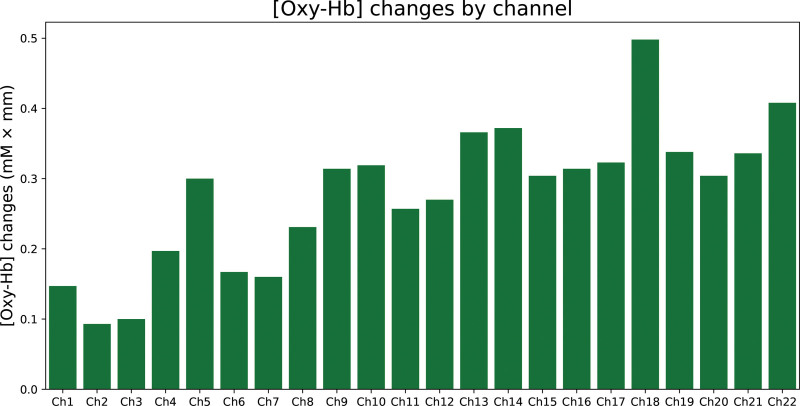
Figure 3 shows that [Oxy-Hb] changes according to the channel. Although [Oxy-Hb] changed, the change was not significantly correlated with VFT performance (Table 2).
Figure 3.
Change in oxygenated hemoglobin concentration by cognitive activation. Each oxygenated hemoglobin concentration change per channel were as follows; #1: 0.147, #2: 0.093, #3: 0.100, #4: 0.197, #5: 0.300, #6: 0.167, #7: 0.160, #8: 0.231, #9: 0.314, #10: 0.319, #11: 0.257, #12: 0.270, #13: 0.366, #14: 0.372, #15: 0.304, #16: 0.314, #17: 0.323, #18: 0.498, #19: 0.338, #20: 0.304, #21: 0.336, #22: 0.408 (each channel number is presented after #).
Table 2.
Correlation between psychological tests and oxygenated hemoglobin changes.
| Channels | SDS | STAI-T | STAI-S | TEIQue-SF | VFT performance | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ρ | P value | ρ | P value | ρ | P value | ρ | P value | ρ | P value | |
| 1 | −0.367 | .065 | −0.258 | .203 | −0.512 | .008 | 0.124 | .547 | 0.092 | .656 |
| 2 | −0.278 | .169 | −0.180 | .379 | −0.401 | .042 | −0.192 | .348 | 0.013 | .950 |
| 3 | −0.391 | .048 | −0.321 | .109 | −0.449 | .021 | 0.016 | .939 | 0.166 | .418 |
| 4 | −0.471 | .015 | −0.406 | .040 | −0.311 | .122 | 0.016 | .936 | 0.074 | .721 |
| 5 | −0.464 | .017 | −0.338 | .091 | −0.466 | .017 | 0.093 | .651 | −0.090 | .661 |
| 6 | −0.354 | .076 | −0.213 | .295 | −0.341 | .088 | −0.030 | .844 | 0.091 | .658 |
| 7 | −0.371 | .062 | −0.258 | .204 | −0.414 | .035 | −0.033 | .875 | −0.078 | .705 |
| 8 | −0.349 | .080 | −0.378 | .057 | −0.323 | .108 | 0.023 | .911 | 0.122 | .553 |
| 9 | −0.457 | .019 | −0.377 | .058 | −0.284 | .160 | 0.029 | .886 | −0.051 | .804 |
| 10 | −0.455 | .019 | −0.303 | .132 | −0.364 | .068 | 0.021 | .919 | 0.056 | .787 |
| 11 | −0.385 | .052 | −0.295 | .144 | −0.353 | .077 | −0.003 | .989 | −0.056 | .787 |
| 12 | −0.354 | .076 | −0.258 | .204 | −0.302 | .133 | 0.040 | .846 | 0.079 | .701 |
| 13 | −0.362 | .069 | −0.254 | .211 | −0.388 | .050 | 0.029 | .886 | 0.132 | .522 |
| 14 | −0.491 | .011 | −0.305 | .130 | −0.376 | .058 | 0.185 | .367 | 0.085 | .679 |
| 15 | −0.461 | .018 | −0.378 | .057 | −0.423 | .031 | −0.038 | .855 | 0.029 | .889 |
| 16 | −0.405 | .040 | −0.401 | .042 | −0.420 | .033 | 0.057 | .783 | −0.012 | .955 |
| 17 | −0.337 | .093 | −0.298 | .139 | −0.383 | .053 | 0.035 | .864 | 0.062 | .763 |
| 18 | −0.278 | .168 | −0.144 | .482 | −0.167 | .415 | 0.076 | .713 | 0.133 | .517 |
| 19 | −0.431 | .028 | −0.327 | .102 | −0.200 | .328 | 0.072 | .728 | 0.120 | .559 |
| 20 | −0.415 | .035 | −0.295 | .144 | −0.398 | .044 | 0.104 | .614 | −0.022 | .916 |
| 21 | −0.336 | .093 | −0.153 | .456 | −0.284 | .160 | 0.194 | .342 | 0.302 | .134 |
| 22 | −0.330 | .136 | −0.115 | .574 | −0.228 | .262 | 0.201 | .324 | 0.205 | .316 |
Statistically significant values and their P values are indicated in bold.
