Abstract
The use of consumer wearable devices (CWDs) to track health and fitness has rapidly expanded over recent years because of advances in technology. The general population now has the capability to continuously track vital signs, exercise output, and advanced health metrics. Although understanding of basic health metrics may be intuitive (eg, peak heart rate), more complex metrics are derived from proprietary algorithms, differ among device manufacturers, and may not historically be common in clinical practice (eg, peak , exercise recovery scores). With the massive expansion of data collected at an individual patient level, careful interpretation is imperative. In this review, we critically analyze common health metrics provided by CWDs, describe common pitfalls in CWD interpretation, provide recommendations for the interpretation of abnormal results, present the utility of CWDs in exercise prescription, examine health disparities and inequities in CWD use and development, and present future directions for research and development.
Keywords: athlete, exercise, fitness, photoplethysmography, wearable device
The 21st century has witnessed an explosion in the availability and use of consumer wearable devices (CWDs) capable of providing health and fitness metrics directly to the end user.1,2 While competitive athletes have utilized CWD metrics to measure, monitor, and improve their exercise performance for decades, these data are increasingly extending from the niche realm of elite-athlete training into the broader arena of monitoring health and wellness in the general population. Although this trend represents a profound democratization of access to physiological data, it also presents unique challenges for practicing clinicians, because patients frequently seek medical advice regarding the accuracy, interpretation, and therapeutic applications of CWD health metrics. These challenges are compounded by the rapid evolution of CWD technology, the diverse array of reported metrics, the frequent use of proprietary calculations and algorithms, and the lack of normative data for most CWD-derived parameters.
This review seeks to provide a tangible primer on CWD metrics for the practicing cardiovascular clinician with an emphasis on the following: 1) measurement techniques and accuracy of common health metrics; 2) pitfalls in CWD interpretation; 3) recommendations for interpretation of abnormal results; 4) the use of CWDs in exercise prescription; 5) important considerations pertaining to health disparities and inequity in the use of CWDs; and 6) future considerations for CWD development.
HISTORY OF WEARABLE DEVICES
CWDs capable of heart rate monitoring and the derivation of related health metrics have their origins in early work from Norman Holter. Holter’s first creation was an 85-pound device capable of radio transmission of electrocardiography (ECG) waves that he wore on his back while riding a stationary bicycle.3 This technology formed the basis of the eponymous ambulatory Holter monitor, which ultimately reached commercial production in the 1960s.4 The first wireless heart rate monitors, designed as a training aid for competitive endurance athletes, were developed in the late 1970s by the Finnish company Polar Electro. These CWDs, available for purchase by 1982, measured heart rate using 2 diodes embedded in an adjustable fabric chest strap which communicated with a wristwatch-based output. Although similar chest-strap technology remains an accurate CWD method of heart rate monitoring, newer generated CWDs more frequently rely on optical photoplethysmography (PPG) measured at the wrist or finger, with which a wide variety of health and fitness metrics can be generated.
HEALTH METRICS
The following sections summarize common cardiac and extracardiac health and wellness metrics reported by CWDs with an emphasis on measurement technique, accuracy, and normative data (Central Illustration). A detailed search strategy for this review can be found in the Supplemental Appendix.
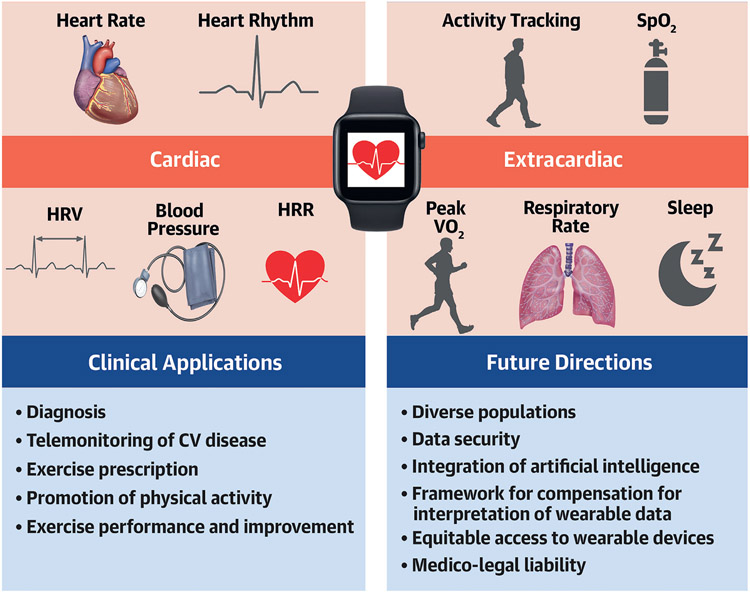
CENTRAL ILLUSTRATION. Common Health Metrics Provided by Consumer Wearable Devices.
Top left show common cardiac health metrics provided by consumer wearable devices, while top right highlights common extracardiac health metrics provided by consumer wearable devices. Clinical applications and future considerations are additionally presented. HRR = heart rate recovery; HRV = heart rate variability; SpO2 = oxygen saturation; VO2 = oxygen uptake.
CARDIAC HEALTH METRICS.
Heart rate.
Heart rate is provided by most CWDs and is used to derive many secondary variables. Normal resting heart rate ranges between 60 to 100 beats/min, but habitual exercise training often results in marked reductions of resting heart rate in the absence of pathology. Maximal obtainable heart rate is determined by age, sex, and genetics, and cannot be increased by exercise training. This parameter is commonly used as a reference value to prescribe and to gauge the intensity of exercise and can be measured during exercise testing in both laboratory and field settings. Maximal predicted heart rate (MPHR) is often calculated as 220 - age (years), although this approach produces highly variable estimates on an individual basis.
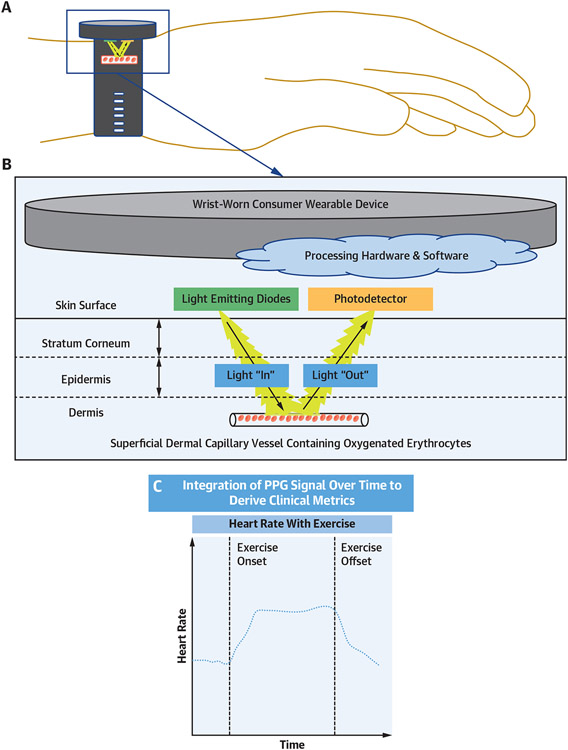
CWDs measure heart rate using either PPG or ECG. PPG relies on the use of light-emitting diodes, which emit light at specific wavelengths toward the skin. The intensity and pulsatility of light reflected from blood vessels is then measured by a photodetector and converted into estimates of blood flow and heart rate (Figure 1). PPG has the ability to provide continuous heart rate data (depending on the CWD software and device sampling rate) and is most commonly used by wrist and finger-worn CWDs.5 Commercial chest straps most commonly use electrode-based ECG methods to measure heart rate, which is consistently more accurate in heart rate measurement than wrist-worn PPG-based devices.6,7 Although some wrist-worn CWDs possess ECG capabilities, they typically require on-demand activation and thus do not offer continuous heart rate measurements. Alternative heart rate measurement methods include smartphone camera-based PPG measurement and standalone ECG devices that connect wirelessly to a smartphone.6
FIGURE 1. Basic Methodology of PPG.
Schematic of a wrist-worn consumer wearable device (A) and basic methodology of photoplethysmography (PPG) for a consumer wearable device (B). PPG signals are integrated over time to derive common clinical metrics such as heart rate (C).
Numerous studies have examined the validity of PPG-based heart rate measurements by CWDs. These data are challenging to interpret and implement in clinical practice because of the variety of CWDs studied, testing conditions, and gold-standard comparators. In one systematic review assessing the accuracy of wrist-worn CWD heart rate data provided by 9 different manufacturers, PPG-based heart rate measurement demonstrated ±3% measurement error in controlled settings compared with reference standard devices (conventional ECG, electrode-based chest straps, pulse oximetry) among the majority of studies.8 One wrist-worn CWD also met accuracy recommendations in free living settings (measurement error ±10%) when compared with an electrode-based chest strap.8 Although these data are compelling, the gold-standard technique to assess heart rate remains the use of conventional clinically approved ECG, because electrode-based chest straps and finger pulse oximeters have variable accuracy. Studies assessing popular wrist-worn CWDs compared with ECG have found that CWDs may slightly underestimate heart rate,5 but interestingly may be more accurate than research-grade CWDs.9 Wrist-worn CWDs utilizing PPG for heart rate measurement have also been found to be less accurate during activity, with one study suggesting a 30% reduction in accuracy.9 This therefore remains an area in need of future innovation. With the rapid growth of finger-worn CWDs, further studies should look to include both wrist- and finger-worn devices in a variety of different patient demographics and environmental settings.10
CWDs can detect abnormally low and high heart rates, which may suggest pathology and/or abnormal heart rhythms (Table 1). In general, we recommend interpreting CWD heart rate data based on the circumstances that prompted data collection and considering individual patient-level trends over time, because heart rate measurements may be affected by patient-specific, environmental, and device-related factors (see section, “Pitfalls in Interpretation of Wearable Devices”). If a patient wants to closely monitor heart rate during exercise, a chest strap is recommended because accuracy is improved compared with current wrist-worn CWD technology.6 Follow-up testing may be warranted if a patient has a higher or lower than expected heart rate (based on patient-level trends) associated with cardiopulmonary symptoms (eg, palpitations, shortness of breath, chest pain, lightheadedness/dizziness) or if there is a significant unexplained deviation from baseline heart rate trends in the absence of device-related quality issues (Table 1).
