Background:
A high fresh gas flow of >5 L/minute is commonly used for emergence from inhalation anesthesia. In addition, a high fresh gas flow may have detrimental effects on climate change. However, no study has determined the optimal fresh gas flow for emergence from inhalation anesthesia. Therefore, we compared the effect of fresh gas flows of 5 L/minute and 10 L/minute on emergence time from sevoflurane anesthesia.
Methods:
Patients who were scheduled for transurethral resection of bladder tumor were randomly assigned to receive fresh gas flows of 5 L/minute (group 5) or 10 L/minute (group 10) during emergence. Emergence time was defined as the time from discontinuation of sevoflurane to tracheal extubation. The primary outcome was the emergence time, and the secondary outcomes were the time to self-movement and the time to eye-opening.
Results:
A total of 54 patients were included. In groups 5 and 10, emergence time (12.1 ± 2.9 minutes vs 11.1 ± 2.7 minutes, respectively; P = .232), time to self-movement (9.4 ± 3.8 minutes vs 8.5 ± 4.6 minutes, respectively; P = .435), and time to eye-opening (11.5 ± 3.1 minute vs 10.6 ± 3.0 minutes, respectively; P = .252) were not significantly different.
Conclusions:
Emergence time, time to self-movement, and time to eye opening were not significantly different between fresh gas flow rates of 5 L/minute and 10 L/minute in transurethral resection of bladder tumor, thus suggesting that fresh gas flow of 5 L/minute is sufficient for emergence from sevoflurane anesthesia.
Trial registration:
ClinicalTrials.gov (NCT05376631).
Keywords: emergence, fresh gas flow, sevoflurane anesthesia
1. Introduction
Upon discontinuation of inhalation agent, patients typically remain unconscious for several minutes and then progress through a series of stages concluding with full consciousness. In general, the stages of emergence include the return of patient behaviors prompting extubation, eye-opening, and the ability to respond to questions.[1,2] Recovery from general anesthesia depends on the decreasing concentration of the anesthetic agent in brain tissue or blood. Inhalation agents are mainly eliminated through the lungs.[3] Consequently, discontinuation of the inhalation agent and usage of higher fresh gas flow are used in order to recover from general anesthesia.
During the maintenance period, low fresh gas flow is also widely used because it has several advantages including reduction of inhalation agent consumption, prevention of workplace contamination and atmospheric pollution, decrease in costs, and better support of mucociliary clearance by maintaining heat and moisture in the respiratory system.[4,5] In contrast, high fresh gas flow is commonly used during the induction of general anesthesia to increase the rates of anesthetic delivery and alveolar and brain anesthetic partial pressure.[6,7] Furthermore, a high fresh gas flow is also used during the emergence of general anesthesia.[3] For rapid recovery from general anesthesia, the anesthetic agent within the breathing circuit should be washed out, and the exhaled air of the patient, which contains an anesthetic agent, should not be rebreathed. These processes can be aided by using high inflow rates of oxygen. Generally, a high fresh gas flow above 5 L/minute has been recommended for emergence from inhalation anesthesia,[8] and various flows of fresh gas are used for the emergence of general anesthesia in clinical circumstances. However, there is no study that reports which fresh gas flow would be better for emergence from inhalation anesthesia.
In this randomized controlled study, we compared the effect of fresh gas flows of 100% oxygen of 5 L/minute and 10 L/minute on emergence time from sevoflurane anesthesia in patients undergoing transurethral resection of bladder tumor. Emergence time was defined as the time from discontinuation of sevoflurane to tracheal extubation.
2. Methods
This study was approved by the Institutional Review Board of Asan Medical Center, Seoul, Republic of Korea, on May 9, 2022 (approval number: 2022-0606). Written informed consent was obtained from all patients participating in the trial. The trial was registered prior to patient enrollment at clinicaltrials.gov (NCT05376631, Date of registration: 05/17/2022; principal investigator: Young-Kug Kim). The study trial was conducted in accordance with the original study protocol.
2.1. Participants
All patients were enrolled between May 2022 and July 2022. The inclusion criteria were as follows: patients aged between 20 and 79 years with American Society of Anesthesiologists physical status I to II and scheduled transurethral resection of bladder tumor under general anesthesia. Surgical operations lasting more than 2 hours were excluded because long durations of surgery may affect the emergence from inhalation anesthesia.[9,10] In addition, patients with hearing disturbances, cognitive disorders, psychiatric substance abuse, and who were not willing to participate were also excluded.
2.2. Randomization, concealment, and blinding
Participants enrolled in this study were randomized. Before initiation of participant recruitment, the first investigator produced a random-numbers table using a web-based randomization software (Random Allocation Software version 1.0; Isfahan University of Medical Sciences, Isfahan, Iran). Eligible participants were allocated to either group 5 (patients received a fresh gas flow of 5 L/minute during the emergence period) or group 10 (patients received a fresh gas flow of 10 L/minute during the emergence period).
