Abstract
Abnormal endocardial cushion formation is a major cause of congenital heart valve disease, which is a common birth defect with significant morbidity and mortality. Although -catenin and BMP2 are two well-known regulators of endocardial cushion formation, their interaction in this process is largely unknown. Here, we report that deletion of -catenin in myocardium results in formation of hypoplastic endocardial cushions accompanying a decrease of mesenchymal cell proliferation. Loss of -catenin reduced Bmp2 expression in myocardium and SMAD signaling in cushion mesenchyme. Exogenous BMP2 recombinant proteins fully rescued the proliferation defect of mesenchymal cells in cultured heart explants from myocardial -catenin knockout embryos. Using a canonical WNT signaling reporter mouse line, we showed that cushion myocardium exhibited high WNT/-catenin activities during endocardial cushion growth. Selective disruption of the signaling function of -catenin resulted in a cushion growth defect similar to that caused by the complete loss of -catenin. Together, these observations demonstrate that myocardial -catenin signaling function promotes mesenchymal cell proliferation and endocardial cushion expansion through inducing BMP signaling.
Keywords: Endocardial cushion formation, Congenital heart valve disease, β-Catenin, BMP
1. Introduction
Heart valves control the unidirectional blood flow in the heart and are essential for normal cardiac function [1–4]. In mice, heart valve development begins with endocardial to mesenchymal transformation (EMT) to generate endocardial cushions within atrioventricular canal (AVC) and outflow tract (OFT) between embryonic (E) 9.5 and E10.5 [2,3,5–7]. From E11.5, these cellularized cushions undergo complex remodeling by proliferation and apoptosis of mesenchymal cells, as well as by deposition and organization of extracellular matrix, to form thin heart valve leaflets at birth [8–11]. Formation of endocardial cushions by EMT and remodeling is tightly regulated by regional molecular signals among endocardium, endocardium-derived mesenchyme, and myocardium [12]. Dysregulation of these signals can cause congenital heart valve defects, which are the most common congenital heart disease [12–16]. Therefore, characterization of the molecular signals among the valve-forming cells underlying the development of endocardial cushions will provide a better understanding of the etiology of congenital heart valve defects and help develop potential early disease diagnosis and interventions.
-catenin is encoded by the Ctnnb1 gene and has two distinct functions: maintaining cell-cell adhesion and mediating the canonical WNT (WNT/-catenin) signaling [17]. WNT/-catenin signaling plays critical roles in endocardial cushion formation. Key WNT pathway genes are expressed in the endocardium, mesenchyme, and myocardium of developing heart valves [8,18–20]. Multiple transgenic mouse models (TOPGAL, BATGAL, and Axin2LacZ) that report WNT/-catenin signaling indicate high nuclear activities of -catenin in the endocardial cushion regions during heart valve development [20–22]. In particular, WNT/-catenin signaling in endocardial lineage promotes EMT and post-EMT cell proliferation during endocardial cushion formation in zebrafish [23], chicken [24], and mouse [20,21]. In contrast to the intensive studies on WNT/-catenin signaling in endocardial-lineage, the roles of myocardial WNT/-catenin signaling in endocardial cushion development is less defined.
BMP signaling is another key regulator of endocardial cushion development. Multiple BMP ligands and receptors are expressed in cushion region during heart valve development [25]. Bmp2 and Bmp4 are expressed in AVC and OFT myocardium, respectively and required for EMT and post-EMT cushion growth [26–30]. Recently, BMP2 in endocardial lineage has also been reported to regulate AVC cushion maturation [31]. Interactions between WNT/-catenin and BMP signaling have been reported to regulate early events of cardiogenesis including the formation of first and second heart field, as well as the looping morphogenesis of the heart tube [32]. Bmp4 is a downstream target of WNT/-catenin signaling in second heart field [32]. Our previous ex vivo studies have suggested an endocardial–WNT to myocardial-BMP signaling axis underlying EMT process [33].
In this study, by using a myocardial-specific -catenin knockout mouse model, we provide the genetic evidence to support that Bmp2 is a mediator of WNT/-catenin signaling in AVC cushion myocardium to promote mesenchymal cell proliferation and cushion growth in a paracrine manner.
2. Methods
2.1. Experimental mouse models
All mouse experiments were performed according to the guideline of the National Institute of Health and the protocol approved by the Institutional Animal Care and Use Committee of Albert Einstein College of Medicine. Noontime on the day of detecting vaginal plugs was designated as E0.5. Adult mice and mouse embryos were PCR genotyped using tail and yolk sac DNA, respectively.
2.2. Histology and immunofluorescence staining
Embryos were dissected between E9.5 and E15.5, and processed for paraffin sections as described previously [8]. Hematoxylin and eosin (H &E) and immunofluorescence staining were performed using standard protocol. The stained tissue sections were examined and photographed using a Zeiss Axio Observer Z1 and a Leica confocal microscope.
2.3. EdU and TUNEL assays
Cell proliferation and apoptosis were detected by using EdU and TUNEL assays respectively. Pregnant female mice were injected with EdU (Life Technology) through intraperitoneal injection at a concentration of 100 mg/kg. After a 2-h pulse, embryos were collected and processed for frozen sections. Serial sections crossing the entire cushion region were first stained with PECAM1 antibody followed by EdU staining with EdU imaging Kit (Life Technology) and counterstained with DAPI (Vector lab). The stained sections were photographed using a confocal microscope. The TUNEL assays were performed according to the manufacture’s instruction.
2.4. RNA extraction and quantitative PCR (qPCR)
Total RNAs were isolated from pooled heart tissues using Trizol (Invitrogen). First strand cDNA was synthesized using the Superscript II Reverse Transcriptase Kit (Invitrogen). qPCR was performed using the Power SYBR Green PCR Master Mix (ABI). Gene specific primers were used (Table S1).
2.5. RNA in situ hybridization
RNA in situ hybridization (ISH) was performed as previously described [33]. Digoxigenin-labeled complementary RNA probes for Bmp2, Msx1, and Msx2 were prepared from the linearized plasmids by reverse transcription. The stained embryos were then post-fixed, dehydrated, embedded in paraffin, sectioned and photographed using the Zeiss Axio Observer Z1 inverted microscope.