TABLE 1.
Differential Diagnosis and Recommended Testing to Consider for Abnormal Health Metrics From Consumer Wearable Devices
| Metric | Differential Diagnosis | Confirmatory Diagnostic Testing to Be Considered |
Additional Testing Considerationsa |
|---|---|---|---|
| Low heart rate (resting conditions) | Wearable device heart rate measurement failure Bradyarrhythmia Sinoatrial node dysfunction, high grade atrioventricular block Sinus bradycardia Anorexia/malnutrition, CNS disease, hypothermia, hypothyroidism, exercise training, medication effect, sleep apnea, increased vagal tone |
|
Bradyarrhythmia Refer to AHA/ACC guidelines following arrhythmia confirmationb Sinus bradycardia
|
| High heart rate (resting conditions) | Wearable device heart rate motion artifact Tachyarrhythmia Paroxysmal supraventricular tachycardia, ventricular tachycardia Sinus tachycardia Anxiety, dehydration, anemia, medication effect (eg, beta-blocker withdrawal, beta agonist), infection, heart failure, hyperthyroidism, pain, POTS, pregnancy, PE, shock, stimulant use, inappropriate sinus tachycardia |
|
Tachyarrhythmia Refer to AHA/ACC guidelines following arrhythmia confirmationb Sinus tachycardia
|
| Low SpO2 (resting conditions) | Wearable device SpO2 measurement failure High altitude Hypoventilation Chest wall pathology (eg, kyphoscoliosis), CNS disease, sedatives, hypothyroidism, metabolic alkalosis, OSA, respiratory muscular weakness (eg, ALS) V/Q mismatch or shunt ARDS, asthma, atelectasis, pulmonary AVM, bronchiectasis, COPD, pulmonary edema (cardiac and noncardiac), ILD, intracardiac shunt, mucus plugging, PE, PNA |
|
|
| Irregular heart rhythm (resting conditions and during exercise) | Wearable device heart rate measurement failure Wearable device heart rate motion artifact Arrhythmia Respiratory sinus arrhythmia |
|
Arrhythmia Refer to AHA/ACC guidelines following arrhythmia confirmationb Sinus arrhythmia No further testing recommended |
| HRV (low) | Wearable device heart rate measurement failure aging Physical deconditioning inadequate recovery/overtraining infection Medication effect Mental stress Poor sleep Systemic illness Toxins (eg, EtOH) Thermal stress (warmer temperatures) |
|
No further testing recommended |
| HRR (low) | Wearable device heart rate measurement failure Physical deconditioning Medication effect Systemic illness Tachyarrhythmia |
|
|
| Peak (low) | Wearable device algorithm inaccuracy Physical deconditioning Cardiopulmonary disease Anemia Skeletal muscular disease (myopathy) |
|
|
| High respiratory rate (resting conditions and during exercise) | Wearable device RR motion artifact Anxiety Cardiac disease (arrhythmia, ischemia, heart failure) Diabetic ketoacidosis Anemia Exercise Fever/sepsis Pulmonary disease (asthma, COPD, effusion, ILD, PE) Toxins |
|
|
| Low respiratory rate (resting conditions and during exercise) | Wearable device RR measurement failure CNS disease Hypothyroidism Medication effect Sleep apnea Toxins (eg, opiates) |
|
|
| Sleep (poor sleep) | Wearable device heart rate and/or RR measurement failure Dehydration Medication effect (eg, stimulants) Sleep disorder (eg, restless leg, syndrome, sleep apnea) Stress/anxiety Toxins (eg, EtOH) |
|
|
A thorough history and physical examination is recommended as the starting point in the evaluation of all abnormal testing.
For confirmed bradyarrhythmia, refer to the 2018 AHA/ACC/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay.102 For confirmed tachyarrhythmia, refer to the 2015 AHA/ACC/HRS guideline for the management of adult patients with supraventricular tachycardia103 or the 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death104 or the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation105/2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation.106
ABG = arterial blood gas; ARDS = acute respiratory distress syndrome; AVM = arteriovenous malformation; BMP = basic metabolic panel; CMP = comprehensive metabolic panel; CNS = central nervous system; COPD = chronic obstructive pulmonary disease; EtOH = alcohol; ILD = interstitial lung disease; OSA = obstructive sleep apnea; PE = pulmonary embolism; PFT = pulmonary function test; PNA = pneumonia; POTS = postural orthostatic tachycardiac syndrome; RR = respiratory rate; TSH = thyroid stimulating hormone; TTE = transthoracic echocardiography.
Heart rate recovery.
Heart rate recovery (HRR) is an increasingly popular fitness-related metric derived from raw heart rate data. HRR reflects cardiac autonomic function and is defined as the difference between heart rate at the immediate cessation of exercise and heart rate at a later time interval.11,12 For example, HRR at 60 seconds (often designated HRR60) is the difference between heart rate at exercise cessation and heart rate 60 seconds later. HRR after exercise is conventionally divided into “fast” and “slow” phases that correspond to HRR following the first minute of exercise cessation and subsequent HRR during the more prolonged period of time required to reach baseline heart rate.11,12 The fast phase of HRR is primarily mediated by rapid vagus nerve reactivation, whereas the slow phase represents the more gradual withdrawal of sympathetic tone and decline in catecholamine levels.11-13
There are no universally accepted normative values for HRR. Key factors that influence HRR include measurement time interval of data acquisition; variability of the exercise stimulus preceding HRR acquisition (ie, mode, intensity, and duration); subject position during recovery (upright vs supine); ambient temperature; and patient demographics, training, hydration, and recent sleep quality.11,12,14-16 The vast majority of published HRR data has focused on its prognostic utility. Numerous studies examining mortality among people referred for clinically indicated exercise testing have used failure to achieve a 12 beats/min reduction at 1 minute as a cutpoint for risk stratification.11,12 Alternative HRR cutpoints for mortality prediction have in general ranged from 12 to 30 beats at 1 minute to 22 to 42 beats at 2 minutes.11,12,17 Similar data in healthy populations are limited. Among healthy, active men ages 20 to 65 years, a median HRR60 of 13 beats/min (IQR: 9-20 beats/min) has been reported.18 Numerous studies have also demonstrated greater (ie, faster) HRR among trained athletes compared with untrained or less trained people.12,13
At the present time, there are sparse primary data examining the accuracy of HRR derived from CWDs. Given that HRR is calculated as the difference between 2 discrete heart rate measurements, factors that influence CWD heart rate measurement are broadly applicable to HRR (see section “Pitfalls in Interpretation of Wearable Devices”). Any clinical utilization of HRR must be interpreted relative to the specific time interval used by a given CWD. Given the paucity and heterogeneity of normative data, intraindividual changes over time likely represent the most meaningful use of CWD HRR data. Future research should assess the effect of intraindividual changes in HRR on outcomes and the response to therapeutic interventions. If a patient presents with a sustained significant reduction from prior HRR values, a careful history and physical examination (H&P) may diagnose the majority of causes (eg, physical deconditioning, systemic illness, medication effect), and exercise testing with ECG may be considered if there is a high suspicion for exercise-associated tachyarrhythmia (Table 1).
Heart rate variability.
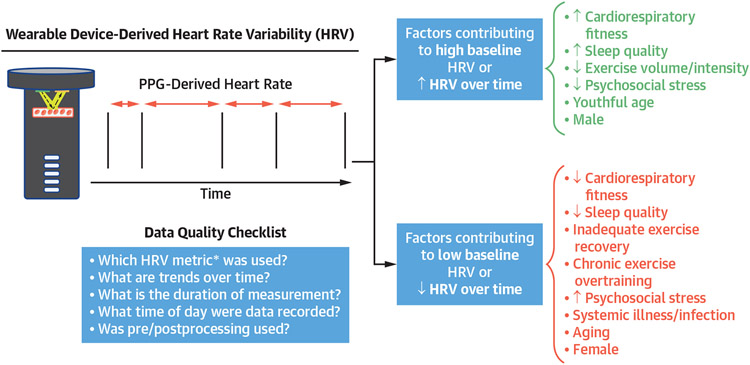
Heart rate variability (HRV) broadly reflects the temporal magnitude of beat-by-beat variability over a specified time period. Historically, HRV has been examined in research contexts for prognostication in cardiovascular disease. For example, lower time-domain HRV indices are associated with increased mortality after myocardial infarction19 and among patients with heart failure.20 HRV is affected by numerous factors that modulate the autonomic nervous system independently of cardiovascular disease, including age, sex, mental stress, infection, caffeine and nicotine use, sleep quality, recent exercise training patterns, hydration, and time of day (Figure 2).21-23 Despite its prognostic value in research settings, challenges related to interpretation and utilization of HRV data have limited use of HRV in clinical practice.
FIGURE 2. Common Factors Affecting HRV and Data Quality Checklist.
Data quality checklist for interpretation of heart rate variability (HRV) results from consumer wearable devices and common factors leading to high baseline/increased HRV and low baseline/decreased HRV. *See Supplemental Table 1. PPG = photoplethysmography.
