Abstract
Background
Vaccine-induced immune thrombocytopenia and thrombosis (VITT) is a new syndrome associated with the ChAdOx1 nCoV-19 adenoviral vector vaccine against severe acute respiratory syndrome coronavirus 2. Data are lacking on the clinical features of and the prognostic criteria for this disorder.
Methods
We conducted a prospective cohort study involving patients with suspected VITT who presented to hospitals in the United Kingdom between March 22 and June 6, 2021. Data were collected with the use of an anonymized electronic form, and cases were identified as definite or probable VITT according to prespecified criteria. Baseline characteristics and clinicopathological features of the patients, risk factors, treatment, and markers of poor prognosis were determined.
Results
Among 294 patients who were evaluated, we identified 170 definite and 50 probable cases of VITT. All the patients had received the first dose of ChAdOx1 nCoV-19 vaccine and presented 5 to 48 days (median, 14) after vaccination. The age range was 18 to 79 years (median, 48), with no sex preponderance and no identifiable medical risk factors. Overall mortality was 22%. The odds of death increased by a factor of 2.7 (95% confidence interval [CI], 1.4 to 5.2) among patients with cerebral venous sinus thrombosis, by a factor of 1.7 (95% CI, 1.3 to 2.3) for every 50% decrease in the baseline platelet count, by a factor of 1.2 (95% CI, 1.0 to 1.3) for every increase of 10,000 fibrinogen-equivalent units in the baseline d-dimer level, and by a factor of 1.7 (95% CI, 1.1 to 2.5) for every 50% decrease in the baseline fibrinogen level. Multivariate analysis identified the baseline platelet count and the presence of intracranial hemorrhage as being independently associated with death; the observed mortality was 73% among patients with platelet counts below 30,000 per cubic millimeter and intracranial hemorrhage.
Conclusions
The high mortality associated with VITT was highest among patients with a low platelet count and intracranial hemorrhage. Treatment remains uncertain, but identification of prognostic markers may help guide effective management. (Funded by the Oxford University Hospitals NHS Foundation Trust.)
As of June 6, 2021, approximately 175 million severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections and 3.8 million deaths from coronavirus disease 2019 (Covid-19) have been reported worldwide.1 Programs providing unprecedented rapid clinical development of vaccines have been established. A number of vaccines against SARS-CoV-2 have been approved, and many persons in the world have been vaccinated.2 One of the most widely administered vaccines is ChAdOx1 nCoV-19 (Oxford–AstraZeneca), which uses a nonreplicating chimpanzee adenovirus to deliver the spike antigen to the recipient.3,4 Rollout of the ChAdOx1 nCoV-19 vaccine started in the United Kingdom on January 4, 2021.5 The vaccine was first administered to older adults and then to younger age groups; by June 6, approximately 16 million first doses had been administered to persons 50 years of age or older and 8 million had been administered to those younger than 50 years of age. The latter population mainly comprised healthcare and social workers and vulnerable persons.
In March 2021, concerns developed regarding an increased risk of thrombosis associated with thrombocytopenia among persons who had received ChAdOx1 nCoV-19. By the end of the month, groups from Norway, Germany, Austria, and the United Kingdom reported on persons admitted to the hospital 5 to 24 days after vaccination with ChAdOx1 nCoV-19. These patients had thrombosis at unusual sites, thrombocytopenia, disproportionately elevated d-dimer levels, and reduced fibrinogen levels.6-8 Most of these patients were previously fit, healthy young persons.
This reported syndrome has been given various names, but we have used the term vaccine-induced immune thrombocytopenia and thrombosis (VITT) to acknowledge its pathogenic similarities with heparin-induced thrombocytopenia (HIT), even though VITT occurs in the absence of heparin.9 The clinical and laboratory resemblances to HIT are supported by the finding in the patients’ serum of high-titer antibodies to platelet factor 4 (PF4) that activate platelets.6-8 Because of this unusual and severe thrombotic syndrome and the likely importance of the early population rollout of the ChAdOx1 nCoV-19 vaccine, we convened an expert hematology panel to evaluate patients as they presented, discuss treatment, and develop consensus management guidance based on observations of patients and extrapolation of knowledge of other forms of immune-mediated thrombocytopenia and thrombosis.10 Here, we describe the first 220 cases of definite or probable VITT reported in the United Kingdom.