SDS = Zung Self-rating Depression Scale, STAI-S = State-Trait Anxiety Inventory Scale Trait anxiety, STAI-T = State-Trait Anxiety Inventory Scale State anxiety, TEIQue-SF = trait emotional intelligence questionnaire-short form, TEIQue = Trait Emotional Intelligence Questionnaire-Short Form, VFT = verbal fluency task.
3.4. Correlation between [Oxy-Hb] changes and psychological tests
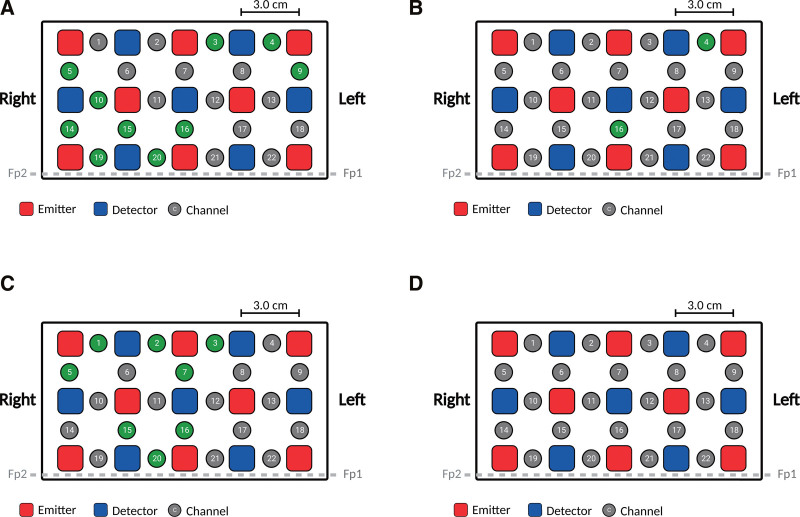
Table 2 shows the correlation between [oxy-Hb] changes and psychological test results. The significantly correlated channels for each psychological test are shown individually (Fig. 4). The SDS scores were negatively correlated with [Oxy-Hb] changes in channels 3, 4, 5, 9, 10, 14, 15, 16, 19, and 20. STAI-T scores were negatively correlated with changes in [Oxy-Hb] in channels 4 and 16. STAI-S scores were negatively correlated with [Oxy-Hb] changes in channels 1, 2, 3, 5, 7, 15, 16, and 20. The TEIQue-SF scores were not significantly correlated with [Oxy-Hb] changes in any channel.
Figure 4.
Correlation between psychological tests and changes in oxy-Hb changes. Correlations between psychological tests (SDS, STAI-T, STAI-S, and TEIQue-SF) and [Oxy-Hb] was displayed according to the probe setting. The green channel indicates a significant correlation between the [Oxy-Hb] changes and (A) SDS, (B) STAI-T, (C) STAI-S, and (D) TEIQue-SF. [Oxy-Hb] = oxygenated hemoglobin concentration, SDS = Zung self-rating depression scale, STAI = state-trait anxiety inventory, STAI-S = state anxiety or anxiety about an event of STAI, STAI-T = trait anxiety or anxiety level as a personal characteristic of STAI, TEIQue-SF = trait emotional intelligence questionnaire-short form.
