Abstract
Background
This meta-analysis was conducted to evaluate the efficacy of the treat-repair-treat (TRT) strategy in the treatment of severe pulmonary arterial hypertension with congenital heart disease (PAH-CHD).
Methods
PubMed, EMBASE, Cochrane and Web of Science online databases were searched by two independent investigators for studies that used the TRT strategy for PAH-CHD, and the retrieved studies were reviewed by a third investigator. The main outcomes were pulmonary artery pressure (PAP), pulmonary vascular resistance (PVR), 6-minute walk distance (6MWD), and transcutaneous oxygen saturation (SpO2). The changes were compared between follow-up and baseline. Stata version 14.0 was used for data analysis. A random-effects model was selected for meta-analysis. Subgroup analysis and meta-regression were used to find the source of heterogeneity.
Results
A total of 335 patients from 9 single-arm studies were included. Meta-analysis showed significant reductions in PAP and PVR and improvements in 6MWD and SpO2 (PAP: SMD -2.73 95% CI -2.97, − 2.50 p = < 0.001; PVR: SMD -1.27 95% CI -1.53, − 1.02 p = < 0.001; 6MWD: SMD 1.88 95% CI 1.49, 2.27 p = < 0.001; SpO2: SMD 3.72 95% CI 3.13, 4.32 p = < 0.001). Subgroup analysis showed that younger patients had better efficacy, and the change in SpO2 was an indication for patient selection. The combined mortality rate was 5% at follow-up.
Conclusions
In this meta-analysis, we demonstrated that the TRT strategy may have positive effects on haemodynamics and cardiac function in patients with severe PAH-CHD at short-term follow-up. Our analysis suggests that changes in age and SpO2 may be related to patient prognosis.
Trial registration
The protocol was registered on the PROSPERO website with the registration number CRD42022366552. The relevant registration information can be obtained from the website https://www.crd.york.ac.uk/prospero/#searchadvanced.
Keywords: Pulmonary arterial hypertension, Congenital heart disease, Treat-repair-treat, Targeted drugs
Background
Pulmonary arterial hypertension associated with congenital heart disease (PAH-CHD) is a serious cardiovascular disease. The left-to-right shunt results in increased blood flow and pressure in the pulmonary vasculature [1]. Persistent exposure of the pulmonary vasculature to increased blood flow, as well as increased pressure, may result in pulmonary obstructive arteriopathy [2]. Surgical repair at an early stage can correct the left-to-right shunt and improve patient prognosis. In the advanced stages of the disease, the pulmonary artery is irreversibly diseased, and the patient will lose the opportunity for surgery. Patients experience reduced exercise tolerance, heart failure [3], and even death. Targeted drugs, which have been invented in the last 20 years, include endothelin receptor antagonists (ERAs), phosphodiesterase type-5 inhibitors (PDE5is), prostacyclin (PC), and soluble guanylate cyclase stimulators (sGC). Combined drug therapy can improve patients’ exercise capacity and reduce pulmonary vascular resistance (PVR) [4]. In some cases, patients with severe PAH-CHD have successfully completed surgery after receiving targeted drug therapy [5–7]. In 2010, a large case series from China suggested that patients who are sensitive to targeted drug therapy have access to surgery [8]. The development of targeted drugs has brought new hope for patients [8–16]. It has been suggested that endothelin receptor antagonists may have antiproliferative effects causing reverse remodelling in the pulmonary circulation [17]. This concept has made surgical treatment possible. The preoperative and postoperative administration of targeted drugs to enable patients with severe PAH-CHD to complete surgery for congenital heart disease is called the TRT strategy, and the treatment guidelines have changed as a result. According to the 2022 ESC/ERS Guidelines for the diagnosis and treatment of PAH [18], in patients with ASD and a PVR > 5 Wu that declines to < 5 Wu with PAH treatment, shunt closure may be considered. In patients with ASD and a PVR > 5 WU despite PAH treatment, shunt closure is not recommended. However, there are many questions about the efficacy of the TRT strategy. First, pulmonary hypertension crisis is an important cause of perioperative death in patients with CHD [19, 20]. Second, many patients continue to have PAH after surgery. In Takaya et al. [9] report, 69% of patients were still taking targeted drugs at follow-up, and 19% were increasing their drug use. Therefore, the purpose of this study was to collect relevant clinical studies for meta-analysis to evaluate the efficacy of the TRT strategy for patients with PAH-CHD. We summarized the prognostic factors of patients, hoping to help doctors judge the feasibility of surgery.
Methods
This study was conducted in adherence with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [21]. Three reviewers independently conducted the literature search, screening evaluation, and data extraction.
Retrieval strategy
The PubMed, Embase, Cochrane and Web of Science online databases were searched for relevant studies by three reviewers. The following keyword combinations were used to retrieve relevant studies: (“Treat Repair” or “Treat Close” or “specific medications” or “targeted drugs” or “specific drugs” or “targeted medications”) and (“pulmonary arterial hypertension associated with congenital heart disease” or “PAH-CHD” or “CHD-PAH”). Included were articles published in all languages, studies carried out on human subjects and articles published from the establishment of the database to November 1, 2022. The search strategies were adapted according to the characteristics of the databases. The reference lists of the included articles were manually retrieved to identify additional relevant studies.
Inclusion and exclusion criteria
Studies that met the following criteria were included: (1) population: patients with PAH associated with congenital heart disease and a mean PVR > 5 Wu or a mean mPAP> 45 mmHg without treatment; (2) intervention: interventional occlusion or surgical repair after treatment with PAH-specific medications; and (3) comparison: baseline data were compared with data at follow-up. The exclusion criteria were as follows: (1) overviews, reviews, seminar papers, comments, reports, letters, animal experiments and duplicate publications were excluded; (2) lack of right cardiac catheterization data; and (3) PAH after surgery for congenital heart disease.