Methods
Study Design and Oversight
Patient information was gained from the daily meetings of the expert hematology panel, which began on March 22, 2021. The wider hematology community was alerted to the existence of the expert hematology panel by the British Society for Haematology and was encouraged to report suspected cases of VITT. A total of 182 consultant hematologists presented cases from 96 National Health Service trusts. An anonymized electronic reporting form, developed with Public Health England, was completed for each patient by the local attending team. This form captured the presenting features, thrombosis site, laboratory measures, initial treatment, and outcome. All cases were simultaneously reported to the Medicines and Healthcare Products Regulatory Agency.11 Cases that were retrospectively identified as having occurred before recognition of VITT in mid-March were also included.
The study was designed by the core group of the expert hematology panel. The article was written by the first author with input from the core group of the expert hematology panel, and all the authors contributed to the editing of the manuscript. The cases described here were reported up to June 6, 2021.
Case Definition
Cases of VITT were defined according to five criteria as follows: the onset of symptoms 5 to 30 days after vaccination against SARS-CoV-2, the presence of thrombosis, thrombocytopenia (platelet count <150,000 per cubic millimeter), a d-dimer level greater than 4000 fibrinogen-equivalent units (FEUs), and the presence of antibodies to PF4 detected by means of enzyme-linked immunosorbent assay (ELISA). Persons who met all five criteria were considered to have definite VITT. Persons who did not meet all five criteria were judged to have probable, possible, or unlikely VITT according to the classification shown in Table 1. The absence of a criterion could have been a true absence or the test may not have been carried out (e.g., anti-PF4 antibodies may have been measured before VITT was recognized as a syndrome).
Table 1. Case Definition Criteria for Vaccine-Induced Immune Thrombocytopenia and Thrombosis (VITT), According to an Expert Hematology Panel.*.
| Type of VITT | Description |
| Definite VITT |
|
| Probable VITT | d-dimer level >4000 FEU but one criterion not met (timing, thrombosis, thrombocytopenia, or anti-PF4 antibodies) or d-dimer level unknown or 2000–4000 FEU and all other criteria met |
| Possible VITT | d-dimer level unknown or 2000–4000 FEU with one other criterion not met, or two other criteria not met (timing, thrombosis, thrombocytopenia, or anti-PF4 antibodies) |
| Unlikely VITT | Platelet count <150,000 per cubic millimeter without thrombosis with d-dimer level <2000 FEU, or thrombosis with platelet count >150,000 per cubic millimeter and d-dimer level <2000 FEU, regardless of anti-PF4 antibody result, and alternative diagnosis more likely |
ELISA denotes enzyme-linked immunosorbent assay, FEU fibrinogen-equivalent unit, PF4 platelet factor 4, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
Confirmation of the type of vaccine against SARS-CoV-2 received and whether it was the first or second dose was obtained from primary care practitioners or public health bodies. All cases of suspected VITT were independently categorized by two expert hematology panel members; if there was discrepancy, the case was scored by a third expert hematology panel member and final categorization was made by consensus. Our analysis involved patients who were classified as having definite or probable VITT.
Laboratory Tests
Standard tests that were performed in local laboratories included the platelet count, prothrombin time, activated partial-thromboplastin time, fibrinogen level measured by the Clauss method, and d-dimer levels. Normal ranges for coagulation tests were determined at the local laboratories or with the thresholds used by the manufacturers of the reagents. Results for d-dimer levels are reported in FEUs; the normal level is less than 500 FEU (equivalent to <250 ng per milliliter). Many laboratories do not quantitate d-dimer levels above 20,000 FEU; therefore, 20,000 FEU was recorded when the result was above this level.