2.6. Cell proliferation assays on heart explants
AVC tissues were microdissected out from E10.5 control and -catmKO embryos and cultured on the rat-tail collagen gel in 4-well plates. Explants were cultured in media with or without BMP2 (R&D systems, 200 ng/ml) for 48 h. The cell proliferation was detected using EdU assays as described above.
2.7. Statistical analysis
Statistical analysis was performed using Microsoft Excel. All data were presented as mean ± SD. Student’s t-Test was used for comparison between groups. Probability (p) values < 0.05 were considered as significant.
Full methods are available at Supplemental Methods.
3. Results
3.1. Paracrine regulation of endocardial cushion growth by myocardial -catenin
To determine the role of myocardial -catenin in heart valve development, we generated myocardial-specific β-catenin knockout mice (-catmKO, hereafter) by crossing floxed Ctnnb1 (β-catenin) mice with TntCre mice. Lineage tracing the TntCre-marked cells using the Rosa26 lacZ reporter line showed that the Cre-mediated lacZ expression was high in AVC cushion myocardium and low in OFT myocardium, especially in the distal OFT myocardium (Fig. S1A). Immunostaining showed that -catenin was expressed in the endocardium, mesenchyme and myocardium during endocardium cushion formation from E9.5 to E11.5 (Fig. S1B-D). The -catmKO embryos started to show decreased -catenin protein level in the myocardium of AVC and proximal OFT cushions when compared to controls at E9.5 (Fig. S1B), consistent with the low level of Cre-mediated recombination at the distal OFT myocardium (Fig. S1A). By E11.5, -catmKO embryos had completely lost βcatenin protein in the myocardium of AVC and proximal OFT, while residual -catenin protein remained in the myocardium of distal OFT (Fig. S1C and D). As expected, -catenin expression in the endocardium or cushion mesenchyme was not affected in the -catmKO embryos (Fig. S1B-D). Disruption of myocardial β-catenin resulted in embryonic death before E14.5 (Fig. S1E). While gross morphology of E11.5 -catmKO and control (β-cateninf/+ or β-cateninf/f) embryos was indistinguishable, E12.5 -catmKO embryos were underdeveloped compared to their littermate controls and all E14.5 -catmKO embryos were dead (Fig. S1F and G). These results indicate that myocardial -catenin is indispensible for embryo survival.
We then studied cardiac morphology by Hematoxylin and Eosin (HE) staining and found that E9.5 -catmKO embryos had comparable AVC and OFT cushions as their littermate controls (Fig. S2), indicating proper EMT had occurred. However, -catmKO embryos began to develop hypocellular AVC and OFT cushions at E10.5, despite having a normal cushion size (Fig. 1A, Fig. S3A and Fig. S4). By E11.5, -catmKO embryos exhibited smaller AVC and OFT cushions compared to controls (Fig. 1A, Fig. S3A and Fig. S5), suggesting that myocardial -catenin is required for post-EMT cushion growth. Indeed, EdU assays revealed that -catmKO embryos exhibited decreased proliferation of endocardial, mesenchymal and myocardial cells within the AVC and OFT regions (Fig. 1B and Fig. S3B). In addition, TUNEL assays showed that -catmKO embryos had significantly increased apoptosis of cushion mesenchymal cells (Fig. 1C and Fig. S3C). In contrast to the changes in cell fates, alcian blue staining revealed no difference in staining intensity or area in the AVC and OFT cushions between E10.5 control and -catmKO embryos (Fig. S6). Together, these findings demonstrate a critical paracrine function of myocardial -catenin in regulating the proliferation and apoptosis of mesenchymal cells for the post-EMT growth of endocardial cushions.
Fig. 1.

Paracrine regulation of AVC cushion growth by myocardial -catenin.
(A) Representative images from HE stained tissue sections from E10.5 and E11.5 control (-catf/f or -catf/+) and myocardial-specific β-catenin knockout (-catf/f:TntCre, β-catmKO) embryos show atrioventricular canal (AVC) cushions (outlined by dashed line). The bar charts show the quantification of cushion area and cell density. n = 4/group. (B) Representative images of EdU assays show the proliferating cells (green) in E11.5 AVC cushions. The cushion endothelial cells were stained with PECAM1 (red). The bar chart shows the quantification of cell proliferating rate in mesenchyme (Mes), endocardium (Endo) or myocardium (Myo) of AVC cushions. n = 3/group. (C) Representative images of TUNEL analysis show the apoptotic cells (green) in AVC cushion region. The cushion endothelial cells were stained with PECAM1 (red). The bar chart shows the quantification of cell apoptotic rate in mesenchyme, endocardium or myocardium of AVC cushions. n = 3/group. Statistical calculation was performed using unpaired two-way student t-test. * < 0.05. # < 0.01. Δ < 0.001. NS, No significance. The scale bars represent 100 μm.
3.2. Myocardial -catenin regulates endocardial cushion growth through BMP signaling
To identify the factors that could mediate myocardial -catenin to regulate the cushion growth, we performed quantitative PCR (qPCR) to examine the expression of candidate genes in AVC cushions of E9.5, E10.5 and E11.5 -catmKO embryos. Consistent with immunostaining, mRNA level of -catenin was significantly reduced in -catmKO embryos at all three stages (Fig. S7A). The expression of Tbx2 and Tbx3, transcriptional factors involved in AVC myocardium development, was slightly decreased at E9.5 and significantly reduced at E10.5 in β-catmKO embryos. The expression of Ccnd1 (a known target gene of β-catenin), Ve-cadherin (a gene involved in cell-cell adhesion that prevents EMT) and Notch1 (a gene essential for EMT and post-EMT cushion growth) was not affected by loss of myocardial -catenin (Fig. S7A). The expression of Snail or Slug (which encode transcription factors involved in EMT) was not affected at E9.5 and E10.5, but reduced at E11.5 (Fig. S7A). The expression of Msx1 and Msx2, transcriptional factors essential for EMT, were differentially affected. While the expression of Msx1 was not changed in AVC of E9.5 -catmKO embryos, and was slightly decreased in E10.5 and E11.5 -catmKO embryos, the expression of Msx2 was dramatically diminished at all three stages (Fig. S7A). RNA in situ hybridization showed that the expression of Msx2 in the cushion myocardium, but not in mesenchyme, was decreased in E10.5 -catmKO embryos (Fig. S7B). In contrast, Msx1 was expressed predominantly in cushion mesenchyme at a comparable level between E10.5 control and -catmKO embryos (Fig. S7B). The expression of matrix genes (Acan, Vcan, Has2, and Col1a1) was not affected in AVC of -catmKO embryos, except that Acan was upregulated at E9.5. Although the impact of the change of Acan on endocardial cushion formation was not clear, the overall expression of matrix genes were comparable at E10.5 between control and -catmKO embryos.