HRV calculated by CWDs is typically derived from raw heart rate data measured using PPG. HRV can be calculated using time-domain metrics (ie, differences between specific heart rate interval in ms) and frequency-domain metrics (ie, differences in heart rate frequency over specific intervals in ms2). Common examples of time-domain HRV metrics provided by CWDs include the following: the mean normal-normal interval (NN) (ie, the interval between consecutive sinus beats), the standard deviation of NN intervals (SDNN), the standard deviation of NN intervals averaged over a short duration (SDANN), the root mean square of differences in successive NN intervals (RMSSD), and the percentage of successive NN intervals that are >50 ms from the prior interval (pNN50) (Supplemental Table 1).24,25 In contrast, frequency-domain HRV metrics calculate how much of a heart rate signal lies in specific frequency bands or ranges. Frequency-domain measurements are commonly divided into high-frequency power bands (activity in the 0.15- to 0.4-Hz range), which have been proposed to reflect parasympathetic nervous system activity, and low-frequency power bands (activity in the 0.04- to 0.15-Hz range), which have been proposed to reflect sympathetic nervous system activity (Supplemental Table 1).25 Because HRV is a metric of variance, it must be derived from heart rate measurements over a discrete period of time, which are classified as ultra-short-term (<1 minute), short-term (typically <5 minutes), and long-term (typically >24 hours) and are not directly comparable to one another.24,25
Exercise exerts a profound influence on HRV. During the first few days following high-intensity exercise, vagal-related HRV indexes (eg, RMSSD) will usually decrease from baseline values.26 However, over weeks of exercise training in untrained or recreational athletes, HRV tends to increase.25 Interpretation of HRV among competitive athletes is both common and complex. Despite the myriad of factors delineated in the previous text that influence HRV, it is commonly utilized as a guide for training.26 Consistent reductions in HRV during training, in the absence of alternative explanatory factors, have been proposed to reflect accumulated physiological stress (ie, overtraining) and may be used as a rationale to decrease training loads. In contrast, high HRV values have been proposed to indicate a well-recovered physiological state reflective of an appropriate balance of training stress and recovery.25,26
HRV metrics derived from CWDs are subject to the same sources of error and bias as heart rate measurement (see section “Pitfalls in Interpretation of Wearable Devices”). Additionally, HRV assessment requires continuous data acquisition over a defined period, which increases susceptibility to inaccuracy introduced by motion artifact. Measurement of frequency-domain HRV metrics, which require longer durations of continuous data collection, is particularly difficult using CWDs. Accordingly, many CWDs only report HRV during sleep, even for time-domain metrics.27-29 One study examining the effect of missing data on CWD HRV measurements found that mean NN and RMSSD were the metrics least affected by missing data.27 An additional caveat to PPG-based HRV calculation is that many HRV metrics are sensitive to ectopic beats, which can be difficult to identify without simultaneous ECG data.26
PPG-derived HRV may correlate strongly with measurements derived from gold-standard techniques such as ECG under optimal conditions. Specifically, HRV (measured from PPG of the earlobe) performed well (r > 0.95) in healthy subjects in ideal conditions in comparisons with ECG for the measurement of both time and frequency domain HRV metrics.30 A study of 6 CWDs (wrist, finger, and forehead locations) in the setting of polysomnography sleep testing found that CWDs generally show good correlation but tend to underestimate ECG-derived RMSSD.10 Several other studies of wrist- and finger-worn CWDs have found variable correlation of time-domain HRV metrics with ECG or chest-strap reference standards, which in some cases were sensitive to beat filtering methods, the choice of HRV metric, and/or the time period over which measurements were averaged.31-33 Importantly, most prior studies assessing the efficacy of CWD-derived HRV were performed in carefully controlled research settings. The accrual of more robust device- and disease-specific normative data needed to refine clinical use will require future studies in real-world settings.
At present, normative HRV values accounting for age and sex that can be broadly applied across all people and all CWDs do not exist. Supplemental Table 2 provides selected examples illustrating both the general ranges of 2 common HRV metrics (SD of NN intervals and RMSSD) and the variability that results from different measurement durations, sex, age, and time of day. In this context, interpretation of HRV data using absolute values is discouraged. Instead, HRV trends for a given individual should be assessed and integrated with a careful history assessing for the common sources of HRV fluctuation (Figure 2). We therefore recommend that if a patient presents with a lower-than-expected HRV from their baseline, focus should be on a comprehensive H&P to assess for a possible underlying cause (Table 1). Follow-up testing should only be considered if there is a strong suspicion for a secondary cause that requires risk stratification or treatment.
Heart rhythm.
Several popular CWDs have received regulatory approval for the detection of atrial fibrillation (AF).6 Available AF-detection algorithms utilize PPG data to detect an irregular pulse and increase detection specificity by requiring the detection of multiple periods of irregular pulse before notifying the user of possible arrhythmia.34-36 Although PPG-based irregular rhythm detection algorithms operate in the background while the device is worn, they rely on periodic measurements and are susceptible to motion artifact, often requiring periods of relative inactivity before they will alert users of an irregular rhythm.35 As a result, they do not provide truly continuous screening. Notably, these algorithms were initially designed for AF screening and not to detect other arrhythmias or to quantify arrhythmia burden in patients with known AF. There have, however, been studies assessing the potential utility of the detection of non-AF arrhythmias,37 and estimation of AF burden is of active interest.
If an irregular pulse-detection algorithm alerts a patient of a possible arrhythmia, it does not diagnose AF. If the patient’s device has ECG capabilities, the patient may record a single-lead ECG tracing, but this requires user interaction. The ECG tracing can be shared with a physician who may provide an interpretation of whether AF may be present or not. Several popular CWDs have received regulatory approval for ECG acquisition and accompanying algorithms for rhythm classification based on ECG data.6
Automated AF classification algorithms are proprietary to each manufacturer and vary in performance depending on the target audience of the CWD.6,38-40 Notably, in a recent study of 5 different CWDs, sensitivity of AF detection ranged from 58% to 85% and specificity between 69% and 79%.39 CWD algorithms were not able to determine the heart rhythm in 17% to 26% of tracings, but the rhythm was determined upon manual review in 99% of the single-lead ECGs.39 These findings support previous data that demonstrate an improvement in diagnostic performance when single-ECGs from CWDs are over-read by physicians.38,40 There are several other technologies for AF detection through smartphone-based methods such as PPG assessment using the smartphone camera, connection of external electrode-based devices to a smartphone, or mechanocardiography (detection of irregular cardiac motion through accelerometers/gyroscopes),41-43 all of which represent other frontiers for mobile AF detection. A comprehensive overview of CWD heart rhythm detection is out of the scope for this review and can be found elsewhere.6,44
CWDs therefore may lead to the diagnosis of AF or non-AF arrhythmias. If a patient receives a CWD alert for a possible abnormal heart rhythm or has symptoms concerning for an arrhythmia, the patient should be instructed to record a single-lead ECG, if the CWD is ECG capable. After reviewing the CWD ECG and taking a thorough history, providers should consider a 12-lead ECG and/or ambulatory rhythm monitoring as dictated by the clinical circumstance (Table 1).
Blood pressure.
Blood pressure (BP) measurement by CWDs is an area of active interest, but few mainstream consumer wearables currently offer this feature. Several methods of wearable BP measurement exist or are under development, including wrist or finger cuff CWDs, radial artery tonometry, bioimpedance, and PPG-based methods with or without incorporation of ECG data.45,46
One brand of wrist-worn CWDs possesses a BP measurement feature via a proprietary algorithm. This feature requires manual recalibration every 28 days, and a validation study found that BP measurements were systematically biased toward the calibrated value.47 In contrast, a currently available and regulatory-approved watch utilizes an integrated wrist cuff to measure BP, and demonstrated good correlation with manually measured BP (mean absolute difference ≤2.4 mm Hg, SD ≤7.6 mm Hg).48 Finally, a third device employing a machine learning model incorporating PPG and ECG data demonstrated similar performance (mean absolute difference ≤2.2 mm Hg, SD ≤6.1 mm Hg) in a research setting.49 There are also multiple finger-based rings with BP capabilities in development, which represents another exciting new frontier.46 Although emerging technologies have shown promise in the estimation of BP, careful validation studies in diverse cohorts of patients and clinical contexts will be needed to ensure safety and efficacy of devices before widespread use. At present, we are unaware of any robust large-scale data defining use of CWD-derived BP measures in the setting of exercise.
Although there are currently limited BP capabilities among CWDs, if a patient consistently has elevated baseline BP under resting conditions above guideline recommendations of ≥130/80 mm Hg,50 they should consider home blood pressure monitoring with a regulatory-body approved blood pressure cuff to assess for clinically relevant HTN.
EXTRACARDIAC HEALTH METRICS.
Step count and global positioning system data.
Daily step count has emerged as a popular index of physical activity that enables data-driven exercise training and quantitative exercise prescription. In addition, the use of CWDs to track step count has been shown to increase physical activity and improve body composition and fitness.51 Contemporary physical activity guidelines recommend exercise dose as a function of time and intensity (ie, 150-300 minutes of moderate intensity or 75-150 minutes of vigorous intensity exercise weekly) for health and longevity.52 However, higher step count is also associated with lower all-cause mortality and cardiovascular events.53 CWDs offer a direct and user-friendly way of tracking time spent on exercise or other physical activity. A recent meta-analysis of 15 international cohorts found a progressively lower risk of mortality among adults age ≥60 years who achieve up to 6,000 to 8,000 steps/d and among adults age <60 years who achieve up to 8,000 to 10,000 steps/d.54 Therefore, CWDs have the possibility to promote physical activity and improve longevity, which may be extremely beneficial to high-risk populations.55-57
Most contemporary CWDs provide step count estimates either using accelerometers, which are electromagnetic sensors that measure acceleration in a linear plane, or gyroscopes which measure angular velocity. There is a wide variability in the accuracy of step count measurements based on the CWD manufacturer, CWD model, speed of movement, testing environment, and body location of CWD.8,58,59 Although some CWD models consistently overestimate and others consistently underestimate step count, there can also be significant heterogeneity in intradevice reliability when testing and retesting the same CWD.8 The accuracy of CWDs also depends on the reference standard used as a comparator. Numerous studies use research-grade accelerometers as a reference standard,8 although the gold standard remains video-recorded step count,59 because research-grade accelerometers can also vary in accuracy. Therefore, caution should be utilized in interpretation of total step count based on the accuracy of each individual CWD.
Consumer wearable devices utilizing global positioning system (GPS) are often used to track the distance, velocity, and altitude changes during endurance exercise. Studies assessing the accuracy of wrist-worn GPS activity monitors have found that total distance travelled is often underestimated in urban and forested areas where GPS signals can be obstructed by nature or buildings (mean absolute percentage error −1.2% to −8.9%).60,61 In contrast, GPS data typically overestimate distance and velocity at track and field venues (mean absolute percentage error +0.9% to +4.1%).60 These findings are most relevant among individuals who rely on precise measures of training data to optimize fitness and performance.