4. Discussion
VFT performance did not correlate with anxiety, depression, or EI, suggesting that healthy individuals PFC activity is not severely affected by their mental state. Moreover, higher VFT performance did not indicate higher PFC activity, suggesting that cognitive activation successfully activated the PFC, but the activation could not be quantified by cerebral hemodynamics alone. Our findings are consistent with previous research,[20] which indicated that VFT performance did not have a significant main effect on [Oxy-Hb] changes. On the contrary, [Oxy-Hb] changes in the PFC and VFT performance of healthy individuals were greater compared to those in patients with bipolar disorder,[21] major depressive disorder,[16] obsessive-compulsive disorder,[22] and schizophrenia.[23] Therefore, although greater [oxy-Hb] changes do not imply better VFT performance, psychiatric patients often show lower [oxy-Hb] changes with lower VFT performance than healthy individuals. The change in [Oxy-Hb] was negatively correlated with the SDS, STAI-T, and STAI-S scores, suggesting that anxiety and depression have a negative impact on VFT performance, which is consistent with our previous findings. Moreover, structural changes have recently been suggested to be involved in the development of abnormal depression and anxiety-associated circuits.[24,25]
However, the TEIQue-SF showed no statistical correlation with [Oxy-Hb] changes or VFT performance. Therefore, in our study, EI was not associated with PFC activity. Takeuchi et al[26] indicated that the mean diffusivity in the dorsolateral PFC of a magnetic resonance image is significantly correlated with trait emotional intelligence (TEI). TEI involves at least 3 neural circuits of the social cognition network, somatic marker circuitry, and dopaminergic systems, and the social cognition network includes the medial PFC, and the somatic marker circuitry includes the ventromedial PFC.[26]
The middle and superior frontal gyri are involved in mentalizing. Mentalization refers to the ability to understand the mental state of oneself and that of others. The left cerebral hemisphere also plays a significant role in mentalizing.[27] Because of the similarity between mentalizing and EI, mentalizing ability can be measured by EI.[28] If the trait EI was significantly correlated with frontal cortical activation during VFT in healthy men, the role of PFC function in mentalizing was indicated. However, we failed to find any statistically significant correlations. The main reason for this may be the small sample size. Further large-scale studies with adjustments for multiple comparisons are required to confirm our hypotheses. To perform a Spearman correlation analysis with a statistical power of 0.8, a significance level of alpha of 0.05, and an effect size of 0.3, we need 167 participants. In this study, we indicated the necessity for further investigation of the correlation between TEI and hemodynamic responses, which will facilitate stress management in healthy individuals. Turner et al[29] suggested the contribution of psychological stress to health via changes in stress reactivity toward the sympathetic–adrenal–medullary system and hypothalamic–pituitary–adrenal axis. If the sample size is sufficient, a large dataset can be obtained, and machine learning approaches may facilitate using [Oxy-Hb] changes and EI as biomarkers of real-time noninvasive stress management, as shown in a previous study of patients with major depressive disorder.[30]
Other limitations of our study are as follows: We excluded women from this study to avoid the possible effect of menstruation-induced mood fluctuations on our results as the effect is controversial.[31–33] Therefore, our findings may not apply to women; Psychological stress factors were evaluated based on self-rated scores. We did not evaluate participants psychological stress based on a structured diagnostic interview (e.g., a Structured Clinical Interview for Diagnostic and Statistical Manual diagnoses [34]); We did not consider the participants intelligence quotient, which may have influenced the VFT;[35] Because the study was conducted in participants who were able to apply through recruitment posters, there is a possibility that this study population does not clearly represent healthy Japanese males. However, as all candidates who met the inclusion criteria participated in our study, there were no arbitrary selection biases.
5. Conclusion
Our preliminary study is the first to attempt to show the activity of the PFC on EI through a hemodynamic response, although there is a critical sample size shortage. EI may play a vital role in reducing stress associated with depression and anxiety in our social lives.
Acknowledgements
BioRender was used to render the figures (Figure2 and Figure 4) after obtaining a paid license for publication authorization.
Author contributions
Conceptualization: Haruka Amitani, Akihiro Asakawa.
Data curation: Takamasa Fukumoto.
Formal analysis: Takamasa Fukumoto, Haruka Amitani, Ryusei Nishi, Midori Wada.
Funding acquisition: Akihiro Asakawa.
Investigation: Takamasa Fukumoto.
Methodology: Naoya Oishi.
Project administration: Akihiro Asakawa.
Resources: Naoya Oishi.
Supervision: Haruka Amitani, Ryusei Nishi, Naoya Oishi.
Validation: Ryusei Nishi.
Visualization: Midori Wada.
Writing – original draft: Takamasa Fukumoto.
Writing – review & editing: Haruka Amitani, Ryusei Nishi, Midori Wada, Akihiro Asakawa.