The randomization codes were concealed by the first investigator and delivered to the second investigator. The second investigator, who was not blinded to the allocated group, awakened patients from general anesthesia. The emergence time (i.e., time to tracheal extubation), time to self-movement, and time to eye-opening were assessed by the second investigator. In addition, bispectral index (BIS), end-tidal sevoflurane concentration (EtSEV), tidal volume, peak airway pressure, and positive end-expiratory pressure (PEEP) during the emergence period were also assessed by the second investigator. Blood pressure, peripheral oxygen saturation, postoperative nausea/vomiting, and patient satisfaction were assessed by the third investigator, who was blinded to the allocated group in the post-anesthesia care unit. Other investigators and patients were blinded to the allocated group during the study period.
2.3. Study protocol
Preoperatively, patients were educated about emergence from general anesthesia and about outcomes such as postoperative nausea/vomiting and patient satisfaction.
The patients were monitored according to our institutional standards upon arrival at the operating room. General anesthesia was induced by administering 2 mg/kg of propofol followed by 0.6 mg/kg of rocuronium bromide. Once the patient was unconscious and their train-of-four count was zero, endotracheal intubation was performed. During the operation, anesthesia was maintained with sevoflurane in oxygen (inspiratory fraction 0.5 at a flow rate of 2 L/minute) using the Dräger Primus anesthetic machine (Dräger, Lubeck, Germany). Sevoflurane administration was maintained to give an end-tidal concentration of 3 to 4% until termination of inhaled anesthesia.
Approximately 5 minutes before the termination of sevoflurane administration, sugammadex was administered to reverse muscle relaxation until the train of four ratio > 90%. The administration of sevoflurane was terminated at the end of the surgery. After discontinuation of sevoflurane, patients received fresh gas flows of 100% oxygen of 5 L/minute (group 5) or 10 L/minute (group 10). During emergence, all patients received pressure support ventilation. The flow trigger was set at 2 L/minute. Support amount and safety backup ventilation were adjusted according to the patient response to meet the target tidal volume of 7 to 8 mL/kg of predicted body weight and respiratory rate of 10 to 16 breaths/minute and decreased gradually as the patient restored tidal volume and respiratory rate. The partial pressure of end-tidal carbon dioxide was maintained at 30 to 40 mm Hg. Ventilatory support was stopped when the patient showed adequate tidal volume (>6 mL/kg) and respiratory rate (≥10 breaths/minute) without ventilatory support.[11] No stimulation or suction was provided until the patient opened their eyes or moved. Extubation criteria were as follows: adequate oxygenation as indicated by adequate ventilation which is represented by tidal volume >5 mL/kg, spontaneous respiratory rate >7 breaths/minute and <30 breaths/minute, end-tidal carbon dioxide <50 mm Hg, and respiratory rate to tidal volume ratio <105 breaths/minute/L; stable hemodynamics; full reversal of muscle relaxation; intact neurologic status as indicated by following verbal commands and intact airway reflex; normothermia as indicated by body temperature >35.5°C and <38.5°C; surgical considerations (bleeding <100 mL within last 30 minutes).[12] After suction of the oral secretion, the endotracheal tube was removed.
2.4. Assessments
Intraoperative variables such as infused propofol amount, surgery time, anesthesia time, and body temperature during emergence were measured. Respiratory variables during emergence, including tidal volume, minute ventilation, peak airway pressure, and PEEP, were also measured.
Patients were asked to open their eyes every 1 minute after the termination of sevoflurane. The emergence time, time to self-movement, and time to eye-opening were recorded. Emergence time was evaluated as the time from turning off the vaporizer to tracheal extubation.[10,13–16] The time to the self-movement was evaluated as the time from turning off the vaporizer to self-movement.[17] The time to eye opening was evaluated as the time from turning off the vaporizer to eye-opening on verbal command.[18]
The EtSEV and BIS were also recorded every 1 minute until tracheal extubation. In addition, tidal volume, peak airway pressure, and PEEP were also recorded during the emergence period. In the postoperative care unit, blood pressure, peripheral oxygen saturation, postoperative nausea/vomiting, and patient satisfaction were assessed. Patient satisfaction was assessed using a 7-point Likert scale (1 = strongly dissatisfied, 2 = moderately dissatisfied, 3 = slightly dissatisfied, 4 = neutral, 5 = slightly satisfied, 6 = moderately satisfied, 7 = extremely satisfied) at 6 hours postoperatively.[19]
2.5. Primary and secondary outcomes
The primary outcome was the emergence time. The secondary outcomes were the time to self-movement and time to eye-opening.
2.6. Statistical analysis
This study was designed to evaluate the effect of fresh gas flow on the emergence time. As per our experience, emergence time was 11.7 ± 3.3 minutes using a fresh gas flow of 5 L/minute. We assumed that a fresh gas flow of 10 L/minute would decrease the emergence time by 30%.[20,21] This 30% difference (i.e., approximately 3 minutes) was considered a clinically relevant difference in emergence time in clinical circumstances. According to this assumption, we calculated that a sample size of 21 patients in each group would yield statistical significance, with a 2-sided α = 0.05 and β = 0.1. Considering a 20% dropout rate, 27 patients were included in each group.