Among the BMP pathway genes examined, Bmp2 and Tgfβ2 were significantly downregulated in AVC cushions of -catmKO embryos at all three stages (Fig. 2A and Fig. S7A). Consistently, RNA ,in situ hybridization revealed that Bmp2 was highly expressed in the AVC myocardium of E10.5 and E11.5 control embryos, and such expression was markedly reduced in -catmKO embryos (Fig. 2B). Importantly, immunostaining revealed significantly reduced levels of phosphorylatedSMAD1/5/9 (pSMAD1/5/9) in the AVC cushions of E10.5 and E11.5 -catmKO embryos compared to controls (Fig. 2C and D), indicating reduced BMP signaling in the AVC cushion of -catmKO embryos and suggesting that BMP signaling may mediate the positive effect of myocardial -catenin on AVC cushion growth.
Fig. 2.
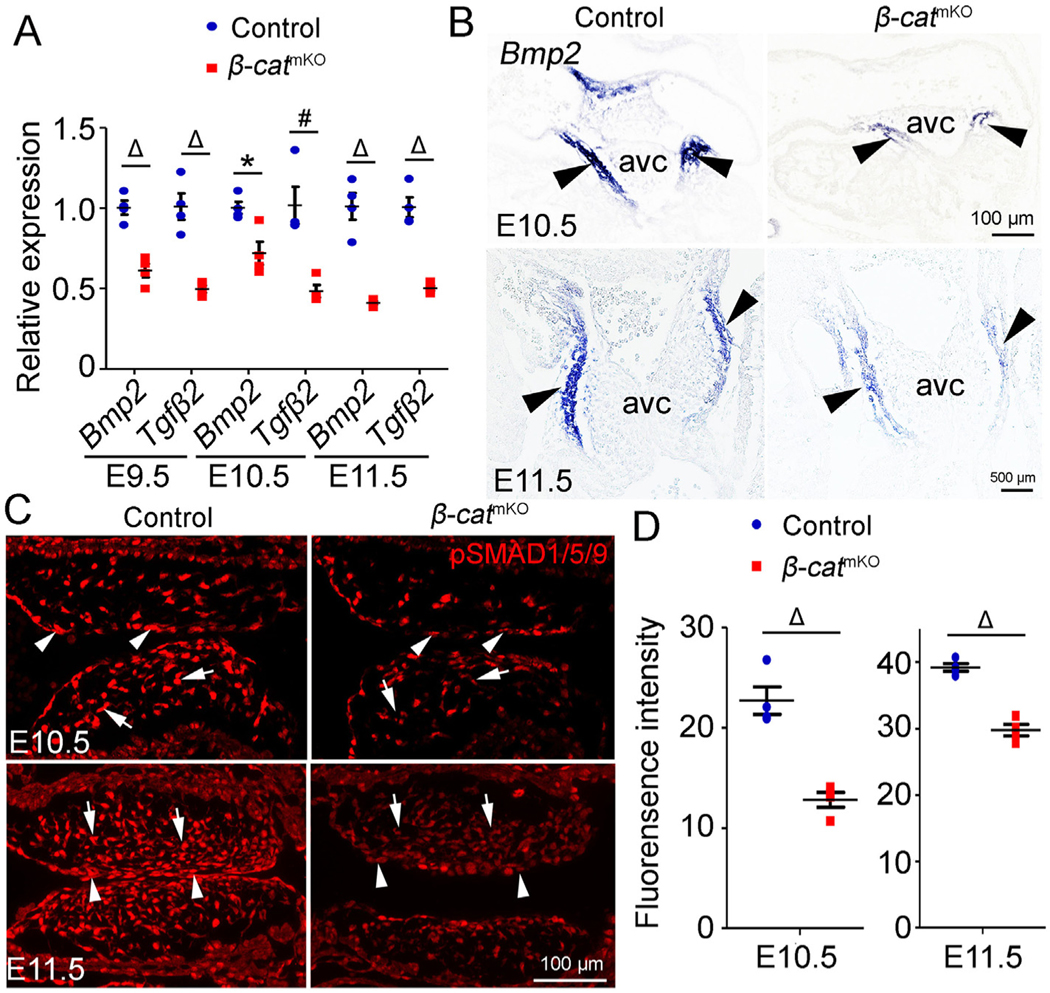
Loos of myocardial -catenin disrupts Bmp2 expression in myocardium and the BMP/SMAD signaling in mesenchyme.
(A) Quantitative PCR (qPCR) analysis of the expression of Bmp2 and Tgfb2 genes in AVC cushion tissues from E9.5, E10.5 and E11.5 control (-catf/f or -catf/+) and -catmKO (-catf/f:TntCre) embryos. The expression of Bmp2 and Tgfb2 was normalized to that of Gapdh. Tissues from two to four embryos were pooled as one sample. n = 4/group. (B) Representative images of RNA in situ hybridization analysis show Bmp2 expression in myocardium of AVC cushion (arrowheads) of E10.5 and E11.5 control and -catmKO embryos. n = 3–5/group. (C and D) immunofluorescence shows the expression of phospho-SMAD1/5/9 (p-SMAD1/5/9) (red) in AVC cushions of E10.5 and E11.5 control and -catmKO embryos. Arrows and arrowheads indicate pSMAD1/5/9 positive mesenchymal and endocardial cells, respectively. The bar charts show the quantification of the mean fluorescence intensity of pSMAD1/5/9 staining. n = 4/group. Statistical calculation was performed using unpaired two-way student t-test. * < 0.05. # < 0.01. Δ < 0.001.
We therefore performed rescue experiment to determine whether BMP2 mediates myocardial -catenin-dependent mesenchymal cell proliferation. We cultured AVC tissues of E10.5 control and -catmKO embryos on collagen gel using media with or without recombinant BMP2 proteins. Of note, at this stage EMT is complete within the AVC cushion, allowing us to examine the post-EMT proliferation of mesenchymal cells. In this ex vivo system, the proliferation of mesenchymal cells is independent on the working function of cardiac chamber myocardium. Consistent with the in vivo findings (Fig. 1B and Fig. S3B), EdU labeling of explants showed that mesenchymal cells from -catmKO embryos exhibited significantly reduced proliferation and this reduction was fully rescued by adding BMP2 (Fig. 3A and B). These findings demonstrate that -catenin in myocardium promote AVC cushion growth through inducing myocardial BMP2 expression and BMP/SMAD signaling in the cushion mesenchymal cells.