Abbreviations:
- [Oxy-Hb]
- oxygenated hemoglobin concentration
- EI
- emotional intelligence
- fNIRS
- functional near-infrared spectroscopy
- PFC
- prefrontal cortex
- SDS
- Zung self-rating depression scale
- STAI-S
- state anxiety or anxiety about an event of STAI
- STAI-T
- trait anxiety or anxiety level as a personal characteristic of STAI
- TEI
- trait emotional intelligence
- TEIQue
- trait emotional intelligence questionnaire
- TEIQue-SF
- trait emotional intelligence questionnaire-short form
- VFT
- verbal fluency task
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
This study was supported by a KAKENHI Grant-in-Aid for Scientific Research on Innovative Areas in Japan (“Willdynamics” Grant 16H06401). The funders played no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
The authors have no conflicts of interest to disclose.
How to cite this article: Fukumoto T, Amitani H, Nishi R, Wada M, Oishi N, Asakawa A. Correlation between trait emotional intelligence and prefrontal activation during a verbal fluency task: A functional near-infrared spectroscopy study. Medicine 2023;102:29(e34418).
Contributor Information
Takamasa Fukumoto, Email: k5632275@kadai.jp.
Haruka Amitani, Email: amitani@m3.kufm.kagoshima-u.ac.jp.
Ryusei Nishi, Email: k6450967@kadai.jp.
Midori Wada, Email: k7902731@kadai.jp.
Naoya Oishi, Email: noishi@kuhp.kyoto-u.ac.jp.
References
- [1].Cohen S, Gianaros PJ, Manuck SB. A stage model of stress and disease. Perspect Psychol Sci. 2016;11:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mayer JD, Salovey P, Caruso DR. Emotional intelligence: theory, findings, and implications. Psychol Inq. 2004;15:197–215. [Google Scholar]
- [4].O’Connor PJ, Hill A, Kaya M, et al. The measurement of emotional intelligence: a critical review of the literature and recommendations for researchers and practitioners. Front Psychol. 2019;10:1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bru-Luna LM, Martí-Vilar M, Merino-Soto C, et al. Emotional intelligence measures: a systematic review. Healthcare (Basel). 2021;9:1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liu WZ, Zhang WH, Zheng ZH, et al. Identification of a prefrontal cortex-to-amygdala pathway for chronic stress-induced anxiety. Nat Commun. 2020;11:2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nishi R, Fukumoto T, Asakawa A. Possible effect of natural light on emotion recognition and the prefrontal cortex: a scoping review of near-infrared (NIR) spectroscopy. [Published online April 2023]. Adv Clin Exp Med. [DOI] [PubMed] [Google Scholar]
- [9].Periáñez JA, Lubrini G, García-Gutiérrez A, et al. Construct validity of the stroop color-word test: influence of speed of visual search, verbal fluency, working memory, cognitive flexibility, and conflict monitoring. Arch Clin Neuropsychol. 2021;36:99–111. [DOI] [PubMed] [Google Scholar]
- [10].Tran BX, Nguyen TT, Boyer L, et al. Differentiating people with schizophrenia from healthy controls in a developing country: an evaluation of portable functional near infrared spectroscopy (fNIRS) as an adjunct diagnostic tool. Front Psychiatry. 2023;14:1061284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zung WWK, Richards CB, Short MJ. Self-rating depression scale in an outpatient clinic: further validation of the SDS. Arch Gen Psychiatry. 1965;13:508–15. [DOI] [PubMed] [Google Scholar]
- [12].Iwamoto M, Dodo H, Yoneda J, et al. A study of the state-trait anxiety inventory (STAI) and its application to noise stress. Jpn J Hyg. 1989;43:1116–23. [DOI] [PubMed] [Google Scholar]
- [13].Abe K, Wakabayashi H, Saiki T, et al. Trait validity and reliability of Japanese emotional intelligence questionnaire-SF and Jefferson scale of physician empathy. J Med Educ (Jpn). 2012;43:351–9. [Google Scholar]
- [14].Siegling AB, Vesely AK, Petrides KV, et al. Incremental validity of the trait emotional intelligence questionnaire-short form (TEIQue-SF). J Pers Assess. 2015;97:525–35. [DOI] [PubMed] [Google Scholar]
- [15].Takizawa R, Fukuda M, Kawasaki S, et al. Neuroimaging-aided differential diagnosis of the depressive state. Neuroimage. 2014;85:498–507. [DOI] [PubMed] [Google Scholar]