In aggregate, patients whose CWD shows a low step count (<6,000-8,000 steps/d for age ≥60 years, 8,000-10,000 steps/d for age <60 years) or those who do not meet guideline recommendations for physical activity (150-300 minutes moderate or 75-150 minutes vigorous intensity activity) should be counseled to increase activity levels in accordance with contemporary guidelines to optimize health and longevity.52,54
Oxygen saturation.
Oxygen saturation (SpO2) can be used to establish hypoxemia in patients with cardiovascular, pulmonary, and hematologic disorders. The normal SpO2 in the general population at sea level is ≥95%, while lower SpO2 goals (88%-92%) may be clinically targeted in certain disease states such as chronic obstructive pulmonary disease.62 Among healthy competitive athletes, mild to moderate exercise-induced arterial hypoxemia at high workloads is common. Theorized explanatory mechanisms include rapid pulmonary capillary red blood cell transit time, extravascular lung water accumulation, increased blood viscosity, cytokine release, and pulmonary capillary stress failure.63 Competitive male runners (age 18-39 years) at sea level have a reported exercise-induced arterial hypoxemia prevalence of ranging from 70% to 84% depending on the trait definition (SpO2 ≤91% vs ≤93%), rendering this a normal finding in otherwise healthy asymptomatic athletes.64
Consumer wearable devices measure SpO2 using PPG by emitting light-emitting diode lights of 2 different wavelengths and then measuring subsequent light absorption, which differs based on the oxygenation status of hemoglobin.65 Current guidance from the U.S. Food and Drug Administration stipulates that the variability between SpO2 measurement from a clinically approved PPG device and an invasive arterial blood gas (ABG) should have a root mean square error ≤3.5%.66 Several studies have reported that select commercially available wrist-worn CWDs may meet these U.S. Food and Drug Administration thresholds at rest or during sleep.67-69 However, the majority of these studies compared CWDs to medical-grade pulse oximeters rather than the gold standard invasive ABG, and definitive studies assessing the accuracy of SpO2 during exercise are lacking.
Evaluation of the accuracy of wrist-worn CWDs with simulated altitude (normobaric hypoxia),70 as well as actual high altitude,71 has shown poor correlation between resting SpO2 measured by a wrist-worn PPG measurement and commercial pulse oximetry and/or ABG. Data documenting the tendency of wrist-worn PPG to overestimate SpO2 are of particular concern,70 because commercial wrist-worn CWDs are often marketed for mountaineering during which hypoxemia may predict the onset of high altitude illness. Further rigorous evaluation comparing CWD SpO2 to gold standard ABG in diverse cohorts of patients, conditions, and levels of exertion are needed before recommendations for clinical use.72 Data also suggest that individuals with darker skin tones may be particularly susceptible to inaccurate measurement of SpO2 (discussed in depth in the following text—see section “Health Equity and Disparities in the Use and Accuracy of Wearable Devices”). Heterogeneity in the accuracy of SpO2 measurements across available CWDs necessitates that users determine the performance of their own device in specific environments.
Given limited data on the accuracy of CWD-derived SpO2 during exertion, results during exercise and/or physical activity should be interpreted with caution. However, an abnormal resting or exercise SpO2 from a CWD associated with significant cardiopulmonary symptoms may warrant further work-up to assess for underlying pathology (Table 1).
Respiratory rate.
Respiratory rate, a fundamental vital sign that largely drives minute ventilation (the product of respiratory rate and tidal volume), is a sensitive predictor of clinical deterioration.73 Normal respiratory rate for an adult at rest is defined as 12 to 20 breaths/min. Physiological increases in respiratory rate occur in the setting of increased metabolic activity such as stress and exercise.
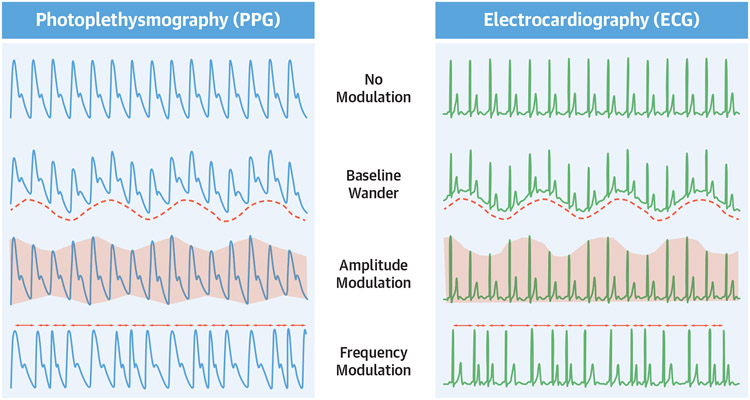
Respiratory rate can be measured using chest straps with biosensors to detect chest wall movement (elastomeric, impedance, or respiratory inductive plethysmography),74 or indirectly using algorithms that detect alterations of PPG and/or ECG signals with breathing. Wrist-worn CWDs can estimate respiratory rate by assessing several different types of cyclic variation in PPG and ECG waveforms. The 3 major variables assessed as part of respiratory rate algorithms include the following: 1) baseline wander; 2) amplitude modulation; and/or 3) frequency modulation (also known as respiratory sinus arrhythmia) (Figure 3).75 These changes result from respirophasic cardiac motion within the thoracic cavity, hemodynamic changes, and alterations in vagal tone.75
FIGURE 3. Waveform Analysis to Estimate Respiratory Rate From PPG and ECG.
Diagram depicting natural physiological changes in photoplethysmography (PPG) (left) and electrocardiography (ECG) tracings (right) with normal respiration that can be utilized to estimate respiratory rate. Adapted with permission from Charlton et al.107
Chest straps that estimate respiratory rate using heart rate data have been shown to be more accurate than wrist-worn CWDs among healthy subjects at rest.76 Data examining wrist-worn CWDs are limited but suggest that respiratory rate during sleep can be reliably obtained (bias −0.24%; 95% limits of agreement: −1.5 to +1.0/min in 1 study).77,78 We are unaware of large-scale published data examining respiratory rate accuracy among common CWDs during movement or exercise where increased error from artifact is anticipated. Accordingly, estimations of respiratory rate from widely available CWDs obtained during conditions other than prolonged quiet rest and sleep should be interpreted with caution.
Given the limitations described in the previous text, we recommend only considering targeted secondary testing based on the suspected etiology among those with symptoms and corollary abnormalities of respiratory rate during wakened hours under resting conditions (Table 1).
Peak .
Peak oxygen consumption is the gold standard objective measure of functional capacity and is an integrative metric reflecting the precise coordination and health of the cardiovascular, pulmonary, hematologic, and musculoskeletal systems. Peak is utilized in the clinical setting in the assessment of exertional symptoms of unclear etiology, prognosis and risk stratification in the setting of established pathology, and the assessment of response to specific therapeutic interventions.79 Given the robust association between peak and exercise performance, this metric is also commonly utilized to assess the response to exercise training. Habitual endurance exercise can lead to more efficient oxygen utilization and can increase peak . Improvements in peak are also related to an individual’s baseline activity level, because sedentary patients may see much greater improvement in peak with habitual exercise training than patients who are starting from a higher baseline of physical activity.
Direct quantification of peak is performed using measurements of metabolic gas exchange during maximal effort cardiopulmonary exercise testing (CPET). Peak is estimated by CWDs using a variety of different, typically proprietary methods which rely on some combination of age, sex, body size, heart rate metrics (resting heart rate, peak heart rate, HRV), and physical activity level. Estimates of peak using CWDs are derived both under resting conditions (utilizing resting heart rate and HRV) and by the user exercising with CWD monitoring (utilizing physical activity levels and heart rate metrics).80 Peak estimated by exercise-based algorithms demonstrates higher accuracy but generally underestimates true peak (bias −0.1, 95% limits of agreement: −9.9 to +9.7 mL/kg/min). Peak estimated based on resting conditions has lower accuracy and tends to overestimate peak (bias +2.2; 95% limits of agreement −13.1 to +17.4 mL/kg/min).80 Regardless of the estimation technique, there is significant error at the individual level when compared with CPET-derived measurements.80 Further quality improvement is needed before confident use of peak derived by CWDs in the clinical or performance setting, and the gold standard remains direct measurement through CPET. CWDs also do not often provide estimation of submaximal exercise parameters such as the ventilatory threshold, which can have an important prognostic and therapeutic role, particularly in the use of a customized exercise prescription.
A low peak can be found among those with low cardiac output (CO = stroke volume × heart rate) or low peripheral O2 extraction (Ca-VO2; per the Fick Equation ). As there is significant intraindividual variation in peak estimation by CWDs, we recommend assessing trends over time rather than absolute values. A patient presenting with estimated peak newly reduced from their baseline should undergo a thorough H&P to assess for physical activity level (eg, deconditioning), cardiopulmonary symptoms suggestive of pathology, excessive muscle fatigue suggestive of myopathy, and signs/symptoms of anemia (Table 1). Follow-up testing should be guided by the clinical scenario, and definitive measurement with a CPET is only recommended if it will change clinical management or prognosis.
Assessment of sleep.
Sleep quality and duration are increasingly recognized as vital contributors to overall health and wellness.81 Current guidelines from the National Sleep Foundation and American Academy of Sleep Medicine recommend at least 7 hours of sleep per night for adults but highlight that heterogeneity of optimal sleep duration exists at the individual level.82,83 The gold standard method for assessing sleep quality is polysomnography (PSG) in a sleep laboratory. PSG integrates brain waive signals (electroencephalography), eye movement signals (electrooculography), muscle activity (electromyography), cardiac tracings (ECG), and finger PPG to monitor hemodynamics and determine the duration of different stages of sleep. However, PSG is not practical outside of a sleep laboratory and is associated with high costs, specialized training for interpretation, and significant time required to perform the studies.