Fig. 3.
BMP2 mediates myocardial -catenin to promote mesenchymal cell proliferation.
(A and B) AVC cushions from E10.5 control (-catf/f or -catf/+) and -catmKO (-catf/f:TntCre) embryos were cultured on collagen gels with or without BMP2 (200 ng/ml) for 48 h. Representative images from EdU analysis of these explants show proliferating cells (green). Mesenchymal cells were highlighted by immunostaining of αSMA (red), while cell nuclei were stained with Hoechst (blue). The bar chart shows the quantification of cell proliferating rate in indicated conditions. n = 5/group. Statistical calculation was performed using unpaired two-way student t-test. Δ < 0.001.
In contrast to AVC cushions, the OFT cushions of -catmKO embryos showed unchanged or slightly increased expression of BMP/Tgfβ ligands including Bmp2, Bmp4, Bmp5, Bmp6, Bmp7, Tgfβ1, Tgfβ2, and Tgfβ3 (Fig. S8). The -catmKO embryos showed increased expression of mesenchymal cell markers (Snail and Slug) and the matrix genes (Acan, Vcan and Has2) within OFT cushions (Fig. S8). This differential gene expression change between AVC and OFT cushions suggests that myocardial -catenin regulates the development of AVC and OFT cushions through different mechanisms.
3.3. Autocrine regulation of endocardial cushion growth by -catenin in endocardium and mesenchyme
To determine whether -catenin in endocardium and mesenchyme is required for cardiac cushion formation, we generated endocardial lineage-specific -catenin knockout mice (-cateKO, hereafter) by crossing floxed Ctnnb1 mice with the Nfatc1Cre mice. Immunostaining showed that -catenin protein in AVC endocardium of E9.5 -cateKO embryos was slightly decreased when compared to controls, while its expression in OFT endocardium of E9.5 -cateKO embryos was not affected at this stage (Fig. S9). At E10.5, -catenin level in the endocardium and mesenchyme within AVC and proximal OFT regions was greatly diminished (Fig. S9). -cateKO embryos were lethal before E15.5 (Fig. S10A), but developed normally by E11.5, with gross morphology comparable to their littermate controls (-cateninf/+ or -cateninf/f) (Fig. S10B). Of particular note, -cateKO embryos developed normal vasculature in the yolk sac at E10.5 (Fig. S10B), and are unlike the embryos with the pan-endothelial deletion of -catenin that have vascular defects in the yolk sac [34]. -cateKO embryos began exhibiting a sign of underdevelopment at E12.5 and dying between E13.5 and E14.5 (Fig. S10B). These results demonstrate that -catenin in the endocardium and mesenchyme is indispensible for embryo survival.
We then studied cardiac morphology by HE staining and found that E10.5 -cateKO embryos developed normal AVC and OFT cushions, evident by similar cushion area and number of mesenchymal cells to those of littermate controls (Fig. S10C and D, and Fig. S11). However, the subsequent development of AVC and OFT cushions in E11.5 and 12.5 -cateKO embryos was arrested (Fig. 4A, Fig. S12A, S13 and S14). EdU assays revealed significantly reduced proliferation of endocardial and mesenchymal cells in AVC and OFT of E11.5 -cateKO embryos compared to controls (Fig. 4B and Fig. S12B). TUNEL labeling showed that endocardial deletion of -catenin resulted in increased apoptosis of mesenchymal cells in AVC and OFT cushions of E11.5 embryos (Fig. 4C and Fig. S12C). In contrast, endocardial deletion of -catenin had no impact on alcian blue staining (Fig. S15). At the molecular level, qPCR analysis showed that E11.5 -cateKO embryos had decreased mRNA levels of Bmp6, Msx1 and Id3 in their hearts (Fig. S16). Together, these results reveal a cell autonomous regulation of endocardial cushion growth by -catenin in endocardium and mesenchyme during heart valve development.
Fig. 4.

Autocrine regulation of AVC cushion growth by -catenin in the endocardium and mesenchyme.
(A) Representative images from HE stained tissue sections of E11.5 and E12.5 control (-catf/f or -catf/+) and endocardial-specific -catenin knockout (-catf/f:Nfatc1Cre, -cateKO) embryos show the regions of atrioventricular canal (AVC) cushions (outlined by dashed line). The bar charts show the quantification of cushion area. n = 3/group. (B) Representative images of EdU assay show the proliferating cells (green) in E11.5 AVC cushions. The cushion endothelial cells were stained with PECAM1 (red). The bar chart shows the quantification of cell proliferating rate in mesenchyme (Mes), endocardium (Endo) or myocardium (Myo) of AVC cushions. n = 3/group. (C) Representative images of TUNEL analysis show the apoptotic cells (green) in AVC cushion region. The cushion endothelial cells were stained with PECAM1 (red). The bar chart shows the quantification of cell apoptotic rate in mesenchyme, endocardium or myocardium of AVC cushions. n = 3/group, Statistical calculation was performed using unpaired two-way student t-test. * < 0.05. # < 0.01. NS, No significance.
3.4. WNT/-catenin signaling is spatiotemporally activated in the heartvalve forming regions
-catenin can form a transcriptional complex with TCF/LEF in nucleus and mediate canonical WNT signaling. The cushion defects resulting from tissue-specific inactivation of -catenin in the endocardium, mesenchyme and myocardium led us to examine the canonical WNT activity in the heart valve-forming regions between E9.5 and E12.5. We traced WNT/-catenin signaling using a transgenic reporter line specific for canonical WNT signaling in which GFP expression is under the control of 6 tandem TCF/LEF binding sites [35]. The results showed that a sustained high level of WNT/-catenin activity was present in the AVC cushion myocardium from E9.5 to E12.5. In contrast, WNT/-catenin activities in the endocardium and mesenchyme of the AVC cushion were undetectable or at a very low level at E9.5 and E10.5, and gradually increased after E10.5 (Fig. 5A and B). We found that canonical WNT/-catenin activities in the OFT had a similar pattern of tissue distribution, although they appeared one day later compared to AVC (Fig. 5A and B). The spatiotemporal WNT/β-catenin activities suggest that -catenin regulates endocardial cushion growth through its signaling function.