Data extraction and quality assessment
The extracted items included (1) details of the articles (first author, publication year, sample size); (2) sample characteristics (age, sex, diagnosis of CHD); and (3) intervention measures (type of targeted drugs, duration of medication, mode of operation and follow-up duration). (4) Main outcomes: mean pulmonary artery pressure (mPAP) or systolic pulmonary artery pressure (sPAP), PVR, 6MWD, SpO2, Qp:Qs and death toll. Baseline and last follow-up data were collected. According to the study of McGrath et al. [22], medians and interquartile ranges were converted into means and standard deviations. If the data were not given in the article and could not be obtained from the author, we used WebPlotDigitizer version 4.1 [23] to estimate the interquartile ranges based on box plots. We calculated the interquartile ranges for each article three times independently and chose the average as the final value for analysis.
The quality of the included studies was evaluated according to the MINORS (Methodological Index for Nonrandomized Studies) checklist [24]. This tool was specifically developed and validated to evaluate the quality of nonrandomized surgical studies. It includes 12 items; the last 4 are specific for comparative studies. The score for the items varies from 0 to 2 (0, not reported; 1, reported but poorly done or inadequate; 2, reported but well done and adequate). The ideal global score was 16 for a noncomparative study and 24 for a comparative study. This evaluation was performed by two independent reviewers who tried to reach a consensus in case of disagreement.
Statistical analysis
Statistical software Stata 14.0 was used for meta-analysis. For continuous variables, the standardized mean difference (SMD) was used for calculations, and a fixed effects model was used for analysis. Heterogeneity was tested by Cochrane’s Q test and quantified by the I2 statistic. When the data had great heterogeneity (I2 > 50%), the random effects model was used for analysis. Subgroup analysis was used to compare the efficacy of different patients, and meta-regression was used to determine the source of heterogeneity. Publication bias was analysed by Egger’s bias tests. A p value< 0.05 was considered statistically significant (significant bias). The mortality rate was recorded, and the estimated effect (ES) for mortality estimates with the corresponding 95% CI was calculated.
Results
Characteristics of eligible studies and quality assessment
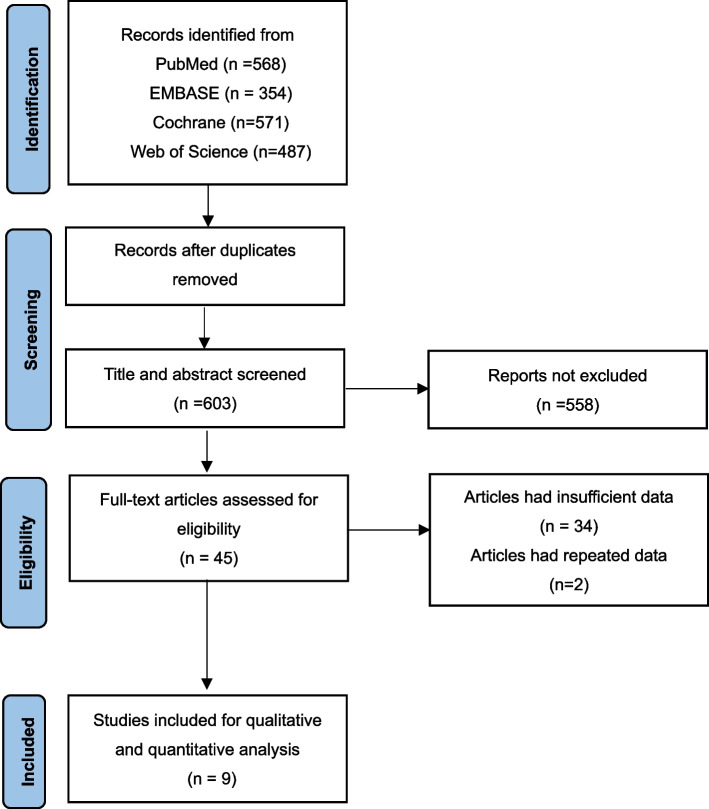
The search retrieved 603 results. A total of 558 irrelevant articles were excluded. We reviewed the full texts of the remaining 45 articles and excluded 36 articles with incomplete and repeated data. Finally, we included 9 single-arm studies with 335 patients. The flow chart of the research selection process is shown in Fig. 1. One study involved eight patients with Eisenmenger syndrome. Because Eisenmenger syndrome is often accompanied by severe PAH and these patients are difficult to treat and have a very poor prognosis, we still included this study to avoid data loss and bias. Details of the articles and sample characteristics are collected in Table 1. Intervention measures and main outcomes are displayed in Table 2. The screening criteria and the number of deaths are shown in Table 3. We also collected the proportion of patients taking targeted drugs at the last follow-up in Table 3. Our meta-analysis included 9 studies with a MINOR score of 12-13 each, suggesting that low-quality studies were not included.
Fig. 1.
Flow diagram for retrieving eligible articles
Table 1.