Data are expressed as means (standard deviation) or numbers (%), as appropriate. We focused on the primary outcome as emergence time, which was compared using the independent t-test. Furthermore, continuous variables were also compared using the independent t-test. Categorical variables were compared using the chi-square test or Fisher exact test as appropriate. Cumulative emergence was also compared between group 5 and group 10 using a reverse Kaplan–Meier survival curve with a log-rank test. All P values were 2-sided, and a value of P < .05 was considered statistically significant. Statistical analyses were performed using MedCalc version 11.3.3.0 (MedCalc Software bvba, Mariakerke, Belgium) and SPSS version 21.0.0 for Windows (IBM Corporation, Chicago, IL).
3. Results
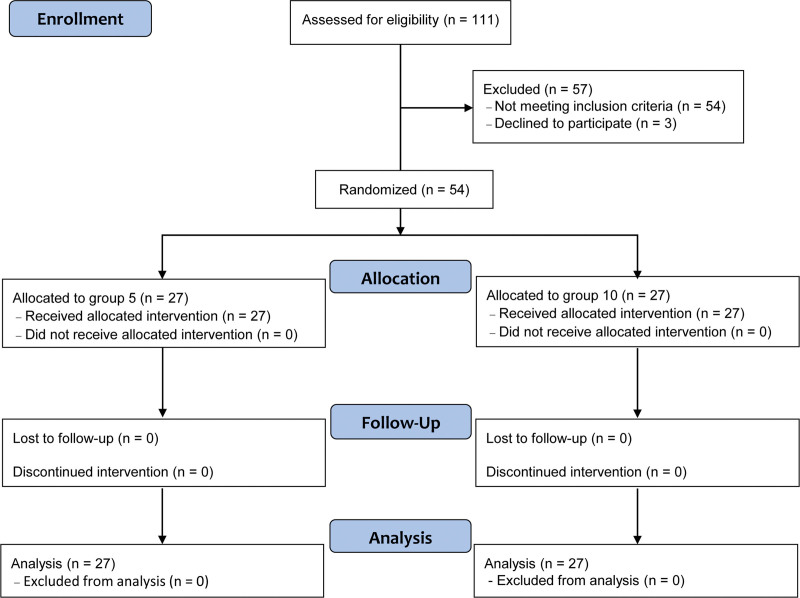
The study flowchart is presented in Figure 1. During the enrollment process, 111 patients were assessed for eligibility, of which 57 patients were excluded. In the final analysis, all 54 randomized patients were included since neither group was lost to follow-up. The patient characteristics such as age, sex, height, weight, body mass index, American Society of Anesthesiologists physical status, hypertension, diabetes mellitus, and chronic kidney disease are shown in Table 1.
Figure 1.
Flow diagram of study participants. Group 5 and group 10 include patients who received a fresh gas flow of 5 L/min and 10 L/min, respectively, during the emergence period.
Table 1.
Patient characteristics.
| Variables | Group 5 (n = 27) | Group 10 (n = 27) |
|---|---|---|
| Age, yr | 63 ± 8 | 63 ± 9 |
| Sex, male/female | 20 (74.1%)/7 (25.9%) | 21 (77.8%)/6 (22.2%) |
| Height, cm | 163.4 ± 8.8 | 164.1 ± 5.3 |
| Weight, kg | 67.2 ± 12.4 | 66.5 ± 8.7 |
| Body mass index, kg/m2 | 25.0 ± 2.6 | 24.6 ± 3.1 |
| ASA physical status, I/II | 18 (66.7%)/9 (33.3%) | 17 (63.0%)/10 (37.0%) |
| Underlying disease | ||
| Hypertension | 8 (29.6%) | 6 (22.2%) |
| Diabetes mellitus | 4 (14.8%) | 3 (11.1%) |
| Chronic kidney disease | 3 (11.1%) | 3 (11.1%) |
Data are presented as mean ± standard deviation or number (%) as appropriate.
ASA = American Society of Anesthesiologists.
Group 5 and group 10 include patients who received a fresh gas flow of 5 L/min and 10 L/min, respectively, during the emergence period.
Intraoperative variables such as infused propofol amount, surgery time, and anesthesia time are assessed in Table 2. Body temperature, tidal volume, minute ventilation, peak airway pressure, and PEEP during emergence are also shown in Table 2. There were no significant differences in intraoperative variables between group 5 and group 10.
Table 2.
