Abstract
Background and Objectives
Psychiatric morbidity is common after a multiple sclerosis (MS) diagnosis. However, little is known about psychiatric comorbidity during the prodromal phase (before MS onset). To compare the prevalence and relative burden of psychiatric morbidity in individuals with MS with matched controls before MS onset.
Methods
Using linked administrative and clinical data from British Columbia, Canada, we identified cases with MS through a validated algorithm or from neurologist-diagnosed MS clinic attendees. Cases were matched by age, sex, and geographical location with up to 5 general population controls. We identified psychiatric morbidity through a validated definition and determined its prevalence in cases/controls in the 5 years before the first demyelinating claim of cases with MS (“administrative cohort”) or symptom onset (“clinical cohort”) and estimated case/control prevalence ratios with 95% CIs. We also compared the yearly number of physician visits for psychiatric morbidity, visits to psychiatrists, psychiatric-related admissions, and psychotropic dispensations pre-MS onset in cases/controls regardless of whether psychiatric morbidity algorithm was fulfilled using negative binomial regression fitted through generalized estimating equations; results were reported as adjusted rate ratios with 95% CIs. We assessed yearly trends through interaction terms between cases/controls and each year pre-MS onset.
Results
The administrative cohort comprised 6,863/31,865 cases/controls; the clinical cohort comprised 966/4,534 cases/controls. Over the entire 5-year period pre-MS onset, 28.0% (1,920/6,863) of cases and 14.9% (4,738/31,865) of controls (administrative cohort) had psychiatric morbidity, as did 22.0% (213/966) of clinical cases and 14.1% (638/4,534) controls. Psychiatric morbidity prevalence ratios ranged from 1.58; 95% CI 1.38–1.81 (clinical cohort) to 1.91; 95% CI 1.83–2.00 (administrative cohort). In the administrative cohort, health care use was higher for cases in each year pre-MS onset (all 95% CIs >1); physician visits were 78% higher in year 5 pre-MS onset and 124% 1 year before; visits to psychiatrists were 132% higher in year 5 and 146% in year 1; hospitalizations were 129% higher in year 5 and 197% in year 1; and prescription dispensations were 72% higher in year 5 and 100% in year 1. Results were not significant in the clinical cohort.
Discussion
Psychiatric morbidity represents a significant burden before MS onset and may be a feature of the MS prodrome.
Introduction
Psychiatric comorbidities, including depression and anxiety, are common in multiple sclerosis (MS), and prevalence rates are higher than expected in the general population.1,2 However, while much work has focused on people who have already developed MS, little is known about psychiatric comorbidity before medical recognition of MS.3-6
In recent years, mounting evidence has pointed to the existence of a prodromal period in MS.4,7,8 Defined as “early symptoms indicating the onset of a disease or illness,” studies have shown that the MS prodrome is measurable at the level of health service use.4,5,7,9-13 These studies demonstrate that individuals with MS steadily increased their health care use at least 5 years before their first “classic” MS symptom5-7,9 or first demyelinating event,5,6,9-13 when compared with a matched general population. In addition, higher serum neurofilament light levels, a biomarker of neuroaxonal damage, can be found up to 6 years before a first classical MS symptom compared with unaffected matched controls, providing biological support for the MS prodrome.14
A psychiatric component of the MS prodrome has also been observed. In the 5 years before MS onset, individuals developing MS were approximately 50% more likely than matched individuals from the general population to visit a psychiatrist and to fill a prescription for a drug related to the nervous system, which included the antidepressants.6 While intriguing, much remains unknown. A better understanding of the extent and burden of early psychiatric morbidity in MS is needed, especially because it can negatively affect quality of life, affect disability progression,15,16 and possibly increase mortality risk.17 In addition, expanding our knowledge of the MS prodrome may allow for earlier disease recognition and treatment.8 The aim of our study was to describe the prevalence and relative burden of psychiatric morbidity in individuals with MS in the 5 years leading up to MS onset relative to matched controls.