Sample characteristics and intervention measures
| Author | Year | N | Sex (m/f) |
Age (year) |
Diagnosis | Duration of medication(m) | Follow-up duration (m) | MINORS |
|---|---|---|---|---|---|---|---|---|
| Takaya et al. [9] | 2021 | 42 | 10/32 | 51 ± 18 | ASD | 9 | 33 | 12 |
| Thomaz et al. [10] | 2019 | 33 | 12/18 | 0.8(median) | VSD\PDA\A-V canal | 6 | 6 | 13 |
| Huang et al. [11] | 2011 | 38 | – | 3.04 ± 1.98 | ASD\VSD\PDA | 5-120 days | 117.6 | 12 |
| Hu et al. [12] | 2015 | 31 | 19/21 | 29.19 ± 8.51 | VSD | 7.29 | 37.9 | 12 |
| 8 | 3/5 | 22.75 ± 3.77 | VSD and ES | 12.38 | 31.50 | 12 | ||
| Kijima et al. [13] | 2015 | 8 | 0/8 | 37 ± 15 | ASD | – | 19 | 12 |
| Bradley et al. [14] | 2018 | 19 | 4/15 | 37 + 23 | ASD | 24 | 52.8 | 12 |
| Liu et al. [15] | 2014 | 56 | 25/31 | 17.2 ± 9.3 | VSD | 4.4 | 27.9 | 13 |
| He et al. [16] | 2020 | 14 | 1/13 | 27.9 ± 7.4 | ASD | 10.7 | 21.1 | 12 |
| Liu et al. [8] | 2010 | 86 | 51/35 | 2 ± 1.8 | d-TGA\TBA | 13.4 days | 42.1 | 12 |
m/f male/female, m month, ASD atrial septal defect, VSD ventricular septal defect, PDA patent ductus arteriosus, A-V canal atrioventricular septal defect, ES Eisenmenger syndrome, d-TGA dextrotransposition of the great arteries, TBA Taussig-Bing anomaly
Table 2.
Main outcomes
| Author | Targeted drug | Mode of operation | mPAP or sPAP (mmHg) | PVR (Wu) | 6MWD (m) | SpO2 | Qp/Qs |
|---|---|---|---|---|---|---|---|
| Takaya et al. [9] | ERA\PDE5i\sGC\PC | intervention |
A:78.00 ± 26.00 B:38.00 ± 10.00 |
A:6.90 ± 3.20 B:3.20 ± 1.70 |
- - |
- - |
A:1.90 ± 0.80 - |
| Thomaz et al. [10] | PDE5i | – |
A:47.77 ± 14.77 B:25.27 ± 7.64 |
A:5.70 ± 3.69 B:2.97 ± 1.78 |
- - |
- - |
A:2.09 ± 1.02 B:2.43 ± 1.03 |
| Huang et al. [11] | ERA\PDE5i\PC | surgery |
A:70.5.0 ± 11.92 B:30.95 ± 10.92 |
A:20.51 ± 8.90 B:9.20 ± 3.63 |
- - |
- - |
- - |
| Hu et al. [12] | ERA\PDE5i\PC | valved patch repair |
A:79.61 ± 6.88 B:< 50 |
- - |
- - |
- - |
A:1.24 ± 0.21 - |
| ERA\PDE5i\PC | valved patch repair |
A:82.54 ± 6.49 B:59.05 ± 14.52 |
- - |
- - |
A:85.79 ± 2.29 B:91.13 ± 3.72 |
A:0.84 ± 0.13 - |
|
| Kijima et al. [13] | ERA\PDE5i\PC | intervention |
A:104.00 ± 27.00 B:40.00 ± 9.00 |
- - |
- - |
- - |
A:1.39 ± 0.41 - |
| Bradley et al. [14] | ERA\PDE5i\sGC\PC | – |
A:48.00 ± 13.00 B:36.63 ± 12.76 |
A:7.50 ± 4.30 B:5.05 ± 2.88 |
A:366 ± 137 B:486 ± 89 |
- - |
A:2.2 ± 1.5 B:1.11 ± 0.46 |
| Liuet al [15]. | ERA\PDE5i | surgery |
A:67.90 ± 9.90 B:22.50 ± 6.40 |
- - |
A:337.6 ± 23.3 B:424.6 ± 48.3 |
A:89.90 ± 2.10 B:97.30 ± 1.10 |
- - |
| He et al. [16] | ERA\PDE5i |
12 cases: intervention 2 cases: surgery |
A:57.10 ± 7.40 B:32.40 ± 14.10 |
A:8.70 ± 2.90 B:4.10 ± 2.70 |
- - |
- - |
A:1.40 ± 0.25 B:1.00 ± 0.00 |
| Liu et al. [8] | ERA\PDE5i | surgery |
A:64.90 ± 13.00 B:22.40 ± 8.70 |
- - |
- - |
- - |
- - |
A: Baseline; B: The last follow-up
Table 3.