In 17 specialist laboratories in the United Kingdom, anti-PF4 antibodies were detected by a HIT ELISA method with the use of Lifecodes PF4 (Immucor), Asserachrom HPIA (Stago), Zymutest HIA (Hyphen BioMed), or Aeskulisa (Aesku Diagnostics) monospecific (IgG) or polyspecific (IgG, IgA, IgM) assays. The positive thresholds were based on the manufacturers’ optical density thresholds or on locally derived normal ranges. Chemiluminescence assays with high sensitivity for HIT were found to be unreliable in detecting anti-PF4 antibodies in patients with VITT; therefore, the results of these assays were not used in this analysis.
Radiologic Imaging
Radiologic imaging was performed at the discretion of the local clinical teams. For patients who presented with headaches, computed tomographic imaging or magnetic resonance venography was recommended.
Statistical Analysis
The outcome variables studied were the number of days from vaccination to presentation; the site of thrombosis, including arterial thrombosis and thrombosis at multiple sites; intracranial hemorrhage; adrenal thrombosis, hemorrhage, or both; and death. Variables measured against these outcomes were sex, days since vaccination, platelet count, fibrinogen level, d-dimer level, the presence of anti-PF4 antibodies, cerebral venous sinus thrombosis, and intracranial hemorrhage.
Variables are described as numbers and percentages or as medians, ranges, and interquartile ranges, as appropriate. A multivariable logistic regression model was used to investigate the association between the variables and mortality. All numerical variables, except age and d-dimer level, were log-transformed for analysis to remove positive skewness. We used chained equations to impute missing values in the predictor variables with multiple imputation. Ten data sets were created with the use of an imputation model that included all the predictor variables and the outcome. Because of the small sample size, only variables with a univariate P value of less than 0.20 were entered into this model. A backward elimination algorithm was then applied with the use of a P value of less than 0.05.
Results
Patient Identification
Of 294 patients who were evaluated, 31 had been identified retrospectively once VITT had been recognized as a syndrome. A total of 57 patients were classified as being unlikely to have VITT because of three missing data points, unmet criteria, or both (50 patients) or because an alternative cause of the patient’s symptoms was likely (7 patients). The alternative causes were chronic disseminated intravascular coagulation from abdominal aortic aneurysm (in 3 patients) and metastatic cancer (in 4 patients). Of the remaining 237 patients who were considered to have suspected VITT, 17 were classified as having possible VITT; 50 were classified as having probable VITT because one of the criteria was not met (in most cases because of missing data) (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org); and 170 were classified as having definite VITT. All the patients who were classified as having definite or probable VITT presented after the first vaccination with ChAdOx1 nCoV-19 (Fig. S2).
By the end of the study, approximately 16 million first doses of ChAdOx1 nCoV-19 had been administered to persons 50 years of age or older, and 8 million first doses had been administered to persons younger than 50 years of age. Thus, the approximate incidence of VITT was at least 1:100,000 among patients 50 years of age or older and at least 1:50,000 among patients in the younger group (<50 years of age).11
Baseline Characteristics of the Patients
The baseline characteristics and clinicopathological features of the 220 patients with definite or probable VITT are shown in Table 2. A total of 97% presented at 5 to 30 days after vaccination; the 3% of patients who presented between 30 and 48 days had isolated venous thromboembolism (deep-vein thrombosis or pulmonary embolism). The age at presentation ranged from 18 to 79 years, with a median of 48 years (interquartile range, 38 to 56). A total of 56% of the patients were younger than 50 years of age, and 85% were younger than 60 years of age. There were slightly more women (54%) than men. The median time from vaccination to presentation was 14 days (interquartile range, 10 to 16). Data on the patients’ age, days since vaccination, and baseline platelet count and fibrinogen and d-dimer levels are shown in Figure 1.
