Abstract
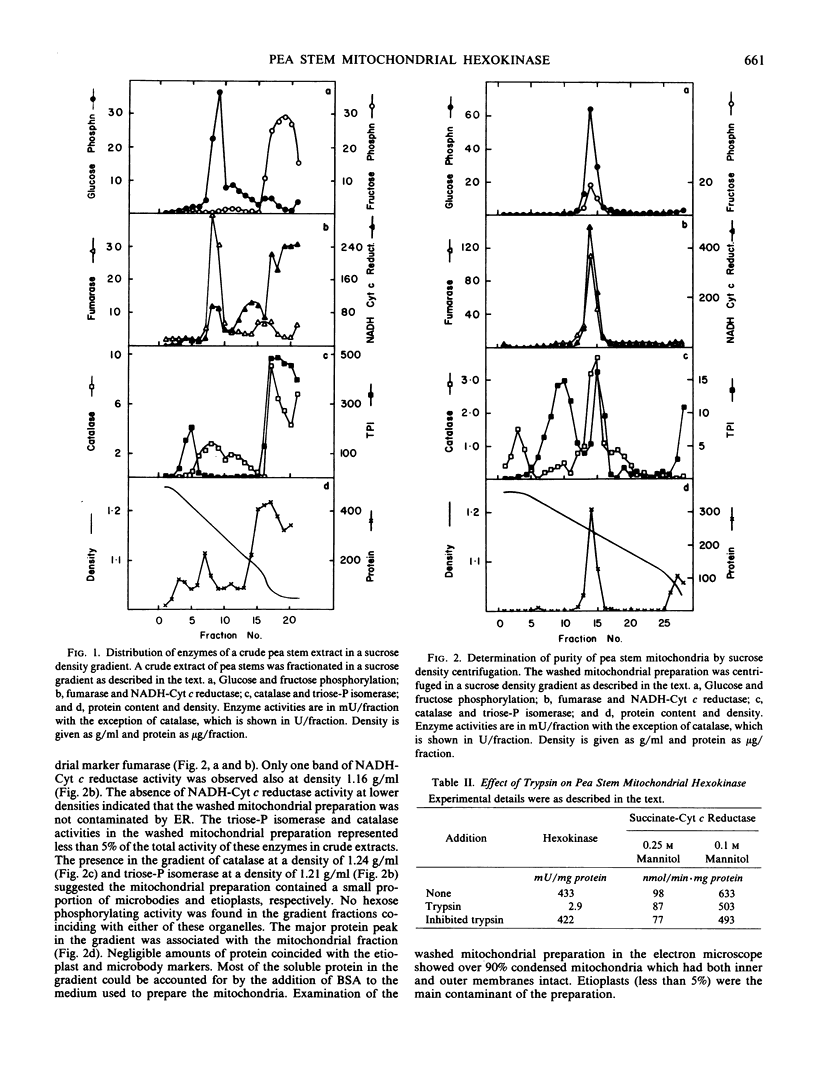
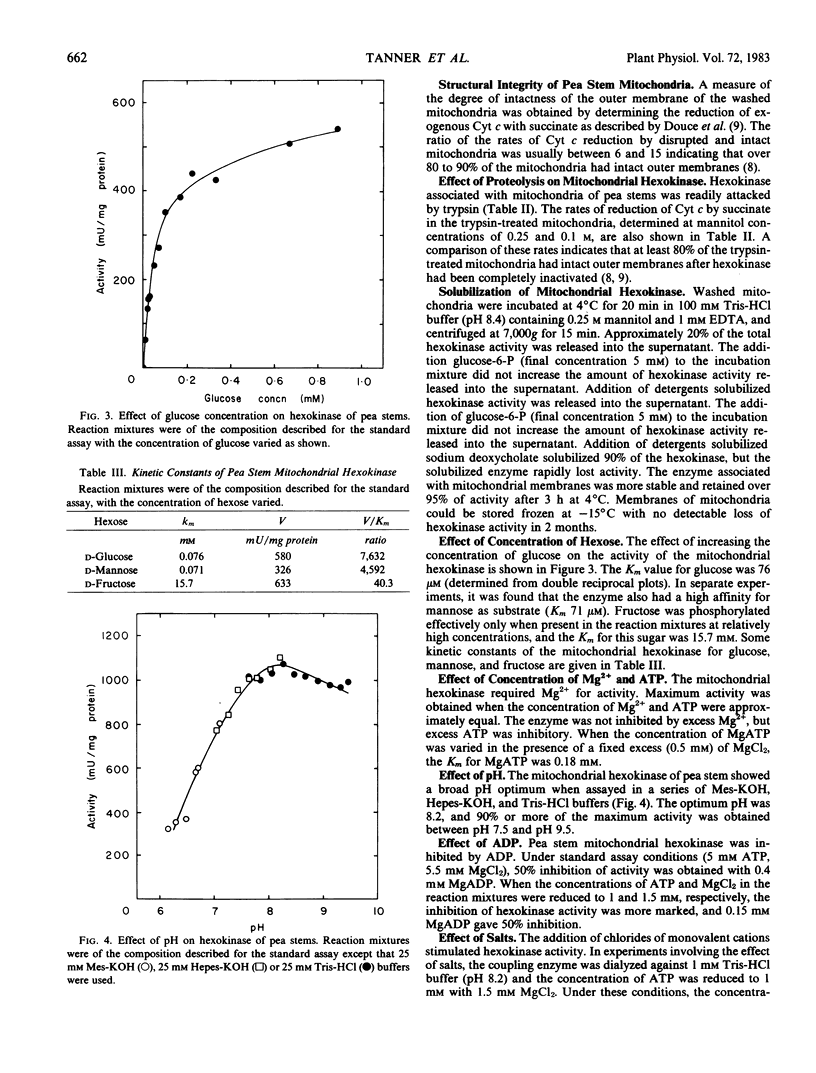
The subcellular localization of hexose phosphorylating activity in extracts of pea stems has been studied by differential centrifugation and sucrose density gradient centrifugation. The hexokinase (EC 2.7.1.1) was associated with the mitochondria, whereas fructokinase (EC 2.7.1.4) was in the cytosolic fraction. Some properties of the mitochondrial hexokinase were studied. The enzyme had a high affinity for glucose (Km 76 micromolar) and mannose (Km 71 micromolar) and a relatively low affinity for fructose (Km 15.7 millimolar). The Km for MgATP was 180 micromolar. The addition of salts stimulated the activity of the hexokinase. Al3+ was a strong inhibitor at pH 7 but not at the optimum pH (8.2). The enzyme was not readily solubilized but, in experiments with intact mitochondria, was susceptible to proteolysis. A location on the outer mitochondrial membrane is suggested for the hexokinase of pea stems.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Copeland L., Harrison D. D., Turner J. F. Fructokinase (Fraction III) of Pea Seeds. Plant Physiol. 1978 Aug;62(2):291–294. doi: 10.1104/pp.62.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven P. A., Goldblatt P. J., Basford R. E. Brain hexokinase. The preparation of inner and outer mitochondrial membranes. Biochemistry. 1969 Sep;8(9):3525–3532. doi: 10.1021/bi00837a007. [DOI] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. The oxidation of malate and exogenous reduced nicotinamide adenine dinucleotide by isolated plant mitochondria. Plant Physiol. 1974 Jan;53(1):104–109. doi: 10.1104/pp.53.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Christensen E. L., Bonner W. D., Jr Preparation of intaintact plant mitochondria. Biochim Biophys Acta. 1972 Aug 17;275(2):148–160. doi: 10.1016/0005-2728(72)90035-7. [DOI] [PubMed] [Google Scholar]
- Douce R., Mannella C. A., Bonner W. D., Jr The external NADH dehydrogenases of intact plant mitochondria. Biochim Biophys Acta. 1973 Jan 18;292(1):105–116. doi: 10.1016/0005-2728(73)90255-7. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Messer J. L., Wilson J. E. Purification of a hexokinase-binding protein from the outer mitochondrial membrane. J Biol Chem. 1979 Jun 25;254(12):4946–4949. [PubMed] [Google Scholar]