Patient screening and prognosis
| Author | Screening of patients | Targeted drugs at the final follow-up | Deaths |
|---|---|---|---|
| Takaya et al. [9] |
CCC:PVR < 5.0–7.0 Wu\Qp:Qs > 1.5 Left-to-right shunt |
100% | 1 |
| Thomaz et al. [10] | VT: PVR < 6.0 Wu and PVR/SVR < 0.3 | 82% | 3 |
| Huang et al. [11] |
SpO2 > 93% Left-to-right shunt |
– | 0 |
| Hu et al. [12] |
VT: Qp/Qs > 1.5 and PVR/SVR < 2/3 Left-to-right shunt |
63% | 2 |
| Kijima et al. [13] | CCC:Qp/Qs ≥1.5 and PVR < 8 Wu | 100% | 0 |
| Bradley et al. [14] | CCC:PVR < 6.4 Wu and 6MWD > 300 m | – | 0 |
| Liu et al. [15] | SpO2 > 93% | 42% | 0 |
| He et al. [16] | VT:PVR < 5 Wu or Qp/Qs > 1.5 | 86% | 0 |
| Liu et al. [8] | increase of SpO2 > 5% | – | 6 |
CCC conventional cardiac catheterization, VT vasodilation test, Qp:Qs pulmonary to systemic blood flow ratio, SVR systemic vascular resistance
Meta-analysis
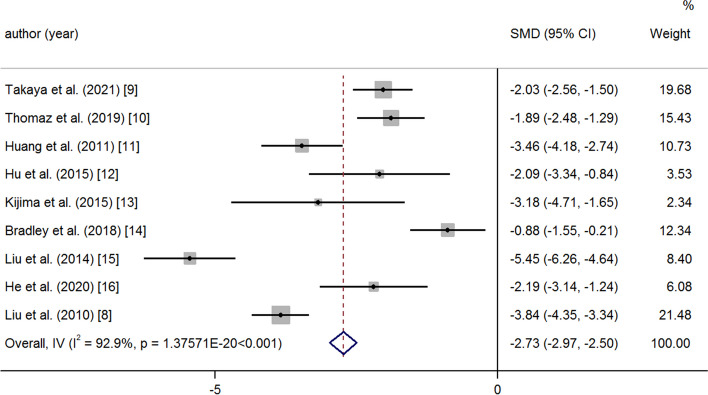
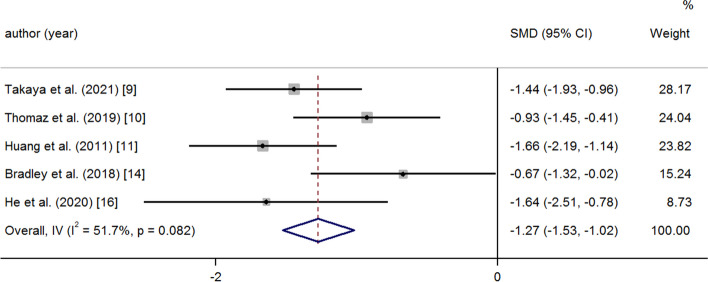
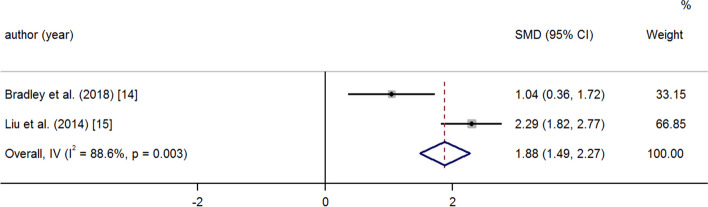
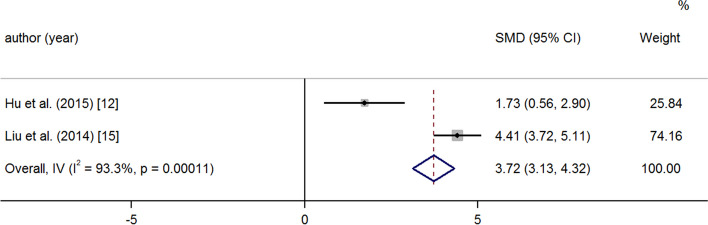
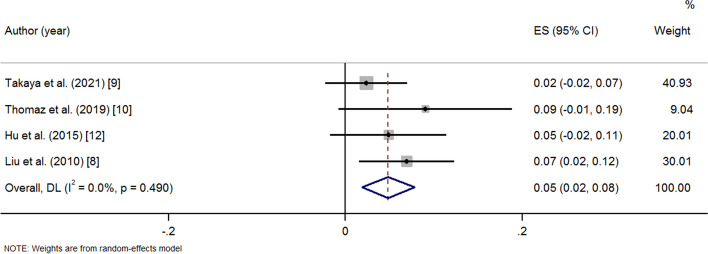
After medication and operation, the PAP and PVR decreased significantly at the last follow-up. A random effects model was used because of great heterogeneity (PAP: SMD -2.73 95% CI -2.97, − 2.50 p = < 0.001 I2 = 92.9%) (Fig. 2); (PVR: SMD -1.27 95% CI -1.53, − 1.02 p = < 0.001 I2 = 51.7%) (Fig. 3). There was a significant increase in 6MWD and SpO2 at the last follow-up compared to baseline (6MWD: SMD 1.88 95% CI 1.49, 2.27 p = < 0.001 I2 = 88.6%); (Fig. 4) (SpO2: SMD 3.72 95% CI 3.13, 4.32 p = < 0.001 I2 = 93.3%) (Fig. 5). The combined mortality rate at follow-up was 0.05 (95% CI 0.02, 0.08 p = 0.01 I2 = 0.0%) (Fig. 6).
Fig. 2.
Meta-analysis of total SMDs based on the PAP
Fig. 3.
Meta-analysis of total SMDs based on the PVR
Fig. 4.
Meta-analysis of total SMDs based on the 6MWD
Fig. 5.
Meta-analysis of total SMDs based on SpO2
Fig. 6.