Table 2. Baseline Clinicopathological Features of the Patients with VITT.*.
| Variable | Value |
|---|---|
| Age — yr | |
| Median (IQR) | 48 (38–56) |
| Range | 18–79 |
| Sex — no./total no. (%) | |
| Female | 119/217 (55) |
| Male | 98/217 (45) |
| Race — no./total no. (%) | |
| White | 90/99 (91) |
| Asian | 9/99 (9) |
| Days since vaccination with ChAdOx1 nCoV-19 | |
| Median (IQR) | 14 (10–16) |
| Range | 5–48 |
| Platelet count — per mm3 | |
| Median (IQR) | 47,000 (28,000–76,000) |
| Range | 6,000–344,000 |
| Prothrombin time — sec | |
| Median (IQR) | 13 (10–14) |
| Range | 10–30 |
| Activated partial-thromboplastin time — sec | |
| Median (IQR) | 29 (22–30) |
| Range | 20–57 |
| Fibrinogen level — g/liter | |
| Median (IQR) | 2.2 (1.2–3.1) |
| Range | 0.3–4.4 |
| d-dimer level — FEU | |
| Median (IQR) | 24,000 (8,000–37,000) |
| Range | 5,000–80,000 |
Shown are the numbers of patients in each category for whom data were known. Data on age and the platelet count were available for 218 patients, data on days since vaccination were available for 219 patients, data on the prothrombin time and activated partial-thromboplastin time were available for 185 patients, and data on the fibrinogen and d-dimer levels were available for 202 patients. IQR denotes interquartile range.
Figure 1. Baseline Distribution of Variables in Patients with Vaccine-Induced Immune Thrombocytopenia and Thrombosis (VITT).
Shown are the variables in patients with VITT, including those who did not survive.
Medical History
Information about medical history was available for 165 of the 220 patients; 68 of these patients (41%) had no previous medical diagnoses. Of the 97 patients (59%) reported to have a past or current medical illness, no diagnoses or use of medications occurred at a frequency that would be unexpected in the general population. Conditions that were specifically examined were autoimmune disease, previous venous thromboembolism, prothrombotic disorders (including thrombophilia and antiphospholipid syndrome), cancer, and previous SARS-CoV-2 infection. Arterial risk factors and the use of hormonal preparations and anticoagulants were also assessed (Table S2). None of the patients had been exposed to heparin in the 3 months preceding their presentation with VITT, and 104 of the patients were not receiving any medication. In the 7 patients who were receiving oral anticoagulation therapy when they presented with VITT, the median d-dimer level was not significantly lower than that documented in the patients with definite VITT (23,850 FEU) or those with probable VITT (20,000 FEU).
Thrombocytopenia
The median baseline platelet count was 47,000 per cubic millimeter (range, 6,000 to 344,000). Eleven of the 220 patients (5%) presented with a normal platelet count (median, 175,000 per cubic millimeter; range, 153,000 to 344,000). All had positive anti-PF4 antibodies; in 9 of these patients, the platelet count subsequently decreased below the lower limit of the normal range (i.e., <150,000 per cubic millimeter). In 2 patients (<1%) the only available platelet counts were those measured at presentation; in 1 patient who presented with middle cerebral artery infarct, the platelet count was 153,000 per cubic millimeter, and in a patient with cerebral venous sinus thrombosis, the platelet count was 173,000 per cubic millimeter. Among 217 patients for whom data on platelet counts were available, the baseline platelet count was less than 30,000 per cubic millimeter at presentation in 56 patients (26%) and the nadir platelet count was less than 30,000 per cubic millimeter in 73 patients (34%).
In 6 patients, thrombocytopenia and all other criteria were met but thrombosis was not documented. Although these patients were classified as having probable, rather than definite, VITT, it is likely that the lack of thrombosis reflected the early recognition and treatment of VITT.
Anti-PF4 Antibodies
Anti-PF4 antibodies were present in 198 of 220 patients (90.0%) with definite or probable VITT. ELISA was not performed in 16 of 220 patients (7.3%) because they died before anti-PF4 antibody testing or had VITT that was diagnosed retrospectively before the recognition of involvement of anti-PF4 antibodies. The result was negative in 6 patients (2.7%). These patients met all other criteria for the diagnosis of VITT, including profound coagulation activation, and they received treatment for VITT. Optical densities were not correlated with disease severity (thrombosis at presentation or death) because different ELISA methods were used.