Given these limitations, techniques have been developed to estimate sleep and wake information from actigraphy, which utilizes accelerometers similar to those in CWDs to measure direct limb movement (typically worn on the wrist or ankle).84 Actigraphy is more readily available and more easily performed than PSG and has revolutionized sleep medicine because measurements can be made at home over extended periods of time (days to weeks). However, actigraphy has numerous limitations compared with PSG: 1) overestimation of sleep time if patients do not have robust limb movements; 2) underestimation of sleep time in patients with movement disorders; and 3) inability to robustly identify stages of sleep.85
CWDs estimate sleep quality and duration through proprietary algorithms, which often integrate accelerometry and PPG-derived data (eg, heart rate). As modern CWDs leverage multiple physiological parameters for this assessment, most CWDs tend to perform better than actigraphy, which solely relies on accelerometer data.86 Modern CWDs have also been shown to improve patient-reported sleep quality.77 In studies comparing CWDs to PSG, CWDs demonstrate good performance in detecting sleep (sensitivity 93%-99%), including defining sleep duration, but are less accurate at both detecting periods of wakefulness and differentiating stages of sleep.86 Therefore, novel algorithms are being created to improve the efficacy of combined accelerometer/PPG data to better approximate PSG data in a variety of sleep disorders.87 Given the rapid growth in CWDs and clinical devices to assess sleep, the American Academy of Sleep Medicine has released a framework for the evaluation of future sleep technologies that should be considered in the assessment of CWDs.88 Future research and technological advances are required to optimize CWDs for the differentiation of sleep stages and to validate efficacy in diverse demographic cohorts and disease states.
CWDs may accurately detect sleep duration. If a patient is consistently not meeting contemporary recommendations for sleep duration (>7 hours),82,83 a thorough H&P should be performed to assess sleep hygiene and to rule out common reversible causes (eg, medication effect, stress/anxiety, toxins) (Table 1). If there are clinical concerns for sleep apnea, PSG should be considered.
Other proprietary metrics.
An overview of other proprietary health metrics (eg, “recovery,” “stress,” “readiness”) is presented in the Supplemental Appendix.
PITFALLS IN INTERPRETATION OF WEARABLE DEVICES
As previously described, PPG is the primary method used by CWDs to measure various health metrics, particularly in the wrist- and finger-worn categories. Therefore, errors in PPG measurement will affect a significant number of measured and estimated variables. Common factors affecting the accuracy of PPG measurements are presented in Table 2.9,89 Although advances in technology have led to numerous techniques to mitigate measurement error (eg, high pass filters, improved calibration, optical shielding, secondary sensors),89 there is still significant error in PPG measurements provided by some CWDs.9
TABLE 2.
Common Factors Affecting the Accuracy of Photoplethysmography Signals
| Obesity |
| Darker skin tone |
| Tattoos |
| Decreased skin perfusion |
| Cold body temperature |
| Wrist body location |
| Inadequate skin contact |
| Motion |
| Ambient light |
A primary determinant of PPG accuracy is the anatomic location of measurement. The PPG-based biosensors worn on the posterior distal wrist, the common location of “wrist-watch” CWDs, have among the largest median error rate at rest and during exercise.90 Wrist motion, in combination with protuberance of the ulnar styloid, may shift CWD position relative to the skin surface, thereby transiently or permanently distancing sensors from their intended measurement site. Error observed during the use of wrist-worn CWDs may be reduced by the addition of an electrode-based or PPG compatible chest strap.7,90 Improper CWD fit is another common source of error. Wrist-worn CWDs that are too loose may not accurately detect PPG signals, and those that are too tight may result in impaired cutaneous blood flow, thereby impairing PPG measurements. An additional source of error is artifact stemming from movement. Artifact can be produced by movement of the CWD in relation to the skin surface, an issue that is common among devices worn loosely. Improper fit leading to device movement applies not only to PPG but other methods of tracking health data including electrode-based chest straps. Motion artifacts are particularly problematic for PPG-based algorithms for AF detection, which has led some CWD producers to rely on measurements primarily during episodes of inactivity.35 CWD error may be particularly pronounced while tracking data during swimming, because water coupled with recurrent arm movements may reduce measurement accuracy.91
Activity tracking using accelerometers or GPS can also be prone to error. Wrist-worn CWDs utilizing accelerometer data may misclassify sedentary time with physical activity (eg, increase step count) when somebody is moving their arms even while seated or alternatively not detect significant movement when someone’s upper body is primarily still (eg, cycling). The accuracy of GPS activity tracking is dependent on the strength and consistency of the GPS signal. Accordingly, activity tracking may be underestimated in locations subject to poor signal strength (eg, urban areas with tall buildings, highly forested recreational areas).60,61
There are also significant limitations among CWDs with single-lead ECG capability. The majority of CWDs require user interaction to record a single-lead ECG. Therefore, asymptomatic paroxysmal arrhythmias may not be diagnosed if the user does not activate the ECG function or if the CWD fails to generate a PDF for a provider to confirm the underlying rhythm.40 Current rhythm diagnostic algorithms from single-lead ECGs also will frequently classify ECGs as “inconclusive.”38-40 This may translate into an increased burden on providers who are asked to review abnormal or inconclusive results. Differentiation of regular tachyarrhythmias (eg, atrial flutter vs atrioventricular nodal re-entrant tachycardia) also remains difficult with single-lead ECG.6 This remains an area of active interest with respect to product development and refinement.
INTERPRETATION AND RECOMMENDATIONS FOR ABNORMAL TESTING
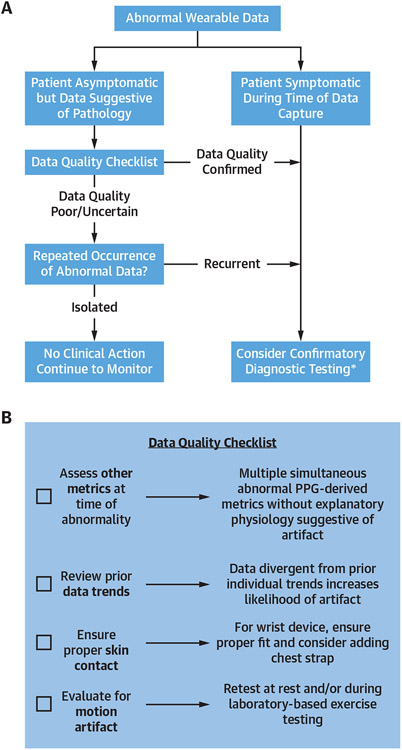
The clinical assessment of all CWD data should begin with careful consideration of possible failure of the CWD to acquire data attributable to the device/patient interface and motion artifact (Figure 4). A differential diagnosis and considerations for subsequent diagnostic testing for common forms of “abnormal” CWD data are presented in Table 1. We recommend that all abnormal testing should be put into clinical context during the decision-making process regarding the role of follow-up testing. Given the lack of device-specific normative data for many CWDs, there should also be a particular emphasis placed on intraindividual changes in a specific health metric over time when evaluating the necessity for secondary testing.
FIGURE 4. Algorithm for Interpretation of Abnormal Testing from CWDs.
Flowchart for the interpretation of abnormal consumer wearable device (CWD) health metrics (A) and checklist to assess for data quality from consumer wearable device health metrics (B). PPG = photoplethysmography.
UTILITY OF CWDs FOR EXERCISE PRESCRIPTION
Routine exercise is an effective strategy for primary and secondary prevention of cardiovascular disease.52 Accordingly, exercise prescription is an essential element of comprehensive cardiovascular care. Effective exercise prescriptions are typically developed using the “FITT” principle (Frequency, Intensity, Time, and Type of exercise).92,93 CWDs enable clinicians and patients to monitor all components of a FITT-based exercise prescription and are more accurate, reproducible, and prognostic than traditional self-reported data, which may improve adherence and corollary clinical outcomes.94,95
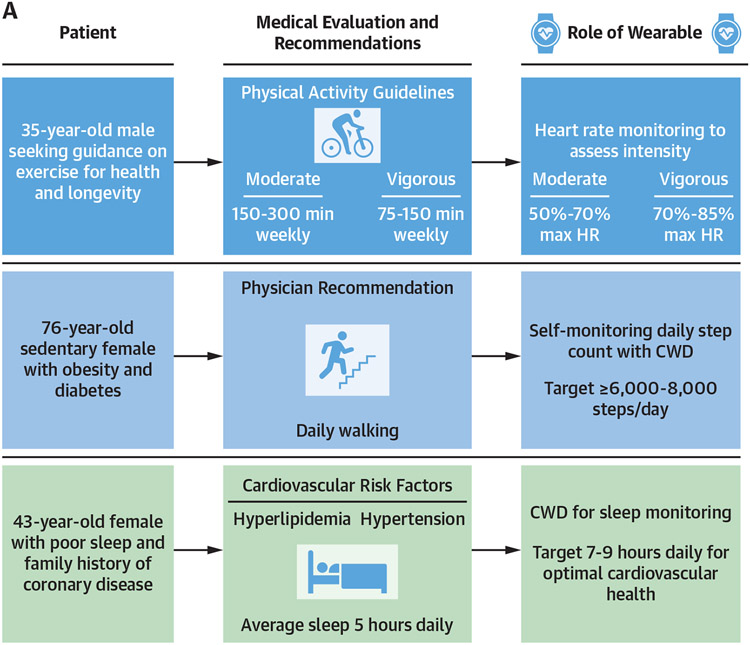
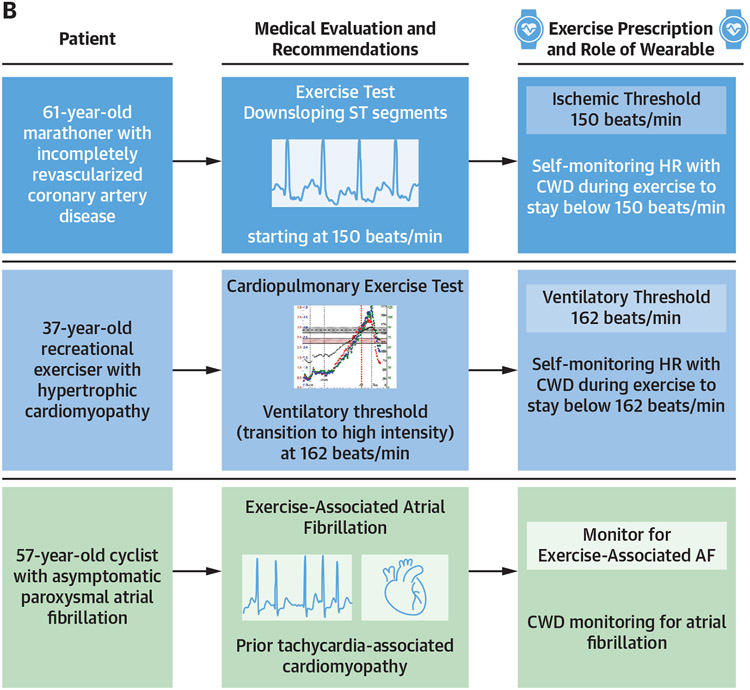
Among patients with cardiovascular disease, CWD heart rate monitoring offers the ability to modulate exercise intensity with the goal of optimizing the safety of therapeutic and recreational exercise. Graded-effort exercise tests can be used to establish workloads, coupled with corollary heart rates, that precipitate myocardial ischemia, malignant arrhythmias, or undesirable symptoms. Such disease- and patient-specific thresholds can subsequently be utilized by practitioners to prescribe exercise intensity ceilings that can be monitored using CWDs during exercise training, with the goal of ultimately improving exercise safety and compliance. Examples of potential utilities of CWDs for exercise prescription are presented in Figure 5.