Intraoperative variables.
| Variables | Group 5 (n = 27) | Group 10 (n = 27) | P value |
|---|---|---|---|
| Infused propofol amount, mg | 127 ± 21 | 126 ± 20 | .896 |
| Surgery time, min | 49.5 ± 22.7 | 48.9 ± 17.4 | .920 |
| Anesthesia time, min | 71.6 ± 23.2 | 71.7 ± 16.9 | .984 |
| Body temperature during emergence period, °C | 35.8 ± 0.4 | 36.0 ± 0.4 | .286 |
| Respiratory variables during emergence period | |||
| Tidal volume, mL | 468 ± 42 | 467 ± 36 | .915 |
| Minute ventilation, L/min | 5.9 ± 1.5 | 6.1 ± 1.3 | .651 |
| Peak airway pressure, cmH2O | 13.4 ± 2.2 | 13.3 ± 2.0 | .949 |
| Positive end-expiratory pressure, cmH2O | 5 ± 0 | 5 ± 0 | >.999 |
Data are presented as mean ± standard deviation.
Group 5 and group 10 include patients who received a fresh gas flow of 5 L/min and 10 L/min, respectively, during the emergence period.
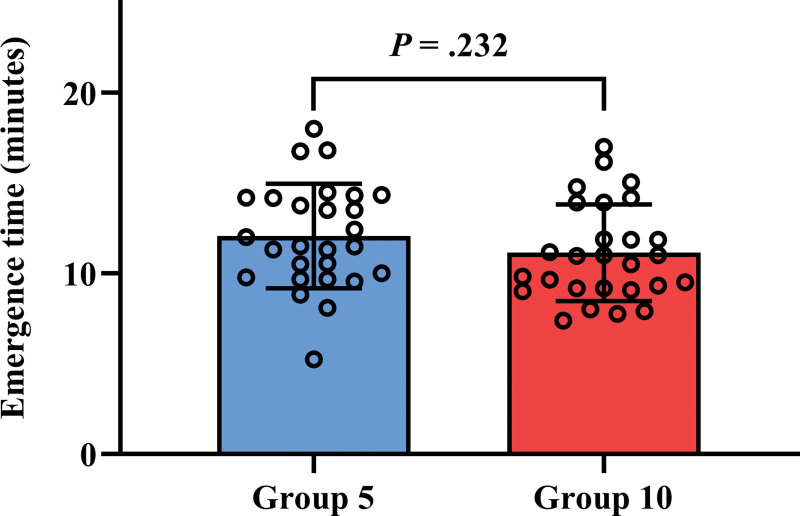
Emergence time was not significantly different between group 5 and group 10 (12.1 ± 2.9 minutes vs 11.1 ± 2.7 minutes, P = .232, Fig. 2). All patients woke up within 20 minutes from discontinuation of sevoflurane anesthesia. Emergence time, time to self-movement, and time to eye-opening among both groups are shown in Table 3. In addition, EtSEV and BIS score at each time point are shown in Table 3. Time to self-movement and time to eye-opening were not significantly different in group 5 and group 10 (P = .435 and P = .252). Furthermore, EtSEV and BIS score were not significantly different in group 5 and group 10 at each time point.
Figure 2.
Comparison of emergence time between group 5 and group 10. Emergence time is defined as the time from discontinuation of sevoflurane to tracheal extubation. Group 5 (blue box) and group 10 (red box) include patients who received a fresh gas flow of 5 L/min and 10 L/min, respectively, during the emergence period. Upper borders and error bars indicate the mean and standard deviation in each group. Dots indicate each emergence time.
Table 3.
Emergence time, time to self-movement, and time to eye-opening.
| Variables | Group 5 (n = 27) | Group 10 (n = 27) | P value |
|---|---|---|---|
| Emergence time, minutes | 12.1 ± 2.9 | 11.1 ± 2.7 | .232 |
| EtSEV at extubation, % | 0.16 ± 0.05 | 0.16 ± 0.07 | .741 |
| BIS at extubation, score | 86 ± 5 | 88 ± 5 | .256 |
| Time to self-movement, minutes | 9.4 ± 3.8 | 8.5 ± 4.6 | .435 |
| EtSEV at self-movement, % | 0.36 ± 0.23 | 0.32 ± 0.18 | .442 |
| BIS at self-movement, score | 60 ± 13 | 59 ± 12 | .908 |
| Time to eye opening, minutes | 11.5 ± 3.1 | 10.6 ± 3.0 | .252 |
| EtSEV at eye opening, % | 0.23 ± 0.08 | 0.20 ± 0.07 | .191 |
| BIS at eye opening, score | 80 ± 8 | 80 ± 5 | .952 |
Data are presented as mean ± standard deviation. BIS = bispectral index, EtSEV = end-tidal sevoflurane concentration.
Group 5 and group 10 include patients who received a fresh gas flow of 5 L/min and 10 L/min, respectively, during the emergence period. Emergence time is defined as the time from discontinuation of sevoflurane to tracheal extubation. Time to self-movement is defined as the time from discontinuation of sevoflurane to self-movement. Time to eye-opening is defined as the time from discontinuation of sevoflurane to eye-opening.