Thrombosis and Thromboembolism
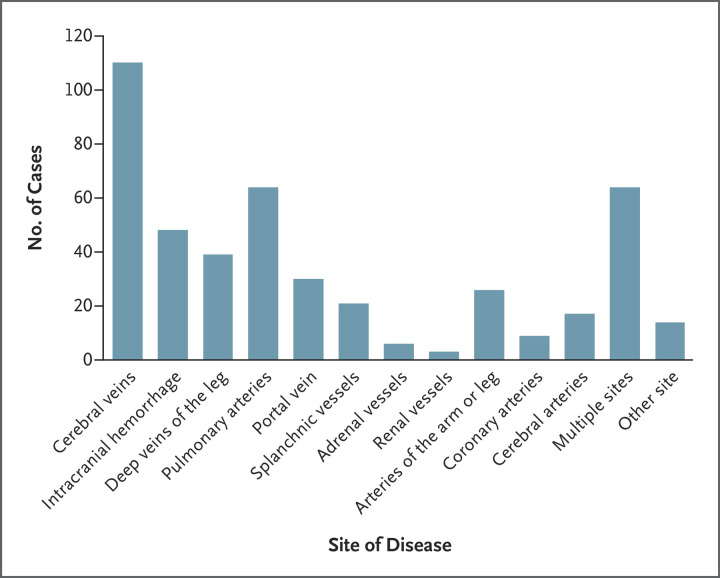
The most common thrombotic site at presentation was the cerebral veins; this was at least one site of thrombosis in 110 of the 220 patients (50%) with definite or probable VITT. In 40 of these patients (36%), cerebral venous sinus thrombosis was complicated by secondary intracranial hemorrhage; in 2 patients, intracranial hemorrhage was reported in association with a cerebrovascular accident. Intracranial hemorrhage was more common in patients with lower platelet counts (median, 34,000 per cubic millimeter) than in those with higher platelet counts (median, 50,000 per cubic millimeter). The deep veins of the legs and the pulmonary arteries were the second most common sites and were affected in 82 of the 220 patients (37%) (40 cases of deep-vein thrombosis and 63 cases of pulmonary embolism; 21 patients had both). A total of 41 patients (19%) had splanchnic-vein thrombosis; this thrombosis most often affected the portal circulation (30 cases of portal-vein thrombosis and 22 cases of other splanchnic-vein thrombosis; 11 patients had both). Forty-seven patients (21%) had one or more arterial thrombotic events; aortic thrombosis or ischemic limb was seen in 26 patients (12%) and cardiac or cerebral arterial events were seen in 26 patients (12%), with 9 myocardial infarctions and 17 cerebrovascular accidents. In 64 of the 220 patients (29%) with definite or probable VITT, two or more vascular beds were involved (Table S3). The number of events according to disease site is shown in Figure 2. Thirty-one patients had isolated venous thromboembolism (all included pulmonary embolism except in 1 patient who had extensive superficial and deep-vein thromboses). No significant differences were noted among patients who presented with cerebral venous sinus thrombosis, those who presented with isolated venous thrombolism, and those who presented with arterial thrombosis with respect to age, platelet count (median and range), d-dimer level, fibrinogen level, or days since vaccination (Table S4).
Figure 2. Sites of Disease in 220 Patients with Definite or Probable VITT.
Sites of disease (thrombosis or bleeding) that are not shown include the bowel and the pelvic, retinal, ophthalmic, and internal jugular veins.
Treatment
The forms of treatment used in patients classified as having definite or probable VITT are shown in Table S6. Intravenous immune globulin was used in 72% of the patients (at all platelet count levels) at a dose of 1 g per kilogram of body weight administered on day 1 of admission. In 11% of the patients, a second dose for ongoing or relapsed disease was administered.
A total of 17 patients with severe disease involving cerebral venous sinus thrombosis, thrombosis at multiple sites, or both underwent plasma exchange; this therapy was associated with 90% survival. Both patients who underwent plasma exchange and died had extensive intracranial hemorrhage at presentation. Octaplas (Octapharma) solvent detergent plasma was used; no allergic reactions, bleeding, hemodynamic compromise, or other complications were seen.