Meta-analysis of total ESs based on the mortality rate
Subgroup analysis
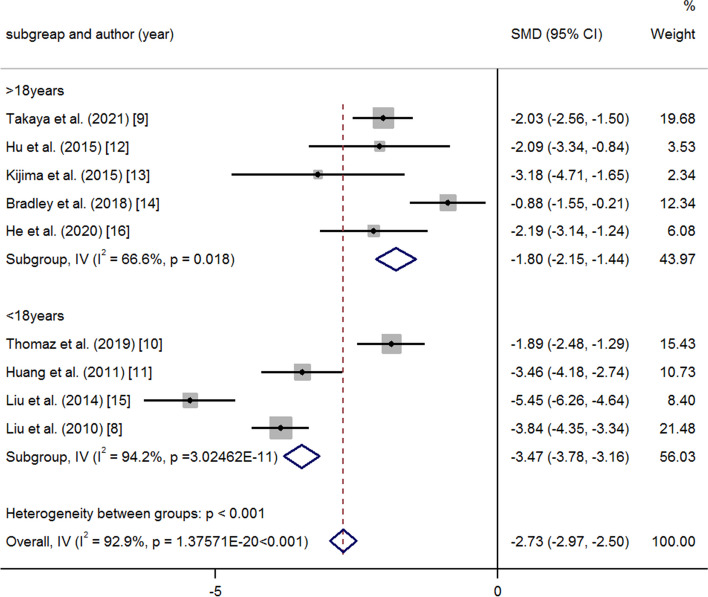
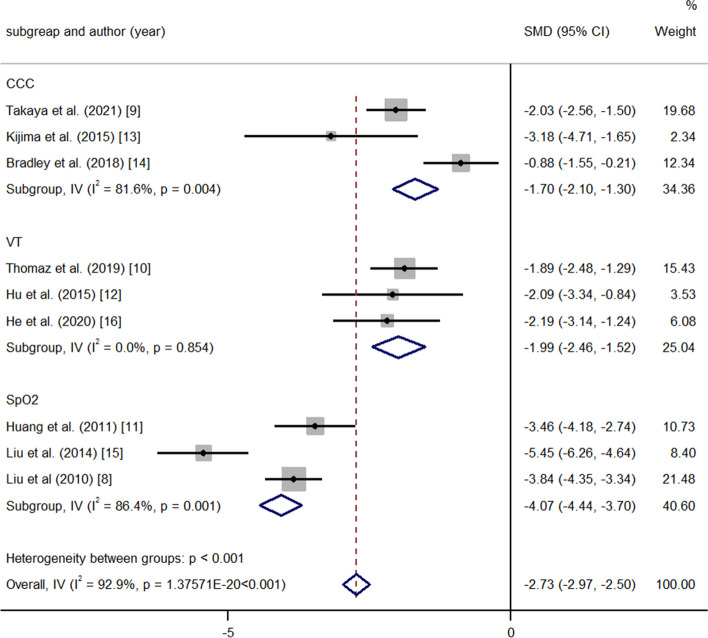
Nine studies described the change in PAP at the time of the last follow-up. Cochrane’s Q test showed that there was significant heterogeneity in the 9 studies. According to the average age of the patients, the nine studies were divided into two groups: average age < 18 years and average age > 18 years. A random-effects model was used for subgroup analysis. The results showed that the decline in PAP was more significant in the subgroup with an average age < 18 years (> 18 years, PAP: SMD-1.80 95% CI-2.15, − 1.44, p = < 0.001, I2 = 66.6%; < 18 years, PAP: SMD-3.47 95% CI-3.78, − 3.16, p = < 0.001, I2 = 94.2%; heterogeneity between groups: p = < 0.001) (Fig. 7). According to the method for selecting patients, the studies were divided into the following groups: conventional cardiac catheterization (CCC), vasodilation test and (VT) and SpO2. The results showed that the SpO2 subgroup had better efficacy (CCC: PAP: SMD -1.70 95% CI -2.10, − 1.30 p = < 0.001 I2 = 81.6%; VT: PAP: SMD -1.99 95% CI -2.46, − 1.52 p = < 0.001 I2 = 0.0%; SpO2: PAP: SMD -4.07 95% CI -4.44, − 3.70 p = < 0.001 I2 = 86.4% heterogeneity between groups: p = < 0.001) (Fig. 8). Heterogeneity decreased after the subgroup analysis. Meta-regression analysis was performed, and the regression coefficient was 1.193 (95% CI: 0.269-2.118 p = 0.019).
Fig. 7.
Meta-analysis of subtotal SMDs based on different ages
Fig. 8.
Meta-analysis of subtotal SMDs based on the different screening methods
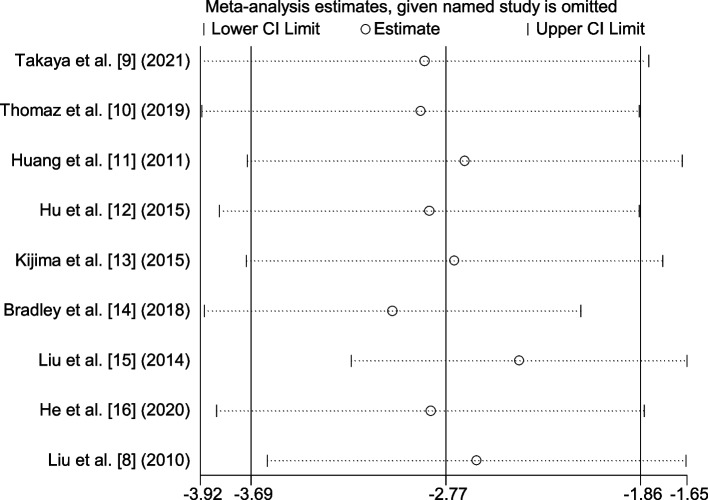
Sensitivity analysis and publication bias
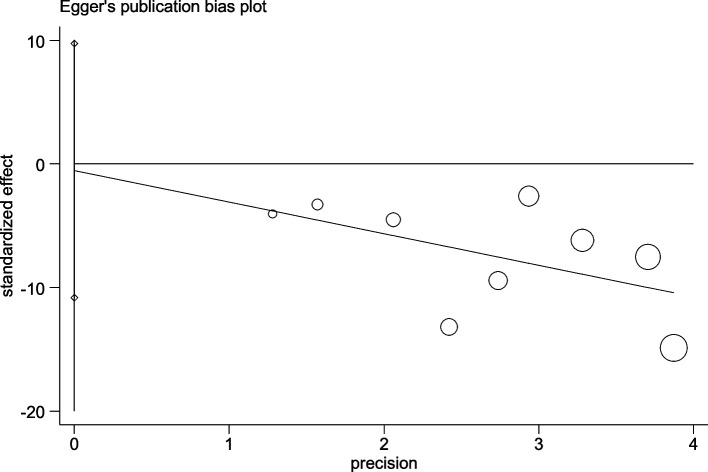
For PAP, the sensitivity analysis results are shown in Fig. 9. Regardless of which study was removed, the combined effect value was still statistically significant. The Egger test model was used for analysis to assess publication bias. For PAP, the results suggested that there was no significant bias (p = 0.907) (Fig. 10). For PVR, p = 0.912. For the mortality rate, p = 0.296.