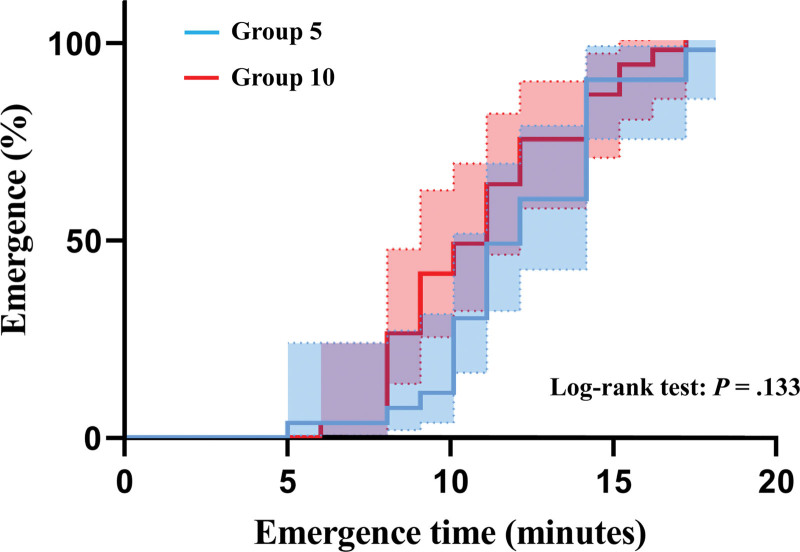
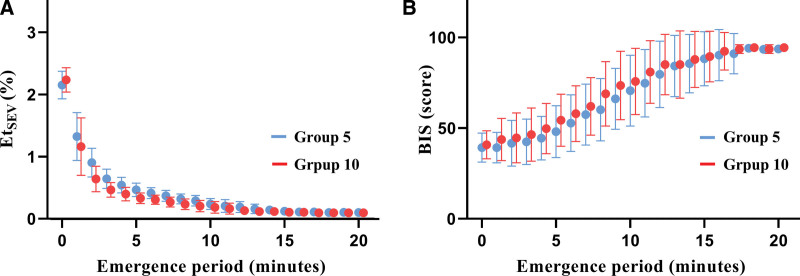
Figure 3 shows the reverse Kaplan–Meier estimates of the cumulative incidence of emergence between group 5 and group 10. No significant difference was found in emergence between the 2 groups (Log-rank test: P = .133). Additionally, Figure 4 shows changes in EtSEV and BIS during the emergence period. There were decreasing and increasing trends of EtSEV and BIS in both groups during emergence.
Figure 3.
Reverse Kaplan–Meier estimates of cumulative incidence of emergence in group 5 and group 10. Emergence time is defined as the time from discontinuation of sevoflurane to tracheal extubation. The time 0 is represented by the discontinuation of sevoflurane; the event corresponds to the emergence from sevoflurane anesthesia. Group 5 (blue line) and group 10 (red line) include patients who received a fresh gas flow of 5 L/min and 10 L/min, respectively, during the emergence period. Dot lines indicate 95% confidence intervals in each group.
Figure 4.
Changes in EtSEV (A) and BIS (B) during the emergence period in group 5 (blue dot) and group 10 (red dot). Error bars indicate the standard deviation in each group. BIS = bispectral index, EtSEV = end-tidal concentration of sevoflurane.
There were no significant differences in systolic blood pressure, diastolic blood pressure, mean blood pressure, peripheral oxygen saturation, postoperative nausea and vomiting, and patient satisfaction between the 2 groups (Table 4).
Table 4.
Blood pressure, peripheral oxygen saturation, postoperative nausea/vomiting, and patient satisfaction.
| Variables | Group 5 (n = 27) | Group 10 (n = 27) | P value |
|---|---|---|---|
| Systolic blood pressure, mm Hg | 130 ± 12 | 132 ± 13 | .618 |
| Diastolic blood pressure, mm Hg | 74 ± 10 | 77 ± 11 | .451 |
| Mean blood pressure, mm Hg | 92 ± 9 | 95 ± 10 | .192 |
| Peripheral oxygen saturation, % | 99.7 ± 0.6 | 99.8 ± 0.4 | .446 |
| Postoperative nausea/vomiting | 1 (3.7%) | 0 (0.0%) | >.999 |
| Patient satisfaction, points | 5.8 ± 0.9 | 5.8 ± 0.7 | >.999 |
Data are presented as mean ± standard deviation or number (%) as appropriate.
Group 5 and group 10 include patients who received a fresh gas flow of 5 L/min and 10 L/min, respectively, during the emergence period.
4. Discussion
In this randomized controlled study, we found that emergence time was not significantly different between fresh gas flows of 5 L/minute and 10 L/minute among patients undergoing transurethral resection of bladder tumor. The time to self-movement and time to eye-opening also were not significantly different between the 2 groups. Moreover, there were no significant differences in blood pressure, peripheral oxygen saturation, postoperative nausea/vomiting, and patient satisfaction measured at the post-anesthesia care unit between the 2 groups.