Systemic glucocorticoids were used in 26% of the patients and in 50% of those with a platelet count below 30,000 per cubic millimeter. Preparations included intravenous methylprednisolone, oral or intravenous dexamethasone, and oral prednisolone. Patients with a baseline platelet count of less than 30,000 per cubic millimeter were three times more likely to receive a platelet transfusion, typically in preparation for neurosurgery or for anticoagulation. In 15% of the patients with extensive cerebral venous sinus thrombosis with or without secondary intracranial hemorrhage, neurosurgery or thrombectomy was performed by interventional radiologists.
Anticoagulation was administered in 91% of the patients; non–heparin-based anticoagulation, including argatroban, fondaparinux, apixaban, and dabigatran, was used in 68%. Heparin was administered at some point during admission in 50 patients (23%). Its use was universal in the patients who presented before mid-March and received a diagnosis retrospectively, and it was administered to other patients before the diagnosis was considered and the anticoagulant was switched. Mortality among these patients was 20%, as compared with 16% among those receiving non–heparin-based anticoagulants.
Death
At the time of the data analysis, 170 of 220 patients with definite or probable VITT were alive and 49 had died (22%). Outcome survival data were unavailable for 1 patient. Clinical and laboratory features with respect to patient outcomes are shown in Table 3 and Table S5. In these 220 patients, the odds of death increased by a factor of 2.7 (95% CI, 1.4 to 5.2) among those with cerebral venous sinus thrombosis, by a factor of 1.7 (95% CI, 1.3 to 2.3) for every 50% decrease in the baseline platelet count, by a factor of 1.2 (95% CI, 1.0 to 1.3) for every increase of 10,000 FEU in the baseline d-dimer level, and by a factor of 1.7 (95% CI, 1.1 to 2.5) for every 50% decrease in the baseline fibrinogen level. Multivariate analysis identified the baseline platelet count and the presence of intracranial hemorrhage as being independently associated with death; the observed incidence of death was 73% among patients with both a platelet count below 30,000 per cubic millimeter and an intracranial hemorrhage.
Table 3. Clinical and Laboratory Features with Respect to Outcomes in Patients with VITT.*.
| Variable | Patients Who Were Alive (N=170) |
Patients Who Died (N=49) |
|---|---|---|
| Age — yr (range) | 48 (40–55) | 47 (32–56) |
| Sex — no./total no. (%) | ||
| Male | 79/97 (81) | 18/97 (19) |
| Female | 90/120 (75) | 30/120 (25) |
| Median days since vaccination (IQR) | 14 (11–17) | 13 (10–16) |
| Cerebral venous sinus thrombosis — no./total no. (%) | ||
| No | 100/117 (85) | 17/117 (15) |
| Yes | 70/102 (69) | 32/102 (31) |
| Intracranial hemorrhage — no./total no. (%) | ||
| No | 145/172 (84) | 27/172 (16) |
| Yes | 25/47 (53) | 22/47 (47) |
| Platelet count <30,000 per mm3 — no./total no. (%) | ||
| No | 135/161 (84) | 26/161 (16) |
| Yes | 33/56 (59) | 23/56 (41) |
| Median platelet count (IQR) — per mm3 | 52,000 (34,000–80,000) | 30,000 (16,000–57,000) |
| Median fibrinogen level (IQR) — g/liter | 2.3 (1.5–3.2) | 1.7 (1.1–2.9) |
| Median d-dimer level (IQR) — FEU | 20,000 (7,000–36,000) | 28,000 (13,000–57,000) |
| PF4 — no./total no. (%) | ||
| Negative | 5/6 (83) | 1/6 (17) |
| Positive | 159/198 (80) | 38/198 (19) |
| Not performed | 6/16 (38) | 10/16 (62) |
Shown are the numbers of patients in each category for whom data were known. Data on age and the platelet count were available for 217 patients (167 who were alive, 49 who had died, and 1 with an unknown outcome), data on days since vaccination were available for 218 patients (168 who were alive, 49 who had died, and 1 with an unknown outcome), and data on the fibrinogen and d-dimer levels were available for 202 patients (160 who were alive, 41 who had died, and 1 with an unknown outcome).