Fig. 9.
Sensitivity analysis plot
Fig. 10.
Egger test plot for PAP
Discussion
In this meta-analysis, we found that the TRT strategy could reduce the PAP and PVR and improve the 6MWD and SpO2 at the short-term follow-up. However, regarding PAP, there was considerable heterogeneity between studies (I2 = 92.9%). We observed significant differences across studies in the patient age distribution, type of CHD, targeted drug therapy, surgical screening criteria, surgical modalities and follow-up time. These may be the causes of heterogeneity. In the study of Rabinovitch et al. [25], patients younger than 2 years of age exhibited reverse remodelling in the pulmonary circulation. Age may be a factor that affects prognosis. Therefore, we conducted a subgroup analysis, and we found that the subgroup with an average age of less than 18 years had a more significant decline in PAP. We thus demonstrated that age may be associated with patient outcomes. CCC was used to screen patients. CCC is the recommended method in the guidelines and can be a good diagnostic modality for PAH [26]. The pulmonary vasodilation test and SpO2 were also used to screen patients. However, regardless of the screening methods, some patients still have PAH after surgery, and the proportion of patients requiring targeted drug therapy at the last follow-up varies from 43 to 100%. We designed subgroups according to different screening methods, hoping to analyse the screening method with the best efficacy. The results showed that the heterogeneity within each subgroup was lower than that within the overall combined effect. We conducted meta-regression, and the results showed that the difference in the screening methods may be the source of heterogeneity. Patients screened according to their SpO2 levels had a greater reduction in PAP. Spo2 is associated with patient prognosis. Based on previous experience, SpO2 has been associated with perioperative death, postoperative PAH, and adverse events such as heart failure and arrhythmia during follow-up [27–29]. Therefore, the change in SpO2 may be an effective screening standard to help us assess whether the patient has the opportunity for operation.
In terms of safety. Four studies found that patients had died during follow-up. The combined mortality rate was 5% at follow-up. Despite rigorous preoperative evaluations, patients still die after surgery. In the study by Thomaz et al. [10], three patients whose lung biopsies showed grades I or II according to the Heath-Edwards classification [30] died after surgery. Two of these deaths were due to pulmonary hypertension crises. Their PVRs were 3.5 Wu, 5.0 Wu and 5.2 Wu, respectively. Although right heart catheterization is the gold standard for diagnosing PAH [31], many patients still have postoperative PAH. At present, there is no effective method to accurately determine the feasibility of surgery in patients with severe PAH-CHD. Surgical indications should be carefully controlled. Risk prediction models that include age, SpO2, haemodynamic parameters and other relevant risk factors may better assist clinicians in decision-making.
Study limitations
First, because of the low incidence of PAH-CHD, the number of articles and subjects included in this meta-analysis was small. As none of the 9 single-arm studies had control group, we were not able to compare long-term outcomes between the TRT strategy and simple targeted drug therapy. More randomized controlled studies are needed to confirm the long-term efficacy. Second, long-term follow-up survival data were not available in the included studies. Some patients still had residual PAH after the operation [32], and they still needed to take PAH-specific medications or even a combination of multiple drugs [33]. High medical expenses will increase the economic burden on patients and reduce their compliance. However, whether the disease will deteriorate rapidly after drug withdrawal remains to be studied. Third, some of the means and standard deviations could not be obtained directly from the included studies, and the corresponding authors could not be contacted by email. We used WebPlotDigitizer version 4.1 to obtain the medians, maximums, minimums, and interquartile values. The mean and standard deviation were obtained by calculation, which may have reduced the reliability of our results. Therefore, we are cautious about drawing conclusions.
Conclusions
In conclusion, this meta-analysis confirms that the TRT strategy may have positive effects on haemodynamics and cardiac function in patients with severe PAH-CHD at short-term follow-up. Our analysis suggests that age and SpO2 changes could be potential predictors of patient prognosis. More research will be performed to build predictive models in the future.
Acknowledgements
No further acknowledgement.