Emergence from general anesthesia is a passive process that depends on the elimination of the drug administered.[1] A key factor in the process of emergence from inhalation anesthesia is the concentration of anesthetic in the brain and alveolus. Moreover, the elimination of inhalation agents is mainly induced by the lungs.[3] Various factors such as prevention of rebreathing, usage of low anesthetic-circuit volume, low absorption property of the anesthetic-circuit, high cerebral blood flow, increased minute ventilation, and high fresh gas flow are known to be associated with emergence from inhalation anesthesia.[2] Recently, central nervous system stimulants such as methylphenidate and dopamine receptor agonists have been studied and proposed as a means of accelerating the emergence from general anesthesia.[22,23] In general, various fresh gas flows of more than 5 L/minute are widely used for emergence from inhalation anesthesia. However, there were scanty studies conducted on whether the use of the fresh gas flow of 5 L/minute is sufficient or not for emergence from inhalation anesthesia.
In our study, emergence time was not significantly different between fresh gas flows of 5 L/minute and 10 L/minute. In the study using a fresh gas flow of 6 L/minute, the emergence time was 11.0 ± 4.3 minutes,[24] whereas in the previous study on the effects of hypercapnic hyperventilation on emergence time using isoflurane using a fresh gas flow of 10 L/minute, the emergence time was >15 minutes.[14] In comparison between the use of sevoflurane and desflurane during outpatient anesthesia using 10 L/minute, emergence times were 11.2 ± 5.1 minutes and 9.3 ± 5.1 minutes, respectively.[25] In our result, emergence times were 12.1 ± 2.9 minutes and 11.1 ± 2.7 minutes using fresh gas flows of 5 L/minute and 10 L/minute, respectively. In most previous studies, hyperventilation or fresh gas flow of 10 L/minute was used for emergence from inhalation anesthesia, although high fresh gas flow above 5 L/minute was recommended.[14,25] During induction with sevoflurane, higher fresh gas flow significantly decreased the time to target sevoflurane concentration.[26] Due to the fact that nearly all the factors that govern the rate at which the alveolar anesthetic concentration rises on induction apply to recovery,[3] the higher fresh gas flow may be associated with faster emergence time. However, in this study, the ventilation setting during the emergence period was the same in both groups to exclude the other factors related to emergence time such as, tidal volume or minute ventilation. Consequently, tidal volume and minute ventilation were similar in both groups, and we evaluated the effect of fresh gas flow on emergence time. As per our study results, there was no significant difference in emergence time between fresh gas flows of 5 L/minute and 10 L/minute. The fresh gas flow of >4 L/minute can prevent rebreathing in the modern anesthesia machine.[27] In our study, a fresh gas flow of 5 L/minute may have been sufficient for minute ventilation of patients and may prevent rebreathing. Consequently, additional fresh gas flow above 5 L/minute does not seem to provide statistically significant additional benefits to reduce emergence time in patients undergoing inhalation anesthesia.
The time to self-movement and time to eye-opening were also not significantly different between fresh gas flows of 5 L/minute and 10 L/minute in patients undergoing transurethral resection of bladder tumor. In the study using a fresh gas flow of 4 L/minute, the time to eye-opening was 9.4 ± 4.5 minutes.[28] In another previous study using a fresh gas flow of 10 L/minute, the time to eye-opening was 9.2 ± 3.6 minutes in patients with sevoflurane.[15] In our result, times to eye-opening were 11.5 ± 3.1 minutes and 10.6 ± 3.0 minutes using a fresh gas flow of 5 L/minute and 10 L/minute, respectively. Recovery from general anesthesia is generally assessed by monitoring physiological and behavioral signs. Furthermore, self-movement and eye-opening are the emergence process of phases 2 and 3.[1] Emergence from general anesthesia is a passive process that depends on the amount of drugs administered. Our results suggest that a fresh gas flow of 5 L/minute is sufficient for emergence from sevoflurane anesthesia.
We found that postoperative nausea/vomiting and patient satisfaction were also not significantly different between fresh gas flows of 5 L/minute and 10 L/minute. Residual inhalation agents can induce postoperative nausea/vomiting and may affect the patient satisfaction. However, given that there was no significant difference in EtSEV in the 2 groups, there would have been little difference in residual sevoflurane. Additionally, postoperative nausea/vomiting was rare because the duration of surgery was short, most of the patients were male, all of the patients were administered antiemetics, and none of the patients were administered opioids in our study. Therefore, the incidence of postoperative nausea/vomiting and patient satisfaction could not be significantly different between fresh gas flows of 5 L/minute and 10 L/minute.