Fig. 5.

Spatiotemporal Wnt/-catenin signaling in cushion-forming region.
(A) Representative images of the heart valve-forming region at AVC or OFT from E9.5 to E12.5 hearts of the transgenic Wnt/-catenin signaling reporter embryos show Wnt/-catenin signaling (GFP) in the cushion myocardium (indicated by arrows), endocardium or mesenchyme (indicated by arrowheads). PECAM1 staining marks the endocardium (red). (B) Schematic summary of Wnt/-catenin signaling in myocardium, mesenchyme and endocardium of AVC and OFT cushions from E9.5 to E12.5. At AVC, high levels of Wnt/-catenin signaling are present in the myocardium at all stages, whereas the mesenchyme or endocardium begins to express low levels of Wnt/-catenin signaling at E10.5, which gradually increases at later stages. Wnt/-catenin signaling is present in OFT myocardium at a low level at E9.5 and gradually increases at later stages. Low levels of Wnt/-catenin signaling in the OFT mesenchyme or endocardium are observed after E10.5.
3.5. Signaling function of -catenin critically regulates endocardial cushion growth
We therefore sought to determine whether the signaling function of -catenin is required for cardiac cushion growth. To this end, we used a mouse model -cateninDM that expresses a mutant -catenin protein containing a single amino acid change (D164A) in the first armadillo repeat and a C-terminal truncation. Because these mutations prevent interacting with specific transcriptional coactivators, -cateninDM lacks any detectable transcriptional activity but retains the intact cell adhesion function. By combining -cateninDM with tissue-specific deletion of -catenin, we were able to interrogate the role of the signaling function of -catenin in endocardial cushion development. First, we crossed -cateninDM and -cateninflox mice with TntCre mice to generate compound -catenin mutant mice -cateninDM/flox:TntCre (-catDM/mKO, hereafter), while the mice with genotype -cateninDM/flox or -cateninDM/+ were used as controls. Histology analysis showed that E11.5 -catDM/mKO embryos had hypoplastic AVC (Fig. 6A and C, and Fig. S17) and OFT (Fig. S18 and S19) cushions similar as that observed in -catmKO embryos, demonstrating that the signaling function of β-catenin in the cushion myocardium was essential for cushion growth. We next crossed -cateninDM and -cateninflox mice with Nfatc1Cre mice to generate compound -catenin mutant mice -cateninDM/flox:Nfatc1Cre (-catDM/eKO, hereafter), while the mice with genotype -cateninDM/flox or -cateninDM/+ were used as controls. Histology analysis showed that E11.5 -catdm/eKO embryos had hypoplastic AVC (Fig. 6B and D, and Fig. S20) and OFT (Fig. S17 and S21) cushions which were similar to the defects observed in -cateKO embryos, confirming that the signaling function of -catenin in the cushion endocardium and mesenchyme was essential for cushion growth. Together, these findings indicate that -catenin in myocardium, endocardium and mesenchyme regulates cushion growth through its signaling function.
Fig. 6.
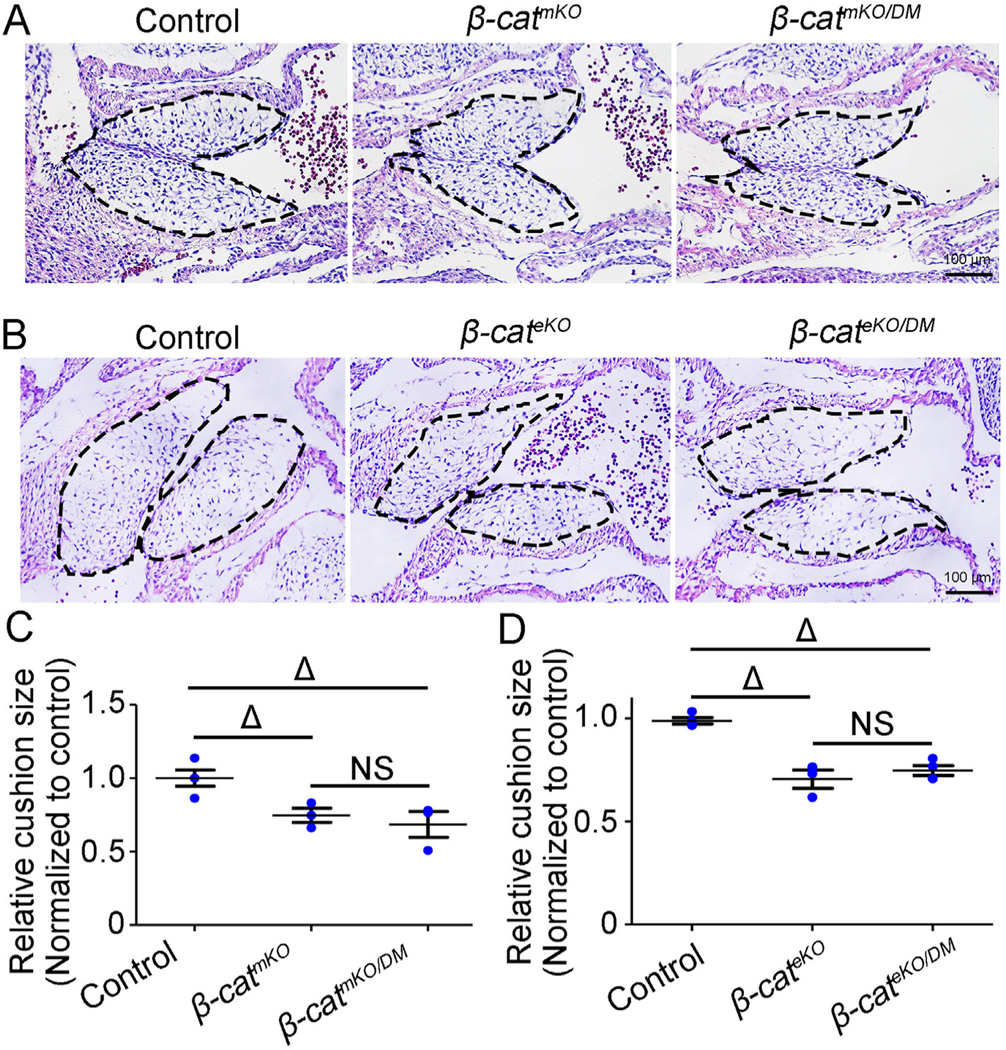
Signaling function of -catenin is essential for endocardial cushion growth.