Abbreviations
- TRT
Treat-repair-treat
- PAH-CHD
Pulmonary arterial hypertension associated with congenital heart disease
- ASD
Atrial septal defect
- VSD
Ventricular septal defect
- PDA
Patent ductus arteriosus
- A-V canal
Atrioventricular septal defect
- ES
Eisenmenger syndrome
- d-TGA
Dextrotransposition of the great arteries
- TBA
Taussig-Bing anomaly
- PAP
Pulmonary artery pressure
- PVR
Pulmonary vascular resistance
- Qp:Qs
Pulmonary to systemic blood flow ratio
- SVR
Systemic vascular resistance
- mPAP
Main outcomes: mean pulmonary artery pressure
- sPAP
Systolic pulmonary artery pressure
- 6MWD
6-minute walk distance
- SpO2
Transcutaneous oxygen saturation
- ERA
Endothelin receptor antagonists
- PDE5i
Phosphodiesterase type-5 inhibitors
- PC
Prostacyclins
- sGC
Soluble guanylate cyclase stimulators
- CCC
Conventional cardiac catheterization
- VT
Vasodilation test
Authors’ contributions
All authors participated in the study design. All the authors have read, critiqued, and approved the manuscript revisions as well as the final version of the manuscript. Xiaobing Li, Jun Peng, and Huijun Zhang retrieved the studies from online databases and screened the reference lists of the original articles. Xiaobing Li and Jun Peng independently extracted the data from all included papers and evaluated the quality of the selected studies using the Methodological Index for Nonrandomized Studies (MINORS) checklist. Zhiyuan Wang and Huijun Zhang wrote the main manuscript text. Zhiyuan Wang prepared all tables and figures. Mengxuan Li wrote part of the text and corrected the grammatical errors.
Funding
This study was not funded by any resource.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Declarations
Ethics approval and consent to participate
This study did not require ethical approval since it was a review of published articles and did not directly involve the use of human or animal subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brida M, Gatzoulis MA. Pulmonary arterial hypertension in adult congenital heart disease. Heart. 2018;104:1568–1574. doi: 10.1136/heartjnl-2017-312106. [DOI] [PubMed] [Google Scholar]
- 2.Galie N, Manes A, Palazzini M, Negro L, Marinelli A, Gambetti S, et al. Management of pulmonary arterial hypertension associated with congenital systemic-to-pulmonary shunts and Eisenmenger's syndrome. Drugs. 2008;68:1049–1066. doi: 10.2165/00003495-200868080-00004. [DOI] [PubMed] [Google Scholar]
- 3.Arnaert S, De Meester P, Troost E, Droogne W, Van Aelst L, Van Cleemput J, et al. Heart failure related to adult congenital heart disease: prevalence, outcome and risk factors. ESC Heart Fail. 2021;8:2940–2950. doi: 10.1002/ehf2.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Issapour A, Frank B, Crook S, Hite MD, Dorn ML, Rosenzweig EB, et al. Safety and tolerability of combination therapy with ambrisentan and tadalafil for the treatment of pulmonary arterial hypertension in children: real-world experience. Pediatr Pulmonol. 2022;57:724–733. doi: 10.1002/ppul.25796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoetzenecker K, Ankersmit HJ, Bonderman D, Hoetzenecker W, Seitelberger R, Klepetko W, et al. Atrial septal defect repair after a 10-month treatment with bosentan in a patient with severe pulmonary arterial hypertension: a case report. J Thorac Cardiovasc Surg. 2009;137:760–761. doi: 10.1016/j.jtcvs.2008.03.064. [DOI] [PubMed] [Google Scholar]
- 6.Taniguchi Y, Emoto N, Miyagawa K, Nakayama K, Kinutani H, Tanaka H, et al. Subsequent shunt closure after targeted medical therapy can be an effective strategy for secundum atrial septal defect with severe pulmonary arterial hypertension: two case reports : strategy for ASD with severe PAH. Heart Vessel. 2014;29:282–285. doi: 10.1007/s00380-013-0351-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim YH, Yu JJ, Yun TJ, Lee Y, Kim YB, Choi HS, et al. Repair of atrial septal defect with Eisenmenger syndrome after long-term sildenafil therapy. Ann Thorac Surg. 2010;89:1629–1630. doi: 10.1016/j.athoracsur.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 8.Liu YL, Hu SS, Shen XD, Li SJ, Wang X, Yan J, et al. Midterm results of arterial switch operation in older patients with severe pulmonary hypertension. Ann Thorac Surg. 2010;90:848–855. doi: 10.1016/j.athoracsur.2010.03.114. [DOI] [PubMed] [Google Scholar]
- 9.Takaya Y, Akagi T, Sakamoto I, Kanazawa H, Nakazawa G, Murakami T, et al. Efficacy of treat-and-repair strategy for atrial septal defect with pulmonary arterial hypertension. Heart. 2022;108:382–387. doi: 10.1136/heartjnl-2021-319096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomaz AM, Kajita LJ, Aiello VD, Zorzanelli L, Galas FR, Machado CG, et al. Parameters associated with outcome in pediatric patients with congenital heart disease and pulmonary hypertension subjected to combined vasodilator and surgical treatments. Pulm Circ. 2019;9:2045894019837885. doi: 10.1177/2045894019837885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang JB, Liu YL, Yu CT, Lv XD, Du M, Wang Q, et al. Lung biopsy findings in previously inoperable patients with severe pulmonary hypertension associated with congenital heart disease. Int J Cardiol. 2011;151:76–83. doi: 10.1016/j.ijcard.2010.04.094. [DOI] [PubMed] [Google Scholar]