- [16].Husain SF, Yu R, Tang TB, et al. Validating a functional near-infrared spectroscopy diagnostic paradigm for major depressive disorder. Sci Rep. 2020;10:9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hock C, Villringer K, Müller-Spahn F, et al. Decrease in parietal cerebral hemoglobin oxygenation during performance of a verbal fluency task in patients with Alzheimer’s disease monitored by means of near-infrared spectroscopy (NIRS) - Correlation with simultaneous rCBF-PET measurements. Brain Res. 1997;755:293–303. [DOI] [PubMed] [Google Scholar]
- [18].Toronov V, Webb A, Choi JH, et al. Investigation of human brain hemodynamics by simultaneous near-infrared spectroscopy and functional magnetic resonance imaging. Med Phys. 2001;28:521–7. [DOI] [PubMed] [Google Scholar]
- [19].Noda T, Yoshida S, Matsuda T, et al. Frontal and right temporal activations correlate negatively with depression severity during verbal fluency task: a multi-channel near-infrared spectroscopy study. J Psychiatr Res. 2012;46:905–12. [DOI] [PubMed] [Google Scholar]
- [20].Kameyama M, Fukuda M, Uehara T, et al. Sex and age dependencies of cerebral blood volume changes during cognitive activation: a multichannel near-infrared spectroscopy study. Neuroimage. 2004;22:1715–21. [DOI] [PubMed] [Google Scholar]
- [21].Fu L, Xiang D, Xiao J, et al. Reduced prefrontal activation during the tower of London and verbal fluency task in patients with bipolar depression: a multi-channel NIRS study. Front Psychiatry. 2018;9:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liao J, Li T, Dong W, et al. Reduced prefrontal-temporal cortical activation during verbal fluency task in obsessive-compulsive disorder: a multi-channel near-infrared spectroscopy study. J Psychiatr Res. 2019;109:33–40. [DOI] [PubMed] [Google Scholar]
- [23].Luo X, Qi Y, Wang H, et al. Prefrontal cortex dysfunction during verbal fluency task after atypicalantipsychotic treatment in schizophrenia: a near-infrared spectroscopy imaging study. Neurosci Lett. 2018;686:101–5. [DOI] [PubMed] [Google Scholar]
- [24].Hare BD, Duman RS. Prefrontal cortex circuits in depression and anxiety: contribution of discrete neuronal populations and target regions. Mol Psychiatry. 2020;25:2742–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pizzagalli DA, Roberts AC. Prefrontal cortex and depression. Neuropsychopharmacology. 2022;47:225–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Takeuchi H, Taki Y, Nouchi R, et al. Mean diffusivity associated with trait emotional intelligence. Soc Cogn Affect Neurosci. 2019;14:871–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Atique B, Erb M, Gharabaghi A, et al. Task-specific activity and connectivity within the mentalizing network during emotion and intention mentalizing. Neuroimage. 2011;55:1899–911. [DOI] [PubMed] [Google Scholar]
- [28].Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behav Brain Sci. 1978;1:515–26. [Google Scholar]
- [29].Turner AI, Smyth N, Hall SJ, et al. Psychological stress reactivity and future health and disease outcomes: a systematic review of prospective evidence. Psychoneuroendocrinology. 2020;114:104599. [DOI] [PubMed] [Google Scholar]
- [30].Li Z, McIntyre RS, Husain SF, et al. Identifying neuroimaging biomarkers of major depressive disorder from cortical hemodynamic responses using machine learning approaches. EBioMedicine. 2022;79:104027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Romans S, Clarkson R, Einstein G, et al. Mood and the menstrual cycle: a review of prospective data studies. Gender Med. 2012;9:361–84. [DOI] [PubMed] [Google Scholar]
- [32].Parry BL, Martínez LF, Maurer EL, et al. Sleep, rhythms and women’s mood. Part I. Menstrual cycle, pregnancy and postpartum. Sleep Med Rev. 2006;10:129–44. [DOI] [PubMed] [Google Scholar]
- [33].Leibenluft E, Fiero PL, Rubinow DR. Effects of the menstrual cycle on dependent variables in mood disorder research. Arch Gen Psychiatry. 1994;51:761–81. [DOI] [PubMed] [Google Scholar]
- [34].Osório FL, Loureiro SR, Hallak JEC, et al. Clinical validity and intrarater and test–retest reliability of the structured clinical interview for DSM-5 – clinician version (SCID-5-CV). Psychiatry Clin Neurosci. 2019;73:754–60. [DOI] [PubMed] [Google Scholar]
- [35].Vaucheret Paz E, Puga C, Ekonen C, et al. Verbal fluency test in children with neurodevelopmental disorders. J Neurosci Rural Pract. 2020;11:95–9. [DOI] [PMC free article] [PubMed] [Google Scholar]