Methods
Study Design and Data Sources
This matched retrospective cohort study was conducted in the province of British Columbia (BC), Canada. All residents in BC have access to publicly funded health care services. We accessed a data platform of prospectively collected health administrative and clinical information from the province that is captured electronically and is accessible for research. Data were linked using each individual's unique personal health care number and provided information on virtually every health encounter for all BC residents. Combined, the data platform captured details regarding physician visits (Medical Services Plan [MSP] Payment Information File)18 and hospitalizations (Discharge Abstract Database),19 with related diagnoses coded using the International Classification of Diseases (ICD) 9/10 system. Medications dispensed at community or outpatient pharmacies in BC were also captured (through PharmaNet),20 coded by their drug identification number and mapped to the Anatomical Therapeutic Chemical (ATC) classification system 5th level. Demographics, socioeconomic and residency status (MSP Registration and Premium Billing Files),21 and death dates (Vital Statistics)22 were also available. Socioeconomic status (SES) estimates were obtained by using an algorithm from Statistics Canada combining each individual's postal code with census-derived mean neighborhood income data measured in quintiles.23 Data obtained from the BC MS Clinical database included the date of MS symptom onset and disease course (relapsing vs progressive onset), as recorded by an MS neurologist during a routine visit to one of BC's 4 MS clinics. All data were deidentified before analyses.
Study Cohorts
Two cohorts were created—an “administrative” cohort and an MS clinic–derived “clinical” cohort. The administrative cohort comprised all persons with MS fulfilling a validated algorithm requiring ≥3 MS-specific hospital, physician, or prescription claims derived from the health administrative data (eTable 1, links.lww.com/WNL/D118).24 This algorithm has a sensitivity of 99.5%, a specificity of 98.5%, a positive predictive value of 99.5%, and a negative predictive value of 97.5%.25 An individual's first demyelinating disease-related claim formed the “administrative index date.” The clinical cohort comprised individuals diagnosed with MS in one of 4 BC's MS clinics. These individuals were then linked to their health administrative data. This administrative information was used to assess the psychiatric-related outcomes (outlined further). The date of MS symptom onset, as recorded by an MS neurologist based on a person's medical history, formed the “clinical index date.” Thus, the index date definitions for the clinical and the administrative cohorts differed; the former is captured retrospectively (MS symptom onset) during patient contact with an MS clinic and the latter prospectively or in “real time” as the patient moves through the health system (e.g., as a demyelinating-related ICD code). The period before MS symptom onset may better represent the “true” MS prodrome because not every symptom of possible early MS necessarily leads to health care system use or to a record of a demyelinating disease-related claim. All individuals within each cohort were matched with up to 5 controls from the general population (who had no MS or other demyelinating disease-related claims or prescriptions filled for MS-specific DMDs over their entire registered period) by sex, exact year of birth, and postal code at the index year. Controls were randomly selected without replacement from the BC general population. Each control was assigned the same index date as their matched patient with MS. To select incident individuals with MS, both patients with MS and their matched controls were required to be resident in BC for ≥90% of the days in each of the 5 years before the index date. The administrative and clinical cohorts were not mutually exclusive. However, because the definition of index dates differed, each cohort was analyzed separately. Motivated by our prior work,5,7 we capitalized on an already available dataset; all data were available from January 1, 1996, through until December 31, 2013, for the administrative cohort and through until December 31, 2008, for the clinical cohort. See “Data availability” for details regarding data access.
Outcomes
First, the presence of any psychiatric morbidity (depression, anxiety, bipolar disorder, or schizophrenia) was identified by applying a validated case definition requiring ≥1 relevant psychiatric-related hospital or ≥5 physician claims (on different days) within a 5-year period, with at least 1 claim falling within 5 years preindex date (eTable 2, links.lww.com/WNL/D118).26 The psychiatric morbidity was considered present during the earliest claim and thereafter. Second, we determined the relative burden of psychiatric morbidity in each of the 5 years preindex dates regardless of whether individuals fulfilled the algorithm for psychiatric morbidity, according to the yearly number of the following: (1) physician visits for any psychiatric morbidity (i.e., any code summarized in eTable 2); (2) visits to psychiatrists, (3) psychiatric-related hospital admissions (eTable 2), and (4) “psychotropic” prescriptions filled (counts, based on the total number of prescriptions filled [ATC fifth level], which were then grouped and reported by pharmacologic class [ATC third level], eTable 3). These yearly numbers were compared between patients with MS and controls. To prevent double counting, physician visits occurring on the same day and with the same ICD code for the same individual were considered as a single visit. Overlapping hospital stays were also counted as 1 hospitalization. Similarly, any prescriptions filled on the same day and with the same drug identification number were counted once. Psychiatric morbidity outcomes were assessed using the same approach in both the administrative and clinical cohorts.