- 12.Hu Z, Xie B, Zhai X, Liu J, Gu J, Wang X, et al. Midterm results of "treat and repair" for adults with non-restrictive ventricular septal defect and severe pulmonary hypertension. J Thorac Dis. 2015;7:1165–1173. doi: 10.3978/j.issn.2072-1439.2015.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kijima Y, Akagi T, Takaya Y, Akagi S, Nakagawa K, Kusano K, et al. Treat and repair strategy in patients with atrial septal defect and significant pulmonary arterial hypertension. Circ J. 2016;80:227–234. doi: 10.1253/circj.CJ-15-0599. [DOI] [PubMed] [Google Scholar]
- 14.Bradley EA, Ammash N, Martinez SC, Chin K, Hebson C, Singh HS, et al. "treat-to-close": non-repairable ASD-PAH in the adult: results from the north American ASD-PAH (NAAP) multicenter registry. Int J Cardiol. 2019;291:127–133. doi: 10.1016/j.ijcard.2019.03.056. [DOI] [PubMed] [Google Scholar]
- 15.Liu A, Li Z, Li X, Fan X, Su J, Zhang J, et al. Midterm results of diagnostic treatment and repair strategy in older patients presenting with nonrestrictive ventricular septal defect and severe pulmonary artery hypertension. Chin Med J. 2014;127:839–844. [PubMed] [Google Scholar]
- 16.He Y, Li Q, Zhang C, Keller BB, Gu H. Successful outcomes for atrial septal defect associated with pulmonary arterial hypertension using a “treat-repair-treat” strategy. Int J Cardiol Congenit Heart Dis. 2021;2:100075. doi: 10.1016/j.ijcchd.2020.100075. [DOI] [Google Scholar]
- 17.Galiè N, Beghetti M, Gatzoulis MA, Granton J, Berger RM, Lauer A, et al. Bosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled study. Circulation. 2006;114:48–54. doi: 10.1161/CIRCULATIONAHA.106.630715. [DOI] [PubMed] [Google Scholar]
- 18.Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618–3731. doi: 10.1093/eurheartj/ehac237. [DOI] [PubMed] [Google Scholar]
- 19.Brunner N, de Jesus Perez VA, Richter A, Haddad F, Denault A, Rojas V, et al. Perioperative pharmacological management of pulmonary hypertensive crisis during congenital heart surgery. Pulm Circ. 2014;4:10–24. doi: 10.1086/674885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadeghpour A, Alizadehasl A. Post atrial septal defect closure pulmonary hypertension crisis. J Pak Med Assoc. 2013;63:1204. [PubMed] [Google Scholar]
- 21.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020;29:2520–2537. doi: 10.1177/0962280219889080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drevon D, Fursa SR, Malcolm AL. Intercoder reliability and validity of webplotdigitizer in extracting graphed data. Behav Modif. 2017;41:323–339. doi: 10.1177/0145445516673998. [DOI] [PubMed] [Google Scholar]
- 24.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 25.Rabinovitch M, Keane JF, Norwood WI, Castaneda AR, Reid L. Vascular structure in lung tissue obtained at biopsy correlated with pulmonary hemodynamic findings after repair of congenital heart defects. Circulation. 1984;69:655–667. doi: 10.1161/01.CIR.69.4.655. [DOI] [PubMed] [Google Scholar]
- 26.D'Alto M, Dimopoulos K, Coghlan JG, Kovacs G, Rosenkranz S, Naeije R. Right heart catheterization for the diagnosis of pulmonary hypertension: controversies and practical issues. Heart Fail Clin. 2018;14:467–477. doi: 10.1016/j.hfc.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Kempny A, Hjortshøj CS, Gu H, Li W, Opotowsky AR, Landzberg MJ, et al. Predictors of death in contemporary adult patients with eisenmenger syndrome: a multicenter study. Circulation. 2017;135:1432–1440. doi: 10.1161/CIRCULATIONAHA.116.023033. [DOI] [PubMed] [Google Scholar]
- 28.Bando K, Turrentine MW, Sharp TG, Sekine Y, Aufiero TX, Sun K, et al. Pulmonary hypertension after operations for congenital heart disease: analysis of risk factors and management. J Thorac Cardiovasc Surg. 1996;112:1600–1607. doi: 10.1016/S0022-5223(96)70019-3. [DOI] [PubMed] [Google Scholar]
- 29.Schuijt MTU, Blok IM, Zwinderman AH, Van Riel A, Schuuring MJ, de Winter RJ, et al. Mortality in pulmonary arterial hypertension due to congenital heart disease: serial changes improve prognostication. Int J Cardiol. 2017;243:449–453. doi: 10.1016/j.ijcard.2017.05.101. [DOI] [PubMed] [Google Scholar]
- 30.Tahara M, Sanada K, Morita R, Hawaka H, Urayama K, Sugino M, et al. Insufficient development of vessels and alveoli in lungs of infants with trisomy 18-features of pulmonary histopathological findings from lung biopsy. Am J Med Genet A. 2021;185:1059–1066. doi: 10.1002/ajmg.a.62060. [DOI] [PubMed] [Google Scholar]
- 31.Barańska-Pawełczak K, Wojciechowska C, Jacheć W. Diagnostic and predictive value of right heart catheterization-derived measurements in pulmonary hypertension. Wiad Lek. 2021;74:546–553. doi: 10.36740/WLek202103130. [DOI] [PubMed] [Google Scholar]
- 32.Lammers AE, Bauer LJ, Diller GP, Helm PC, Abdul-Khaliq H, Bauer UMM, et al. Pulmonary hypertension after shunt closure in patients with simple congenital heart defects. Int J Cardiol. 2020;308:28–32. doi: 10.1016/j.ijcard.2019.12.070. [DOI] [PubMed] [Google Scholar]
- 33.Gao C, Liu J, Zhang R, Zhao M, Wu Y. The efficacy of bosentan combined with vardenafil in the treatment of postoperative pulmonary hypertension in children with congenital heart disease: a protocol of randomized controlled trial. Medicine (Baltimore). 2021;100:e23896. doi: 10.1097/MD.0000000000023896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.