FIGURE 5. Potential Clinical Applications for CWDs in Cardiovascular Health.
Three potential applications for the use of consumer wearable devices (CWDs) (A) in general cardiovascular health and (B) in exercise prescription for cardiovascular disease.
HEALTH EQUITY AND DISPARITIES IN THE USE AND ACCURACY OF WEARABLE DEVICES
Emerging health care technology necessitates continuous assessment of disparities in quality, education, and access to care. Although the rapid expansion of CWDs has provided exciting new opportunities for remote diagnostics and physiologic monitoring, technological advances have often outpaced the rigorous scientific research needed to ensure efficacy and safety across all patient populations. CWD users are more likely to be younger, White, wealthier, and of higher educational status compared with people without devices.96,97 This ownership imbalance has perpetuated disparities in the participant demographics of scientific studies examining CWD performance. Many of these studies, attempting to increase participant enrollment and thus statistical power, have relied on “bring your own device” participant recruitment strategies.98 The clinical impact created by these structural inequities remains incompletely understood, underscoring the need for future study across more diverse populations and environmental conditions.9,89
A growing body of literature documents technological limitations of PPG among people with darker skin tones.89 Mechanistically, PPG sensors using a green light-emitting diode light source have been shown to have decreased accuracy in those with darker skin tone because of increased light absorption by cutaneous melanin. Multiple studies have shown that PPG-derived SpO2 measurements may be inaccurate among people with darker skin.99,100 This phenomenon may have led to significant clinical consequences during the COVID-19 pandemic, during which wearable-derived SpO2 was commonly used to determine the need for in-person clinical evaluation and potential hospitalization, and studies have suggested that there may have been delays in treatment for those with darker skin tone.101 In summary, CWDs using PPG to measure health metrics have the potential to exacerbate existing structural health disparities and contribute to structural racism by failing to account for differences between patients like skin pigmentation. As our society continues to embrace CWDs, ongoing focus on equity concerns will be required in efforts to close the existing digital divide and to avoid the risk of access to CWDs becoming another social determinant of health.
FUTURE CONSIDERATIONS
A major feature of the CWD market remains the significant heterogeneity in device accuracy, capabilities, and measurement techniques. Although rapid technological advances have led to many clinically relevant innovations, CWDs often come to market before rigorous scientific testing to fully define CWD efficacy and safety with regard to measurement of common health metrics. This leaves patients and clinicians with the challenge of interpreting CWD data in the absence of definitive measures of CWD accuracy. As previously emphasized, some commonly reported health metrics (eg, heart rate) demonstrate significant heterogeneity across CWD manufacturers and patient populations.8 In addition, more complex variables (eg, peak ) are often derived from proprietary algorithms that lack standardization and rigorous comparison with clinically-accepted gold standards.80 Other innovative health metrics provided by CWDs (eg, stress scores, daily readiness scores) have no clear gold-standard of measurement, rendering clinical validation extremely difficult. As CWDs continue to grow in popularity and clinical use, it is imperative that practicing medical professionals and CWD technology industries collaborate together to refine and innovate the most clinically-useful data. A comprehensive list of future considerations for CWD design, research, and implementation is presented in Table 3.
TABLE 3.
Future Considerations for Consumer Wearable Device Design, Research, and Implementation
| Promote studies comparing CWD technology with gold-standard reference devices in diverse cohorts of patients and environmental conditions.5,72,99 |
| Assess the safety and outcomes of CWDs for telemonitoring in patients with established forms of cardiovascular disease.108,109 |
| Optimize and study the accuracy of CWD technology in the measurement of health metrics during exercise (eg, exercise-induced arrhythmias).110 |
| Assess how intraindividual alterations in CWD-derived health metrics affect outcomes in those with and without cardiovascular disease. |
| Study the effect of artificial intelligence on health metric measurement and interpretation.109 |
| Involve HCPs in CWD design and implementation to ensure clinically relevant health metrics and data reporting.2,109 |
| Prioritize data security for patients and accessibility to CWD data for HCPs in electronic health records.2,6 |
| Promote transparency surrounding medico-legal liability for HCPs being sent and interpreting data from CWDs.6,44 |
| Ensure equitable distribution of CWDs with consideration of incentives to alleviate costs for CWDs following medical approval.44,111 |
| Promote equitable compensation for HCPs in the interpretation of data from CWDs.111,112 |
| Develop ongoing CME for providers on interpretation of current and emerging CWD technology and to design and study the effect of educational tools to promote digital health literacy.6,44,109 |
CME = continuing medical education; CWD = consumer wearable device; HCP = health care provider.
CONCLUSIONS
Recent decades have seen rapid growth in the availability and use of CWDs that provide a wide range of cardiopulmonary metrics directly to the consumer. As the use of CWDs has expanded from athletes seeking performance gains to the general public, clinicians are increasingly asked to interpret CWD data and to integrate these metrics in diagnostic evaluations and therapeutic planning. This review seeks to assist the broad cardiovascular community with the integration of CWD data into clinical care. The consumer reach, number of health and disease-related metrics, and scientific data documenting accuracy of CWDs will only continue to grow over the coming years, and we anticipate that competency in these topics will become invaluable for the cardiovascular clinician.
Supplementary Material
HIGHLIGHTS.
CWDs have proliferated, but the heterogeneous health metrics they generate makes interpretation challenging.
To optimize their value for patient assessment and management, physicians should become familiar with the measurement techniques, accuracy, clinical relevance, and potential pitfalls inherent in these devices as they continue to evolve.
Along with technological development, clearer delineation of the indications for and appropriate use of monitoring technologies is needed to ensure safety and accurate application of the information provided by devices.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- CPET
cardiopulmonary exercise test
- CWD
consumer wearable device
- ECG
electrocardiogram/electrocardiography
- GPS
global positioning system
- HRR
heart rate recovery
- HRV
heart rate variability
- NN interval
the mean normal-normal interval
- PPG
photoplethysmography
- PSG
polysomnography
- RMSSD
root mean square of differences in successive NN intervals
- SpO2
oxygen saturation
oxygen uptake
APPENDIX
For supplemental Methods as well as tables, please see the online version of this paper.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
REFERENCES
- 1.Dagher L, Shi H, Zhao Y, Marrouche NF. Wearables in cardiology: here to stay. Heart Rhythm. 2020;17:889–895. [DOI] [PubMed] [Google Scholar]
- 2.Seneviratne MG, Connolly SB, Martin SS, Parakh K. Grains of sand to clinical pearls: realizing the potential of wearable data. Am J Med. 2023;136:136–142. [DOI] [PubMed] [Google Scholar]
- 3.Corday E. Historical vignette celebrating the 30th anniversary of diagnostic ambulatory electrocardiographic monitoring and data reduction systems. J Am Coll Cardiol. 1991;17:286–292. [DOI] [PubMed] [Google Scholar]
- 4.Del Mar B. The history of clinical Holter monitoring. Ann Noninvasive Electrocardiol. 2005;10:226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson BW, Low CA, Jacobson N, Aréan P, Torous J, Allen NB. Guidelines for wrist-worn consumer wearable assessment of heart rate in biobehavioral research. NP Digit Med. 2020;3:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Svennberg E, Tjong F, Goette A, et al. How to use digital devices to detect and manage arrhythmias: an EHRA practical guide. Europace. 2022;24:979–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.GILLINOV S, ETIWY M, WANG R, et al. Variable accuracy of wearable heart rate monitors during aerobic exercise. Med Sci Sports Exerc. 2017;49:1697–1703. [DOI] [PubMed] [Google Scholar]
- 8.Fuller D, Colwell E, Low J, et al. Reliability and validity of commercially available wearable devices for measuring steps, energy expenditure, and heart rate: systematic review. JMIR Mhealth Uhealth. 2020;8:e18694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bent B, Goldstein BA, Kibbe WA, Dunn JP. Investigating sources of inaccuracy in wearable optical heart rate sensors. NP Digit Med. 2020;3:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller DJ, Sargent C, Roach GD. A validation of six wearable devices for estimating sleep, heart rate and heart rate variability in healthy adults. Sensors (Basel). 2022;22:6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okutucu S, Karakulak UN, Aytemir K, Oto A. Heart rate recovery: a practical clinical indicator of abnormal cardiac autonomic function. Expert Rev Cardiovasc Ther. 2011;9:1417–1430. [DOI] [PubMed] [Google Scholar]
- 12.Peçanha T, Silva-Júnior ND, Forjaz CL. Heart rate recovery: autonomic determinants, methods of assessment and association with mortality and cardiovascular diseases. Clin Physiol Funct Imaging. 2014;34:327–339. [DOI] [PubMed] [Google Scholar]
- 13.Imai K, Sato H, Hori M, et al. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol. 1994;24:1529–1535. [DOI] [PubMed] [Google Scholar]
- 14.Daanen HAM, Lamberts RP, Kallen VL, Jin A, Van Meeteren NLU. A systematic review on heart-rate recovery to monitor changes in training status in athletes. Int J Sports Physiol Perform. 2012;7:251–260. [DOI] [PubMed] [Google Scholar]