(A) representative images from HE stained tissue sections show atrioventricular canal (AVC) cushions (outlined by dashed line) in E11.5 control (-catf/DM or -catDM/+), -catmKO (-catf/f:TntCre), and -catmKO/DM (-catf/DM:TntCre) embryos. (B) representative images from HE stained tissue sections show AVC cushions (outlined by dashed line) in E11.5 control (-catf/DM or -catDM/+), -cateKO (-catf/f:Nfatc1Cre), and -cateKO/DM (-catf/DM:Nfatc1Cre) embryos. (C and D) The bar charts show the quantification of cushion size showed in panel A (C) and panel B (D). The cushion size was normalized to controls. n = 3/group. Δ < 0.001. NS, No significance.
4. Discussion
Based on the in vitro studies, we have previously proposed an endocardial-WNT to myocardial-BMP signaling axis underlying EMT process of endocardial cushion formation. Here we further investigated this model using a myocardial-specific -catenin knockout mouse model and found the in vivo evidence to support that Bmp2 is a mediator of WNT/-catenin signaling in AVC cushion myocardium to promote mesenchymal cell proliferation and cushion expansion. In addition, we confirmed that WNT/-catenin signaling in endocardium and mesenchyme positively regulates the growth of endocardial cushion growth. Our observations are summarized schematically in Fig. 7.
Fig. 7.
Schematic summaries of autocrine and paracrine regulation of endocardial cushion formation by -catenin.
The schematic model summaries tissue specific roles of -catenin during AVC cushion formation. Black arrows indicate the autocrine regulation while blue arrows indicate paracrine regulation. Myocardial -catenin regulates the proliferation of mesenchyme in a paracrine manner through inducing expression of Bmp2. Meanwhile, -catenin in endocardium and mesenchyme promotes cell proliferation cell autonomously.
In this study, TntCre was used to drive the deletion of -catenin specifically in myocardium. This deletion caused embryonic lethality around E14.5 and had no effect on the gross embryo development before E11.5, evident by normal morphology and embryo size (Fig. S1E-G). In contrast, previous reports have shown that Islet1Cre or Nkx2.5Cre mediated deletion of -catenin results in a more severe cardiac phenotype characterized by early embryonic lethality and delayed embryo development [36]. This difference between our and previous studies could be explained by the different Cre divers used in the studies. The Islet1Cre mice start to express Cre at E7.0-E7.5 in the mesodermal progenitors of second heart field that give rise to right ventricle and OFT [36]. The Nkx2.5Cre mice start to express Cre in cardiac crescent/straight heart tube around E7.5/8.0 of first heart field. Both Cres are thus expressed in cardiac progenitors giving rise to both cardiomyocytes and endocardial cells [36]. In contrast, TntCre is known to be expressed specifically in cardiomyocytes [26]. Our immunostaining showed that significant deletion of -Catenin drove by TntCre started to be observed at E10.5 (Fig. S1B-D). Together, the later and cardiomyocyte-specific deletion may explain the milder myocardial phenotype caused by deletion of -catenin using TntCre than those caused by deletion of -catenin using Islet1Cre or Nkx2.5Cre.
BMP signaling is a well-known paracrine signal from myocardium for EMT and post-EMT mesenchymal cell proliferation during endocardial cushion formation [25,27,28,37,38]. Disruption of myocardial Bmp2 inhibits EMT and results in hypocellular cushions [28,39], suggesting a critical role of Bmp2 in EMT. Similarly, we have reported previously an endocardial to myocardial NOTCH-WNT-BMP signal axis for EMT during endocardial cushion formation [33]. Consistently, we show in this study that Bmp2 expression in AVC cushions of E9.5 -catmKO embryos was decreased to 70% of that in controls. This slight but significant decrease is most likely due to incomplete deletion of -catenin at this early stage. Consistently, previous studies have reported that myocardial -catenin is required for Bmp2 expression and mitral valve formation [21]. In our study, early development of AVC in -catmKO embryos appears normal at E9.5 without changes in the expression of EMT markers (Ve-cad, Notch1, Snail and slug). However, cushion growth after EMT is inhibited in these embryos. These findings suggest that 70% of normal Bmp2 level is sufficient for proper EMT, yet not for maintaining the high proliferation rate during post-EMT cushion growth. On the other hand, further reduction of BMP2 resulted from complete inactivation of -catenin impairs EMT. In contrast to AVC cushions, the OFT cushions in -catmKO embryos showed unchanged or slightly increased expression of BMP/Tgfβ ligands including Bmp2, Bmp4, Bmp5, Bmp6, Bmp7, Tgfβ1, Tgfβ2, and Tgfβ3. These findings suggest that myocardial -catenin regulates the growth of AVC and OFT cushions through different mechanisms.
Our genetic evidence strongly supports that Bmp2 is a downstream target and mediator of WNT/-catenin signaling in the AVC myocardium, although the mechanisms by which -catenin controls the expression of Bmp2 remains unknown. A positive feedback regulation between Tbx2/3 and Bmp2 has been reported to regulate AVC myocardial development [40], suggesting that Tbx2/3 may work intermediately between WNT/-catenin and Bmp2 gene activation. The results of our gene expression analysis, however, indicate that Bmp2 is decreased prior to the downregulation of Tbx2/3 in E9.5 -catmKO embryos. Alternatively, Msx1/2 has been shown to regulate the expression of Bmp2 in AVC myocardium [41]. Our results also show that Msx2 and Bmp2 are decreased in -catmKO embryos simultaneously (Fig. S7A). Based on these observations, we speculate that WNT/-catenin signaling may regulate Bmp2 through Msx2. Nonetheless, further investigation is needed to test whether Bmp2 is a direct target of -catenin in the AVC myocardium as previously suggested in osteoblasts and embryonic stem cells [42,43].