Discussion
Against the backdrop of a successful vaccination program in the United Kingdom, VITT has emerged as a rare but devastating complication. We have found that it often affects young, otherwise healthy vaccine recipients and that it is associated with a high mortality. Our study involving the first 220 patients with VITT reported in the United Kingdom shows that the condition usually manifests 5 to 30 days after the first vaccination with ChAdOx1 nCoV-19, as was also noted in a study performed in Scotland.12 In our cohort, 85% of the patients were younger than 60 years of age, despite the predominance of ChAdOx1 nCoV-19 vaccination in older adults.
The incidence of VITT among persons younger than 50 years of age was 1:50,000, consistent with reports from other countries. However, the incidence could have been higher, because the patients probably presented before the condition was recognized, other cases may not have been reported to us, and some cases may have been misclassified. Data are lacking on the numbers of persons vaccinated per age decade among those younger than 50 years of age, and therefore we cannot determine age-specific incidences. No sex preponderance was noted, and no medical condition was seen at a frequency higher than would be expected for the background population, even though the vaccination program in the United Kingdom prioritized persons with underlying medical conditions.
The extensive nature of the thrombotic events was remarkable, and they often simultaneously involved multiple vascular beds and both the venous and arterial circulations. Although cerebral venous thromboses dominated the clinical presentation, deep-vein thrombosis and pulmonary embolism, portal- and splanchnic-vein thrombosis, and arterial events affecting the peripheral vasculature and the myocardial and cerebral arteries were also common. Cerebral venous sinus thrombosis often involved many of the venous sinuses (Fig. S3), with thrombi extending into the venules. Outflow obstruction with increased venous pressure in patients with thrombocytopenia was probably the dominant explanation for secondary hemorrhage. In some patients, craniectomy or interventional radiology for thrombectomy to improve cerebral venous drainage was warranted.
Laboratory evidence of more severe coagulation activation with lower platelet counts, a low fibrinogen level, and higher d-dimer levels were all associated with worse outcomes. The risk of death was particularly high among patients who presented with a platelet count below 30,000 per cubic millimeter, cerebral venous sinus thrombosis, and intracranial hemorrhage. Our overall case–fatality rate was 23%, which compares favorably with descriptions of earlier, smaller cohorts with a case–fatality rate of 50%.6,7
No therapeutic pathways have shown efficacy for the treatment of VITT. The rationale for the use of intravenous immune globulin is based on preventing antigen–antibody complexes from activating platelets through the Fcγ II receptor; this rationale is extrapolated from approaches to spontaneous HIT. A comparison of the efficacy of anticoagulants was not possible in our series because of the small numbers of patients and nonrandom assignment; however, heparin did not appear to be harmful in patients who received it.
The analysis of our cohort has led us to tailor our therapeutic approach to the various clinical phenotypes. For example, the high mortality among patients with intracranial hemorrhage and a platelet count below 30,000 per cubic millimeter has led us to use plasma exchange in those with these low platelet counts and extensive thrombosis or cerebral venous sinus thrombosis (Fig. S4). Survival after plasma exchange was 90%, considerably better than would be predicted given the baseline characteristics.
The constellation of clinical and laboratory features in VITT appears to stem from the production of anti-PF4 antibodies in response to vaccine components, although it remains unclear which components are involved.13 We used the HIT ELISA to detect the anti-PF4 antibodies after previously finding that although these assays are positive in most cases of VITT, the rapid non-ELISA tests are often negative.14 However, 6 patients with probable VITT tested negative for anti-PF4 antibodies. Our recommendation is to repeat testing with an alternative ELISA method,14 but it is unclear whether these patients have antibodies to an epitope that is not recognized by the currently available assays. A study involving Norwegian health care workers detected anti-PF4 antibodies in 6 of 492 patients (1.2%) after vaccination with ChAdOx1 nCoV-19; all had normal platelet counts, but studies are lacking to determine whether this is a risk factor for VITT.15
A potential weakness of our study is case-ascertainment bias. VITT is a new syndrome, and without a full understanding of the pathophysiology, including why certain vascular beds are more susceptible, and with the real incidence of PF4 antibodies after vaccination in the healthy population unknown, cases may be included that are not true VITT or else they may be missed if they do not meet immediate criteria such as platelet count on admission. Furthermore, since recognition of VITT occurred only in mid-March, when the vaccine rollout reached the younger age groups, it is possible that before this time, older patients with thrombosis, sudden death, or both may have had VITT and may have been missed.