- 15.Lamberts RP, Swart J, Capostagno B, Noakes TD, Lambert MI. Heart rate recovery as a guide to monitor fatigue and predict changes in performance parameters. Scand J Med Sci Sports. 2010;20:449–457. [DOI] [PubMed] [Google Scholar]
- 16.Carnethon MR, Sternfeld B, Liu K, et al. Correlates of heart rate recovery over 20 years in a healthy population sample. Med Sci Sports Exerc. 2012;44:273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lachman S, Terbraak MS, Limpens J, et al. The prognostic value of heart rate recovery in patients with coronary artery disease: a systematic review and meta-analysis. Am Heart J. 2018;199:163–169. [DOI] [PubMed] [Google Scholar]
- 18.Vicente-Campos D, López AM, Nuñez MJ, Chicharro JL. Heart rate recovery normality data recorded in response to a maximal exercise test in physically active men. Eur J Appl Physiol. 2014;114:1123–1128. [DOI] [PubMed] [Google Scholar]
- 19.Kleiger RE, Miller JP, Bigger JT, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256–262. [DOI] [PubMed] [Google Scholar]
- 20.Nolan J, Batin PD, Andrews R, et al. Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart). Circulation. 1998;98:1510–1516. [DOI] [PubMed] [Google Scholar]
- 21.Natarajan A, Pantelopoulos A, Emir-Farinas H, Natarajan P. Heart rate variability with photoplethysmography in 8 million individuals: a cross-sectional study. Lancet Digit Health. 2020;2:e650–e657. [DOI] [PubMed] [Google Scholar]
- 22.Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol. 1998;31:593–601. [DOI] [PubMed] [Google Scholar]
- 23.Castaldo R, Melillo P, Bracale U, Caserta M, Triassi M, Pecchia L. Acute mental stress assessment via short term HRV analysis in healthy adults: a systematic review with meta-analysis. Biomed Signal Process Control. 2015;18:370–377. [Google Scholar]
- 24.Malik M, Bigger JT, Camm AJ, et al. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 25.Singh N, Moneghetti KJ, Christle JW, Hadley D, Froelicher V, Plews D. Heart rate variability: an old metric with new meaning in the era of using mHealth technologies for health and exercise training guidance. Part two: prognosis and training. Arrhythm Electrophysiol Rev. 2018;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buchheit M. Monitoring training status with HR measures: do all roads lead to Rome? Front Physiol. 2014;5:73. 10.3389/fphys.2014.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baek HJ, Shin J. Effect of missing inter-beat interval data on heart rate variability analysis using wrist-worn wearables. J Med Syst. 2017;41:147. [DOI] [PubMed] [Google Scholar]
- 28.WHOOP. Everything you need to know about heart rate variability (HRV). Accessed April 19, 2023. https://www.whoop.com/thelocker/heart-rate-variability-hrv/
- 29.OURA. Heart rate variability. Accessed April 19, 2023. https://support.ouraring.com/hc/en-us/articles/360025441974-Heart-Rate-Variability#h_01GVP1BPFR8R2G6AYQE394ZVKH
- 30.Lu G, Yang F, Taylor JA, Stein JF. A comparison of photoplethysmography and ECG recording to analyse heart rate variability in healthy subjects. J Med Eng Technol. 2009;33:634–641. [DOI] [PubMed] [Google Scholar]
- 31.Bellenger CR, Miller DJ, Halson SL, Roach GD, Sargent C. Wrist-based photoplethysmography assessment of heart rate and heart rate variability: validation of WHOOP. Sensors (Basel). 2021;21:3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao R, Azimi I, Sarhaddi F, et al. Accuracy assessment of Oura ring nocturnal heart rate and heart rate variability in comparison with electrocardiography in time and frequency domains: comprehensive analysis. J Med Internet Res. 2022;24:e27487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernando D, Roca S, Sancho J, Alesanco Á, Bailón R. Validation of the Apple Watch for heart rate variability measurements during relax and mental stress in healthy subjects. Sensors (Basel). 2018;18:E2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez MV, Mahaffey KW, Hedlin H, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lubitz SA, Faranesh AZ, Selvaggi C, et al. Detection of atrial fibrillation in a large population using wearable devices: the Fitbit Heart Study. Circulation. 2022;146:1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo Y, Wang H, Zhang H, et al. Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol. 2019;74:2365–2375. [DOI] [PubMed] [Google Scholar]
- 37.Perino AC, Gummidipundi SE, Lee J, et al. Arrhythmias other than atrial fibrillation in those with an irregular pulse detected with a smartwatch: findings from the Apple Heart Study. Circ Arrhythm Electrophysiol. 2021;14:e010063. [DOI] [PubMed] [Google Scholar]
- 38.Ford C, Xie CX, Low A, et al. Comparison of 2 Smart Watch algorithms for detection of atrial fibrillation and the benefit of clinician interpretation: SMART WARS study. J Am Coll Cardiol EP. 2022;8:782–791. [DOI] [PubMed] [Google Scholar]
- 39.Mannhart D, Lischer M, Knecht S, et al. Clinical validation of 5 direct-to-consumer wearable smart devices to detect atrial fibrillation. J Am Coll Cardiol EP. 2023;9:232–242. [DOI] [PubMed] [Google Scholar]
- 40.Seshadri DR, Bittel B, Browsky D, et al. Accuracy of Apple Watch for detection of atrial fibrillation. Circulation. 2020;141:702–703. [DOI] [PubMed] [Google Scholar]
- 41.Jaakkola J, Jaakkola S, Lahdenoja O, et al. Mobile phone detection of atrial fibrillation with mechanocardiography. Circulation. 2018;137:1524–1527. [DOI] [PubMed] [Google Scholar]
- 42.Gill S, Bunting KV, Sartini C, et al. Smartphone detection of atrial fibrillation using photoplethysmography: a systematic review and meta-analysis. Heart. 2022;108:1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopez Perales CR, Van Spall HGC, Maeda S, et al. Mobile health applications for the detection of atrial fibrillation: a systematic review. Europace. 2021;23:11–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brandes A, Stavrakis S, Freedman B, et al. Consumer-led screening for atrial fibrillation: frontier review of the AF-SCREEN international collaboration. Circulation. 2022;146:1461–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kario K. Management of hypertension in the digital era: small wearable monitoring devices for remote blood pressure monitoring. Hypertension. 2020;76:640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sel K, Osman D, Huerta N, Edgar A, Pettigrew RI, Jafari R. Continuous cuffless blood pressure monitoring with a wearable ring bioimpedance device. NP Digit Med. 2023;6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Falter M, Scherrenberg M, Driesen K, et al. Smartwatch-based blood pressure measurement demonstrates insufficient accuracy. Front Cardiovasc Med. 2022;9:958212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuwabara M, Harada K, Hishiki Y, Kario K. Validation of two watch-type wearable blood pressure monitors according to the ANSI/AAMI/ISO81060-2:2013 guidelines: Omron HEM-6410T-ZM and HEM-6410T-ZL. J Clin Hypertens (Greenwich). 2019;21:853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moon JH, Kang M-K, Choi C-E, Min J, Lee H-Y, Lim S. Validation of a wearable cuff-less wristwatch-type blood pressure monitoring device. Sci Rep. 2020;10:19015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. J Am Coll Cardiol. 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 51.Ferguson T, Olds T, Curtis R, et al. Effectiveness of wearable activity trackers to increase physical activity and improve health: a systematic review of systematic reviews and meta-analyses. Lancet Digit Health. 2022;4:e615–e626. [DOI] [PubMed] [Google Scholar]
- 52.Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320:2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kraus WE, Janz KF, Powell KE, et al. Daily step counts for measuring physical activity exposure and its relation to health. Med Sci Sports Exerc. 2019;51:1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paluch AE, Bajpai S, Bassett DR, et al. Daily steps and all-cause mortality: a meta-analysis of 15 international cohorts. Lancet Public Health. 2022;7:e219–e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teixeira E, Fonseca H, Diniz-Sousa F, et al. Wearable devices for physical activity and healthcare monitoring in elderly people: a critical review. Geriatrics (Basel). 2021;6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jensen MT, Treskes RW, Caiani EG, et al. ESC working group on e-cardiology position paper: use of commercially available wearable technology for heart rate and activity tracking in primary and secondary cardiovascular prevention–in collaboration with the European Heart Rhythm Association, European Association of Preventive Cardiology, Association of Cardiovascular Nursing and Allied Professionals, Patient Forum, and the Digital Health Committee. Eur Heart J Digit Health. 2021;2:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Werhahn SM, Dathe H, Rottmann T, et al. Designing meaningful outcome parameters using mobile technology: a new mobile application for telemonitoring of patients with heart failure. ESC Heart Fail. 2019;6:516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bunn JA, Navalta JW, Fountaine CJ, Reece JD. Current state of commercial wearable technology in physical activity monitoring 2015-2017. Int J Exerc Sci. 2018;11:503–515. [PMC free article] [PubMed] [Google Scholar]
- 59.Toth LP, Park S, Springer CM, Feyerabend MD, Steeves JA, Bassett DR. Video-recorded validation of wearable step counters under free-living conditions. Med Sci Sports Exerc. 2018;50:1315–1322. [DOI] [PubMed] [Google Scholar]
- 60.Gilgen-Ammann R, Schweizer T, Wyss T. Accuracy of distance recordings in eight positioning-enabled sport watches: instrument validation study. JMIR Mhealth Uhealth. 2020;8:e17118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nielsen RO, Cederholm P, Buist I, Sørensen H, Lind M, Rasmussen S. Can GPS be used to detect deleterious progression in training volume among runners? J Strength Cond Res. 2013;27(6):1471–1478. 10.1519/JSC.0b013e3182711e3c [DOI] [PubMed] [Google Scholar]
- 62.Austin MA, Wills KE, Blizzard L, Walters EH, Wood-Baker R. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomized controlled trial. BMJ. 2010;341:c5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petek BJ, Gustus SK, Wasfy MM. Cardiopulmonary exercise testing in athletes: expect the unexpected. Curr Treat Options Cardiovasc Med. 2021;23:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Constantini K, Tanner DA, Gavin TP, Harms CA, Stager JM, Chapman RF. Prevalence of exercise-induced arterial hypoxemia in distance runners at sea level. Med Sci Sports Exerc. 2017;49:948–954. [DOI] [PubMed] [Google Scholar]