- Hernandez A., Crane R. K. Association of heart hexokinase with subcellular structure. Arch Biochem Biophys. 1966 Jan;113(1):223–229. doi: 10.1016/0003-9861(66)90176-7. [DOI] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Localization of enzymes within microbodies. J Cell Biol. 1973 Aug;58(2):379–389. doi: 10.1083/jcb.58.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzen H. M., Soderman D. D., Wiley C. E. Multiple forms of hexokinase. Activities associated with subcellular particulate and soluble fractions of normal and streptozotocin diabetic rat tissues. J Biol Chem. 1970 Aug 25;245(16):4081–4096. [PubMed] [Google Scholar]
- Knull H. R., Taylor W. F., Wells W. W. Insulin effects on brain energy metabolism and the related hexokinase distribution. J Biol Chem. 1974 Nov 10;249(21):6930–6935. [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purich D. L., Fromm H. J., Rudolph F. B. The hexokinases: kinetic, physical, and regulatory properties. Adv Enzymol Relat Areas Mol Biol. 1973;39:249–326. doi: 10.1002/9780470122846.ch4. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose I. A., Warms J. V. Mitochondrial hexokinase. Release, rebinding, and location. J Biol Chem. 1967 Apr 10;242(7):1635–1645. [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Isolation of Plastids from Sunflower Cotyledons during Germination. Plant Physiol. 1972 Jul;50(1):55–59. doi: 10.1104/pp.50.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Turner D. H., Blanch E. S., Gibbs M., Turner J. F. Triosephosphate Isomerase of Pea Seeds. Plant Physiol. 1965 Nov;40(6):1146–1150. doi: 10.1104/pp.40.6.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. F., Chensee Q. J., Harrison D. D. Glucokinase of pea seeds. Biochim Biophys Acta. 1977 Feb 9;480(2):367–375. doi: 10.1016/0005-2744(77)90029-8. [DOI] [PubMed] [Google Scholar]
- Turner J. F., Copeland L. Hexokinase II of Pea Seeds. Plant Physiol. 1981 Nov;68(5):1123–1127. doi: 10.1104/pp.68.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. F., Harrison D. D., Copeland L. Fructokinase (Fraction IV) of Pea Seeds. Plant Physiol. 1977 Nov;60(5):666–669. doi: 10.1104/pp.60.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]