We have defined the clinical and laboratory phenotypes of VITT in a large cohort of patients in the United Kingdom. We did not find any individual risk factors for VITT, but we found increased mortality among patients who presented with severe thrombocytopenia, cerebral venous sinus thrombosis, intracranial hemorrhage, laboratory markers of severe coagulation activation, or all these variables. In our cohort, the cases of VITT were associated only with the first ChAdOx1 nCoV-19 vaccination, and VITT primarily affected patients younger than 60 years of age. Multiple-site thromboses and arterial events were common. Along with anticoagulation, intravenous immune globulin has been the mainstay of therapy; anecdotally, patients with more severe disease may have benefitted from plasma exchange.
Acknowledgments
We thank all our clinical, laboratory, and public health colleagues, in particular the many clinical hematologists who have attended our daily meetings, presented cases, and persevered with data collection; Public Health England, especially Dr. Mary Ramsay; the Medicines and Healthcare Products Regulatory Agency; and Thrombosis UK, whose staff provided psychological support and help to patients.
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This article was published on August 11, 2021, and updated on August 12, 2021, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by the Oxford University Hospitals NHS Foundation Trust.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Worldometer. Covid-19 coronavirus pandemic. July 8, 2021. (https://www.worldometers.info/coronavirus/).
- 2.Mathieu E, Ritchie H, Ortiz-Ospina E, et al. A global database of COVID-19 vaccinations. Nat Hum Behav 2021. May 10 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 3.Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021;396:1979-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gov.UK. First people to receive Oxford University/AstraZeneca COVID-19 vaccine today. Press release. January 7, 2021. (https://www.gov.uk/government/news/first-people-to-receive-oxford-universityastrazeneca-covid-19-vaccine-today-4-january-2021).
- 6.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2124-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021;384:2092-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2202-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makris M, Pavord S, Lester W, Scully M, Hunt B. Vaccine-induced Immune thrombocytopenia and thrombosis (VITT). Res Pract Thromb Haemost 2021;5(5):e12529-e12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavord S, Lester W, Makris M, Scully M, Hunt B. Guidance produced by the Expert Haematology Panel (EHP) focussed on vaccine induced thrombosis and thrombocytopenia (VITT). British Society for Haematology. May 28, 2021. (https://b-s-h.org.uk/about-us/news/guidance-produced-by-the-expert-haematology-panel-ehp-focussed-on-vaccine-induced-thrombosis-and-thrombocytopenia-vitt/).
- 11.Medicines & Healthcare products Regulatory Agency. Coronavirus vaccine — weekly summary of yellow card reporting. 2021. (https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting).
- 12.Simpson CR, Shi T, Vasileiou E, et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med 2021. June 9 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greinacher A, Selleng K, Wesche J, et al. Towards Understanding ChAdOx1 nCov-19 Vaccine-induced Immune Thrombotic Thrombocytopenia (VITT). April 20, 2021. ( 10.21203/rs.3.rs-440461/v1). preprint. [DOI] [PMC free article] [PubMed]
- 14.Platton S, Bartlett A, MacCallum P, et al. Evaluation of laboratory assays for anti-platelet factor 4 antibodies after ChAdOx1 nCOV-19 vaccination. J Thromb Haemost 2021. May 10 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sørvoll IH, Horvei KD, Ernstsen SL, et al. An observational study to identify the prevalence of thrombocytopenia and anti-PF4/polyanion antibodies in Norwegian health care workers after COVID-19 vaccination. J Thromb Haemost 2021;19:1813-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.