Fresh gas flow rates of 5 and 10 L/min showed no significant differences in emergence time. This result suggests that a fresh gas flow of 5 L/min may be sufficient and that a high fresh gas flow of 10 L/min may not be necessary for emergence from sevoflurane anesthesia. The reduction of oxygen flows in general anesthesia was considered important for climate change,[29] because operating rooms often require large amounts of medical equipment, produce large amounts of medical waste, and have large energy requirements.[30] Furthermore, the production of medical oxygen has high energy requirements.[29] Therefore, our results can have meaningful implications for the climate change.
Our study had several limitations. First, the time to extubation was considered an emergence time in this study. Although we analyzed not only time domain outcomes such as emergence time, time to self-movement, and time to eye-opening but also other numerical data such as sevoflurane concentration and BIS score, the emergence time might have been somewhat subjective. However, extubation criteria were determined before our clinical trial and strictly adhered during the study period. Moreover, time to extubation has been widely used for emergence time in many clinical trials.[10,13–16] Second, the same ventilation setting protocol was applied during the emergence period in both groups. However, different minute ventilation and fresh gas flow rate might have influenced our results. Further studies are needed to validate this association. Third, the geometry of the anesthesia circle system could affect the results. Even though we used the same anesthesia circle system, caution should be applied when generalizing our results.
In conclusion, emergence time, defined as the time from discontinuation of sevoflurane to tracheal extubation, was not significantly different between the fresh gas flows of 5 L/minute and 10 L/minute in patients undergoing transurethral resection of bladder tumor. Also, the time to self-movement and time to eye-opening were not significantly different between the 2 groups. These results suggest that a fresh gas flow of 5 L/minute is sufficient for emergence from sevoflurane anesthesia.
Author contributions
Conceptualization: Jun-Young Park, Jihion Yu, Chan-Sik Kim, Ji-Won Baek, Yonggyeong Jo, Young-Kug Kim.
Data curation: Jun-Young Park, Jihion Yu, Chan-Sik Kim, Ji-Won Baek, Young-Kug Kim.
Formal analysis: Jun-Young Park, Jihion Yu, Chan-Sik Kim, Ji-Won Baek, Young-Kug Kim.
Methodology: Jun-Young Park, Jihion Yu, Chan-Sik Kim, Ji-Won Baek, Yonggyeong Jo, Young-Kug Kim.
Project administration: Young-Kug Kim.
Supervision: Young-Kug Kim.
Writing – original draft: Jun-Young Park.
Writing – review & editing: Jihion Yu, Chan-Sik Kim, Ji-Won Baek, Yonggyeong Jo, Young-Kug Kim.
Abbreviations:
- BIS
- bispectral index
- EtSEV =
- end-tidal sevoflurane concentration
- PEEP
- positive end-expiratory pressure
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Park J-Y, Yu J, Kim C-S, Baek J-W, Jo Y, Kim Y-K. Comparison of the effects of 5 and 10 L/minute fresh gas flow on emergence from sevoflurane anesthesia: A randomized clinical trial. Medicine 2023;102:29(e34406).
Contributor Information
Jun-Young Park, Email: parkjy@amc.seoul.kr.
Jihion Yu, Email: jihionyu@amc.seoul.kr.
Chan-Sik Kim, Email: kyk@amc.seoul.kr.
Ji-Won Baek, Email: naruguru@naver.com.
Yonggyeong Jo, Email: yanggoon223@gmail.com.
References
- [1].Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Brioni JD, Varughese S, Ahmed R, et al. A clinical review of inhalation anesthesia with sevoflurane: from early research to emerging topics. J Anesth. 2017;31:764–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Saba R, Kaye AD, Urman RD. Pharmacogenomics in Anesthesia. Anesthesiol Clin. 2017;35:285–94. [DOI] [PubMed] [Google Scholar]
- [4].Brattwall M, Warrén-Stomberg M, Hesselvik F, et al. Brief review: theory and practice of minimal fresh gas flow anesthesia. Can J Anaesth. 2012;59:785–97. [DOI] [PubMed] [Google Scholar]
- [5].Gaya da CM, Kalmar AF, Struys MM. Inhaled anesthetics: environmental role, occupational risk, and clinical use. J Clin Med. 2021;10:1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kennedy RR, French RA, Vesto G, et al. The effect of fresh gas flow during induction of anaesthesia on sevoflurane usage: a quality improvement study. Anaesthesia. 2019;74:875–82. [DOI] [PubMed] [Google Scholar]
- [7].Muzi M, Robinson BJ, Ebert TJ, et al. Induction of anesthesia and tracheal intubation with sevoflurane in adults. Anesthesiology. 1996;85:536–43. [DOI] [PubMed] [Google Scholar]
- [8].Eger EI. Inhaled anesthetics: uptake and distribution. In: Miller RD. Miller’s Anesthesia. 7th ed. Philadelphia: Churchill Livingstone/Elsevier; 2010. p. 558. [Google Scholar]