Previous studies by deleting -catenin in pan-endothelium with Tie2Cre indicate an essential role of -catenin in EMT [22]. However, these -catenin mutant embryos die before E10.5 because of multiple defects in heart as well as yolk sac vessels [34]. In our study, we deleted -catenin specifically in endocardium and its mesenchymal progeny using the Nfatc1cre driver line. Unlike pan-endothelial -catenin knockouts, mice with endocardial deletion of -catenin develop normal yolk sac vasculature and survive till to E15.5, allowing us to study the role of -catenin in post-EMT cushion growth. Our tissue-specific deletion shows definitively that endocardial -catenin is required for endocardial cushion formation through promoting cell proliferation. This finding is consistent with the previous report that inhibition of WNT/-catenin signaling by overexpressing DKK1 inhibits cushion growth after EMT [20].
TOPGAL, BATGAL and Axin2LacZ reporter mouse lines are widely used during heart development with different findings. BATGAL mice show that WNT/-catenin signaling is predominantly present in the endocardium and the cushion mesenchyme at E10.5, suggesting a role for WNT/-catenin signaling in promoting EMT [22]. In contrast, Axin2LacZ mice show that WNT/-catenin signaling is primarily located in the cushion myocardium and is rare in the endocardium at E9.5 and E10.5, which argues against a direct role for WNT/-catenin signaling in EMT [20]. Our study uses a new reporter mouse line to examine the WNT/-catenin signaling in the heart valve-forming region. This line expresses an H2B-EGFP fusion protein under the control of six copies of a T-cell specific transcription factor/lymphoid enhancer-binding factor 1 (TCF/Lef1) response element and a heat shock protein 1B (Hspa1b) minimal promoter [35], thus directly reporting the nuclear transcriptional activation by -catenin. We show here that WNT/-catenin signaling is dominant in cushion myocardium and rare in endocardium at E9.5, suggesting that EMT is unlikely dependent on WNT/-catenin signaling in endocardium. This is consistent with the previous report that inhibition of WNT/-catenin signaling by overexpressing DKK1 has no effect on EMT within the AVC cushion [20]. After E10.5, WNT/-catenin activity is retained at a high level in cushion myocardium and begins to increase in cushion mesenchyme, and together they execute paracrine and autocrine functions necessary for post-EMT cushion growth, as shown by using a strategy of compound deletion of a floxed allele -cateninflox and a mutant allele -cateninDM that inactivates the -catenin signaling function, but retains its cell-cell adhesion function.
The canonical WNT signaling is present in a small cell population of endocardium and mesenchyme during endocardial cushion growth and their loss likely will not cause a dramatic cushion defect. However, the similar cushion defects observed in -cateKO and -catDM/eKO suggest that the signaling function of -catenin in endocardium and mesenchyme is also essential for cushion development. -catenin interacts with many proteins, such as TCF/LEF, B-cell lymphoma9 (BCL9), TATA-binding protein (TBP), Brahma/Brahma-relatedgene-1 (Brg-1), CREB-binding protein (CBP)/p300, Mediatorsubunit 12 (MED12), and Hyrax/Parafibromin [44]. Disruption of these interactions in -catdm allele likely blocks the canonical WNT signaling, as well as other signals. Together, their dysregulation may contribute to the cushion phenotypes.
In conclusion, our findings demonstrated that WNT/-catenin signaling in myocardium promotes mesenchymal cell proliferation and cushion expansion through inducing Bmp2 expression in myocardium and the resulting BMP/SMAD signaling in mesenchyme. This new information would advance our current understanding of the mechanisms underlying endocardial cushion formation and congenial heart valve disease.
Supplementary Material
Funding
This work was supported by grants from the American Heart Association13POST16970056 to Y·W and the National Heart, Lung, and Blood InstituteR01HL111770, R01HL116997, and R01HL133120 to B.Z.
Footnotes
Conflict of interest
There is no conflict of interest to be disclosed.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.yjmcc.2018.09.001.
References
- [1].Armstrong EJ, Bischoff J, Heart valve development: endothelial cell signaling and differentiation, Circ. Res 95 (2004) 459–470, 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Combs MD, Yutzey KE, Heart valve development: regulatory networks in development and disease, Circ. Res 105 (2009) 408–421, 10.1161/CIRCRESAHA.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lin CJ, Lin CY, Chen CH, Zhou B, Chang CP, Partitioning the heart: mechanisms of cardiac septation and valve development, Development 139 (2012) 3277–3299, 10.1242/dev.063495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Steed E, Boselli F, Vermot J, Hemodynamics driven cardiac valve morphogenesis, Biochim. Biophys. Acta 1863 (2016) 1760–1766, 10.1016/j.bbamcr.2015.11.014. [DOI] [PubMed] [Google Scholar]
- [5].Barnett JV, Desgrosellier JS, Early events in valvulogenesis: a signaling perspective, Birth Def. Res. C Embryo Today 69 (2003) 58–72, 10.1002/bdrc.10006. [DOI] [PubMed] [Google Scholar]
- [6].Wirrig EE, Yutzey KE, Transcriptional regulation of heart valve development and disease, Cardiovasc. Pathol 20 (2011) 162–167, 10.1016/j.carpath.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].MacGrogan D, et al. , How to make a heart valve: from embryonic development to bioengineering of living valve substitutes, Cold Spring Harb. Perspect Med 4 (2014) a013912, 10.1101/cshperspect.a013912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang Y, et al. , Notch-Tnf signalling is required for development and homeostasis of arterial valves, Eur. Heart J (2015), 10.1093/eurheartj/ehv520. [DOI] [PMC free article] [PubMed]
- [9].Wu B, Baldwin HS, Zhou B, Nfatc1 directs the endocardial progenitor cells to make heart valve primordium, Trends Cardiovasc. Med 23 (2013) 294–300, 10.1016/j.tcm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wu B, et al. , Nfatc1 coordinates valve endocardial cell lineage development required for heart valve formation, Circ. Res 109 (2011) 183–192, 10.1161/CIRCRESAHA.111.245035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wu B, et al. , Developmental Mechanisms of Aortic Valve Malformation and Disease, Annu. Rev. Physiol (2016), 10.1146/annurev-physiol022516-034001. [DOI] [PubMed]
- [12].Lincoln J, Garg V, Etiology of valvular heart disease-genetic and developmental origins, Circ. J 78 (2014) 1801–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hoffman JI, Kaplan S, The incidence of congenital heart disease, J. Am. Coll. Cardiol 39 (2002) 1890–1900. [DOI] [PubMed] [Google Scholar]