- 65.Chen S, Qi J, Fan S, Qiao Z, Yeo JC, Lim CT. Flexible wearable sensors for cardiovascular health monitoring. Adv Healthc Mater. 2021;10:2100116. [DOI] [PubMed] [Google Scholar]
- 66.U.S. Food and Drug Administration. Pulse oximeters - premarket notification submissions [510(k)s]: guidance for industry and food and drug administration staff. Accessed October 25, 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pulse-oximeters-premarket-notification-submissions-510ks-guidance-industry-and-food-and-drug
- 67.Lauterbach CJ, Romano PA, Greisler LA, Brindle RA, Ford KR, Kuennen MR. Accuracy and reliability of commercial wrist-worn pulse oximeter during normobaric hypoxia exposure under resting conditions. Res Q Exerc Sport. 2021;92:549–558. [DOI] [PubMed] [Google Scholar]
- 68.Pipek LZ, Nascimento RFV, Acencio MMP, Teixeira LR. Comparison of SpO2 and heart rate values on Apple Watch and conventional commercial oximeters devices in patients with lung disease. Sci Rep. 2021;11:18901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jung H, Kim D, Lee W, et al. Performance evaluation of a wrist-worn reflectance pulse oximeter during sleep. Sleep Health. 2022;8(5):420–428. 10.1016/j.sleh.2022.04.003 [DOI] [PubMed] [Google Scholar]
- 70.Hermand E, Coll C, Richalet J-P, Lhuissier FJ. Accuracy and reliability of pulse o2 saturation measured by a wrist-worn oximeter. Int J Sports Med. 2021;42:1268–1273. [DOI] [PubMed] [Google Scholar]
- 71.Schiefer LM, Treff G, Treff F, et al. Validity of peripheral oxygen saturation measurements with the Garmin Fēnix(®) 5X Plus wearable device at 4559 m. Sensors (Basel). 2021;21(19):6363. 10.3390/s21196363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Z, Khatami R. Can we trust the oxygen saturation measured by consumer smartwatches? Lancet Respir Med. 2022;10:e47–e48. [DOI] [PubMed] [Google Scholar]
- 73.Mochizuki K, Shintani R, Mori K, et al. Importance of respiratory rate for the prediction of clinical deterioration after emergency department discharge: a single-center, case-control study. Acute Med Surg. 2017;4:172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dias D, Paulo Silva Cunha J. Wearable health devices-vital sign monitoring, systems and technologies. Sensors (Basel). 2018;18(8):2414. 10.3390/s18082414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Charlton PH, Birrenkott DA, Bonnici T, et al. Breathing rate estimation from the electrocardiogram and photoplethysmogram: a review. IEEE Rev Biomed Eng. 2018;11:2–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cosoli G, Antognoli L, Scalise L. Indirect estimation of breathing rate through wearable devices. 2022. IEEE International Symposium on Medical Measurements and Applications (MeMeA). IEEE. 2022:1–6. [Google Scholar]
- 77.Berryhill S, Morton CJ, Dean A, et al. Effect of wearables on sleep in healthy individuals: a randomized crossover trial and validation study. J Clin Sleep Med. 2020;16:775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Natarajan A, Su H-W, Heneghan C, Blunt L, O'Connor C, Niehaus L. Measurement of respiratory rate using wearable devices and applications to COVID-19 detection. NP Digit Med. 2021;4:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guazzi M, Adams V, Conraads V, et al. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126:2261–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Molina-Garcia P, Notbohm HL, Schumann M, et al. Validity of estimating the maximal oxygen consumption by consumer wearables: a systematic review with meta-analysis and expert statement of the INTERLIVE Network. Sports Med. 2022;52:1577–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lloyd-Jones DM, Allen NB, Anderson CAM, et al. Life's essential 8: updating and enhancing the American Heart Association's Construct of Cardiovascular Health: A presidential advisory from the American Heart Association. Circulation. 2022;146:e18–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation's sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1:40–43. [DOI] [PubMed] [Google Scholar]
- 83.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smith MT, McCrae CS, Cheung J, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2018;14:1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Zambotti M, Cellini N, Menghini L, Sarlo M, Baker FC. Sensors capabilities, performance, and use of consumer sleep technology. Sleep Med Clin. 2020;15:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chinoy ED, Cuellar JA, Huwa KE, et al. Performance of seven consumer sleep-tracking devices compared with polysomnography. Sleep. 2020;44(5):zsaa291. 10.1093/sleep/zsaa291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wulterkens BM, Fonseca P, Hermans LWA, et al. It is all in the wrist: wearable sleep staging in a clinical population versus reference polysomnography. Nat Sci Sleep. 2021;13:885–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schutte-Rodin S, Deak MC, Khosla S, et al. Evaluating consumer and clinical sleep technologies: an American Academy of Sleep Medicine update. J Clin Sleep Med. 2021;17:2275–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fine J, Branan KL, Rodriguez AJ, et al. Sources of inaccuracy in photoplethysmography for continuous cardiovascular monitoring. Biosensors (Basel). 2021;11(4):126. 10.3390/bios11040126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Longmore SK, Lui GY, Naik G, Breen PP, Jalaludin B, Gargiulo GD. A comparison of reflective photoplethysmography for detection of heart rate, blood oxygen saturation, and respiration rate at various anatomical locations. Sensors (Basel). 2019;19:1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cosoli G, Antognoli L, Veroli V, Scalise L. Accuracy and precision of wearable devices for real-time monitoring of swimming athletes. Sensors (Basel). 2022;22:4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hansen D, Abreu A, Ambrosetti M, et al. Exercise intensity assessment and prescription in cardiovascular rehabilitation and beyond: why and how: a position statement from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur J Prev Cardiol. 2022;29:230–245. [DOI] [PubMed] [Google Scholar]
- 93.Liguori G Medicine ACoS. ACSM's Guidelines for Exercise Testing and Prescription. 11th ed. Lippincott Williams and Wilkins; 2020. [Google Scholar]
- 94.Rao P, Seshadri DR, Hsu JJ. Current and potential applications of wearables in sports cardiology. Curr Treat Options Cardiovasc Med. 2021;23(10):65. 10.1007/s11936-021-00942-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tikkanen E, Gustafsson S, Ingelsson E. Associations of fitness, physical activity, strength, and genetic risk with cardiovascular disease: longitudinal analyses in the UK Biobank Study. Circulation. 2018;137:2583–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Al-Alusi MA, Khurshid S, Wang X, et al. Trends in consumer wearable devices with cardiac sensors in a primary care cohort. Circ Cardiovasc Qual Outcomes. 2022;15:e008833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chandrasekaran R, Katthula V, Moustakas E. Patterns of use and key predictors for the use of wearable health care devices by us adults: insights from a national survey. J Med Internet Res. 2020;22:e22443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cho PJ, Yi J, Ho E, et al. Demographic imbalances resulting from the bring-your-own-device study design. JMIR Mhealth Uhealth. 2022;10:e29510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shi C, Goodall M, Dumville J, et al. The accuracy of pulse oximetry in measuring oxygen saturation by levels of skin pigmentation: a systematic review and meta-analysis. BMC Med. 2022;20:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kyriacou PA, Charlton PH, Al-Halawani R, Shelley KH. Inaccuracy of pulse oximetry with dark skin pigmentation: clinical implications and need for improvement. Br J Anaesth. 2023;130(1):e33–e36. 10.1016/j.bja.2022.03.011 [DOI] [PubMed] [Google Scholar]
- 101.Fawzy A, Wu TD, Wang K, et al. Racial and ethnic discrepancy in pulse oximetry and delayed identification of treatment eligibility among patients with COVID-19. JAMA Intern Med. 2022;182:730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kusumoto FM, Schoenfeld MH, Barrett C, et al. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(7):932–987. [DOI] [PubMed] [Google Scholar]
- 103.Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016;67(13):e27–e115. [DOI] [PubMed] [Google Scholar]
- 104.Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72(14):e91–e220. [DOI] [PubMed] [Google Scholar]
- 105.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–e76. [DOI] [PubMed] [Google Scholar]
- 106.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104–132. [DOI] [PubMed] [Google Scholar]
- 107.Charlton PH, Villarroel M, Salguiero F. Waveform analysis to estimate respiratory rate. secondary analysis of electronic health records. In: Secondary Analysis of Electronic Health Records. Cham: Springer International Publishing; 2016:377–390. [PubMed] [Google Scholar]
- 108.Tandon A, Nguyen HH, Avula S, et al. Wearable biosensors in congenital heart disease. JACC: Adv. 2023;2:100267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Leclercq C, Witt H, Hindricks G, et al. Wearables, telemedicine, and artificial intelligence in arrhythmias and heart failure: Proceedings of the European Society of Cardiology Cardiovascular Round Table. Europace. 2022;24:1372–1383. [DOI] [PubMed] [Google Scholar]
- 110.Beavers DL, Chung EH. Wearables in sports cardiology. Clin Sports Med. 2022;41:405–423. [DOI] [PubMed] [Google Scholar]
- 111.Manninger M, Zweiker D, Svennberg E, et al. Current perspectives on wearable rhythm recordings for clinical decision-making: the wEHRAbles 2 survey. Europace. 2021;23:1106–1113. [DOI] [PubMed] [Google Scholar]
- 112.Boriani G, Svennberg E, Guerra F, et al. Reimbursement practices for use of digital devices in atrial fibrillation and other arrhythmias: a European Heart Rhythm Association survey. Europace. 2022;24:1834–1843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.