- [9].Cheng H, Clymer JW, Chen BP-H, et al. Prolonged operative duration is associated with complications: a systematic review and meta-analysis. J Surg Res. 2018;229:134–44. [DOI] [PubMed] [Google Scholar]
- [10].House LM 2nd, Calloway NH, Sandberg WS, et al. Prolonged patient emergence time among clinical anesthesia resident trainees. J Anaesthesiol Clin Pharmacol. 2016;32:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jeong H, Tanatporn P, Ahn HJ, et al. Pressure support versus spontaneous ventilation during anesthetic emergence—effect on postoperative atelectasis: a randomized controlled trial. Anesthesiology. 2021;135:1004–14. [DOI] [PubMed] [Google Scholar]
- [12].Li M, Mei W, Wang P, et al. Propofol reduces early post-operative pain after gynecological laparoscopy. Acta Anaesthesiol Scand. 2012;56:368–75. [DOI] [PubMed] [Google Scholar]
- [13].Lim BG, Lee IO, Ahn H, et al. Comparison of the incidence of emergence agitation and emergence times between desflurane and sevoflurane anesthesia in children: a systematic review and meta-analysis. Medicine. 2016;95:e4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sakata DJ, Gopalakrishnan NA, Orr JA, et al. Hypercapnic hyperventilation shortens emergence time from isoflurane anesthesia. Anesth Analg. 2007;104:587–591. [DOI] [PubMed] [Google Scholar]
- [15].Kim JM, Lee JH, Lee HJ, et al. Comparison of emergence time in children undergoing minor surgery according to anesthetic: desflurane and sevoflurane. Yonsei Med J. 2013;54:732–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee J, Kim S. The effects of ultrasound-guided serratus plane block, in combination with general anesthesia, on intraoperative opioid consumption, emergence time, and hemodynamic stability during video-assisted thoracoscopic lobectomy: a randomized prospective study. Medicine. 2019;98:e15385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sanford TJ, Jr, Smith NT, Dec-Silver H, et al. A comparison of morphine, fentanyl, and sufentanil anesthesia for cardiac surgery: induction, emergence, and extubation. Anesth Analg. 1986;65:259–66. [PubMed] [Google Scholar]
- [18].Peyton PJ, Chao I, Weinberg L, et al. Nitrous oxide diffusion and the second gas effect on emergence from anesthesia. Anesthesiology. 2011;114:596–602. [DOI] [PubMed] [Google Scholar]
- [19].Bhardwaj P, Yadav RK. Measuring pain in clinical trials: pain scales, endpoints, and challenges. Int J Clin Exp Physiol. 2015;2:151. [Google Scholar]
- [20].Dworkin RH, Turk DC, McDermott MP, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain. 2009;146:238–44. [DOI] [PubMed] [Google Scholar]
- [21].Farrar JT, Young JP, Jr, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–58. [DOI] [PubMed] [Google Scholar]
- [22].Solt K, Cotten JF, Cimenser A, et al. Methylphenidate actively induces emergence from general anesthesia. Anesthesiology. 2011;115:791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Taylor NE, Chemali JJ, Brown EN, et al. Activation of D1 dopamine receptors induces emergence from isoflurane general anesthesia. Anesthesiology. 2013;118:30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wu Z-F, Jian G-S, Lee M-S, et al. An analysis of anesthesia-controlled operating room time after propofol-based total intravenous anesthesia compared with desflurane anesthesia in ophthalmic surgery: a retrospective study. Anesth Analg. 2014;119:1393–406. [DOI] [PubMed] [Google Scholar]
- [25].Nathanson MH, Fredman B, Smith I, et al. Sevoflurane versus desflurane for outpatient anesthesia: a comparison of maintenance and recovery profiles. Anesth Analg. 1995;81:1186–90. [DOI] [PubMed] [Google Scholar]
- [26].Shin HW, Yu HN, Bae GE, et al. The effect of fresh gas flow rate and type of anesthesia machine on time to reach target sevoflurane concentration. BMC Anesthesiol. 2017;17:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Baum JA. Low-flow anesthesia: theory, practice, technical preconditions, advantages, and foreign gas accumulation. J Anesth. 1999;13:166–74. [DOI] [PubMed] [Google Scholar]
- [28].Talih G, Yüksek A, Şahin E. Evaluation of emergence agitation after general anaesthesia in rhinoplasty patients: Inhalation anaesthesia versus total intravenous anaesthesia. Am J Otolaryngol. 2020;41:102387. [DOI] [PubMed] [Google Scholar]
- [29].McGain F, Sheridan N, Wickramarachchi K, et al. Carbon footprint of general, regional, and combined anesthesia for total knee replacements. Anesthesiology. 2021;135:976–91. [DOI] [PubMed] [Google Scholar]
- [30].MacNeill AJ, Lillywhite R, Brown CJ. The impact of surgery on global climate: a carbon footprinting study of operating theatres in three health systems. Lancet Planet Health. 2017;1:e381–8. [DOI] [PubMed] [Google Scholar]