- [14].Freeman RV, Otto CM, Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies, Circulation 111 (2005) 3316–3326, 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- [15].Pierpont ME, et al. , Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics, Circulation 115 (2007) 3015–3038, 10.1161/CIRCULATIONAHA.106.183056. [DOI] [PubMed] [Google Scholar]
- [16].Lahaye S, Lincoln J, Garg V, Genetics of valvular heart disease, Curr. Cardiol. Rep 16 (2014) 487, 10.1007/s11886-014-0487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gessert S, Kuhl M, The multiple phases and faces of wnt signaling during cardiac differentiation and development, Circ. Res 107 (2010) 186–199, 10.1161/CIRCRESAHA.110.221531. [DOI] [PubMed] [Google Scholar]
- [18].Alfieri CM, Cheek J, Chakraborty S, Yutzey KE, Wnt signaling in heart valve development and osteogenic gene induction, Dev. Biol 338 (2010) 127–135, 10.1016/j.ydbio.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cai X, et al. , Tbx20 acts upstream of Wnt signaling to regulate endocardial cushion formation and valve remodeling during mouse cardiogenesis, Development 140 (2013) 3176–3187, 10.1242/dev.092502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bosada FM, Devasthali V, Jones KA, Stankunas K, Wnt/beta-catenin signaling enables developmental transitions during valvulogenesis, Development 143 (2016) 1041–1054, 10.1242/dev.130575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gillers BS, et al. , Canonical wnt signaling regulates atrioventricular junction programming and electrophysiological properties, Circ. Res 116 (2015) 398–406, 10.1161/CIRCRESAHA.116.304731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liebner S, et al. , Beta-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse, J. Cell Biol 166 (2004) 359–367, 10.1083/jcb.200403050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hurlstone AF, et al. , The Wnt/beta-catenin pathway regulates cardiac valve formation, Nature 425 (2003) 633–637, 10.1038/nature02028. [DOI] [PubMed] [Google Scholar]
- [24].Person AD, Garriock RJ, Krieg PA, Runyan RB, Klewer SE, Frzb modulates Wnt-9a-mediated beta-catenin signaling during avian atrioventricular cardiac cushion development, Dev. Biol 278 (2005) 35–48, 10.1016/j.ydbio.2004.10.013. [DOI] [PubMed] [Google Scholar]
- [25].Delot EC, Control of endocardial cushion and cardiac valve maturation by BMP signaling pathways, Mol. Genet. Metab 80 (2003) 27–35. [DOI] [PubMed] [Google Scholar]
- [26].Jiao K, et al. , An essential role of Bmp4 in the atrioventricular septation of the mouse heart, Genes Dev. 17 (2003) 2362–2367, 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu W, et al. , Bmp4 signaling is required for outflow-tract septation and branchialarch artery remodeling, Proc. Natl. Acad. Sci. U. S. A 101 (2004) 4489–4494, 10.1073/pnas.0308466101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ma L, Lu MF, Schwartz RJ, Martin JF, Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning, Development 132 (2005) 5601–5611, 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- [29].McCulley DJ, Kang JO, Martin JF, Black BL, BMP4 is required in the anterior heart field and its derivatives for endocardial cushion remodeling, outflow tract septation, and semilunar valve development, Dev. Dyn 237 (2008) 3200–3209, 10.1002/dvdy.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sugi Y, Yamamura H, Okagawa H, Markwald RR, Bone morphogenetic protein-2 can mediate myocardial regulation of atrioventricular cushion mesenchymal cell formation in mice, Dev. Biol 269 (2004) 505–518, 10.1016/j.ydbio.2004.01.045. [DOI] [PubMed] [Google Scholar]
- [31].Saxon JG, et al. , BMP2 expression in the endocardial lineage is required for AV endocardial cushion maturation and remodeling, Dev. Biol 430 (2017) 113–128, 10.1016/j.ydbio.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W, Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis, Proc. Natl. Acad. Sci. U. S. A 104 (2007) 18531–18536, 10.1073/pnas.0703113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wang Y, et al. , Endocardial to myocardial notch-wnt-bmp axis regulates early heart valve development, PLoS One 8 (2013) e60244, 10.1371/journal.pone.0060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cattelino A, et al. , The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility, J. Cell Biol 162 (2003) 1111–1122, 10.1083/jcb.200212157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ferrer-Vaquer A, et al. , A sensitive and bright single-cell resolution live imaging reporter of Wnt/ss-catenin signaling in the mouse, BMC Dev. Biol 10 (2010) 121, 10.1186/1471-213X-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kwon C, et al. , Canonical Wnt signaling is a positive regulator of mammalian cardiac progenitors, Proc. Natl. Acad. Sci. U. S. A 104 (2007) 10894–10899, 10.1073/pnas.0704044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Nakajima Y, Yamagishi T, Hokari S, Nakamura H, Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP), Anat. Rec 258 (2000) 119–127. [DOI] [PubMed] [Google Scholar]
- [38].Allen SP, et al. , Misexpression of noggin leads to septal defects in the outflow tract of the chick heart, Dev. Biol 235 (2001) 98–109, 10.1006/dbio.2001.0291. [DOI] [PubMed] [Google Scholar]
- [39].Rivera-Feliciano J, Tabin CJ, Bmp2 instructs cardiac progenitors to form the heart-valve-inducing field, Dev. Biol 295 (2006) 580–588, 10.1016/j.ydbio.2006.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Singh R, et al. , Tbx2 and Tbx3 induce atrioventricular myocardial development and endocardial cushion formation, Cell. Mol. Life Sci 69 (2012) 1377–1389, 10.1007/s00018-011-0884-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chen YH, Ishii M, Sucov HM, Maxson RE Jr., Msx1 and Msx2 are required for endothelial-mesenchymal transformation of the atrioventricular cushions and patterning of the atrioventricular myocardium, BMC Dev. Biol 8 (2008) 75, 10.1186/1471-213X-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang R, et al. , Wnt/beta-catenin signaling activates bone morphogenetic protein 2 expression in osteoblasts, Bone 52 (2013) 145–156, 10.1016/j.bone.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhang X, Peterson KA, Liu XS, McMahon AP, Ohba S, Gene regulatory networks mediating canonical Wnt signal-directed control of pluripotency and differentiation in embryo stem cells, Stem Cells 31 (2013) 2667–2679, 10.1002/stem.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Valenta T, et al. , Probing transcription-specific outputs of beta-catenin in vivo, Genes Dev. 25 (2011) 2631–2643, 10.1101/gad.181289.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