Statistical Analysis
The characteristics of the administrative and clinical cohort were summarized at the respective index dates. For patients with MS and matched controls, we estimated the prevalence of psychiatric morbidity for each of the 5 years before the index dates. We also used a conditional logistic regression model to examine the association between the presence of any psychiatric morbidity and MS over the entire 5-year period (with each strata containing 1 case and its matched controls). To avoid overestimation of the association when the outcome of interest was common (≥10%), we applied the following formula to convert odds ratio (OR) to prevalence ratio with 95% CI: prevalence ratio = OR/([1 − prevalence in the nonexposed group] + [prevalence in the nonexposed group × OR]). OR CIs were transformed to prevalence ratio CIs applying the same formula as well.27 In addition, the association between MS and the number of yearly physician visits for any psychiatric morbidity, visits to psychiatrists, psychiatric-related hospital admissions, and “psychotropic” prescriptions was examined using negative binomial regression models, fitted through generalized estimating equations with an unstructured working correlation matrix. To take into account any variation in the time spent as a resident in the province during a given year between participants, the logarithm of the time was included as an offset in all negative binomial regression models. To assess the temporal trends, an interaction term between patients with MS and controls and each year of the 5-year prodromal period (−1 to −5) was also included, with “−1” indicating the year before the index date and “−5” indicating the fifth year before the index date. Findings were expressed as adjusted rate ratios (aRRs) with the corresponding 95% CIs. Age at the index date (continuous), sex, SES (quintiles; Q1–5; Q1 as a reference), and index year (as a continuous variable) were included as covariates in the models.
All analyses were conducted using R (version 4.1.0; R Foundation for Statistical Computing, Vienna, Austria).
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Clinical Research Ethics Boards at the University of British Columbia (#H08-01544 and #H93-70103).
Data Availability
Because we are not the data custodians, we are not authorized to make these data available. With the appropriate approvals, data may be accessed through the Population Data British Columbia. For this study, data access was permitted through until end/February 2023 to perform the following: present findings at the Americas Committee for Treatment and Research in Multiple Sclerosis Forum, San Diego 2023 (selected for a platform presentation); complete this study; and submit for peer-review publication. This was in accordance with the BC Ministry of Health requirements surrounding data access. No further analyses or data linkages are permitted.
Results
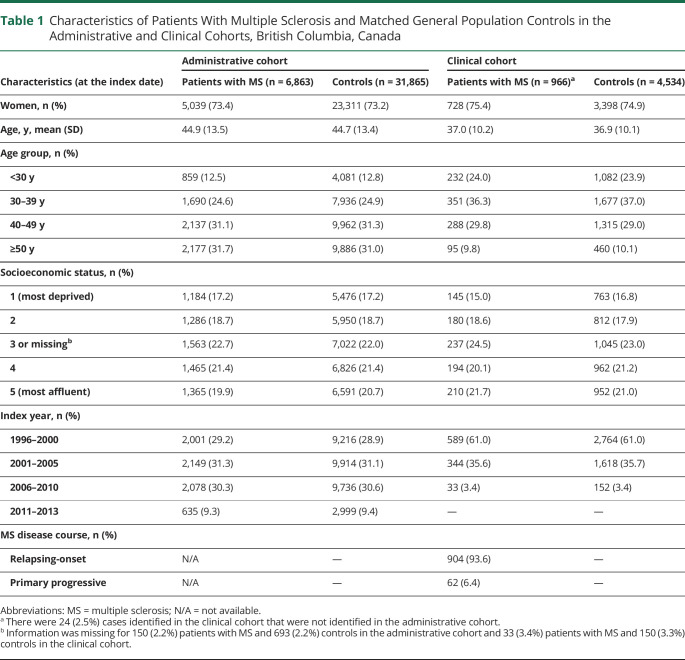
The administrative cohort consisted of 6,863 patients with MS and 31,865 matched controls (Table 1). The clinical cohort included 966 patients with MS and 4,534 controls. In both cohorts, women represented more than 70% of the population. The average age at the index dates was higher in the administrative cohort relative to the clinical cohort (44 vs 37 years). The distribution of SES quintiles was similar between cohorts. More than 90% of patients with MS in the clinical cohort had relapsing onset MS.
Table 1.
Characteristics of Patients With Multiple Sclerosis and Matched General Population Controls in the Administrative and Clinical Cohorts, British Columbia, Canada
| Characteristics (at the index date) | Administrative cohort | Clinical cohort | ||
| Patients with MS (n = 6,863) | Controls (n = 31,865) | Patients with MS (n = 966)a | Controls (n = 4,534) | |
| Women, n (%) | 5,039 (73.4) | 23,311 (73.2) | 728 (75.4) | 3,398 (74.9) |
| Age, y, mean (SD) | 44.9 (13.5) | 44.7 (13.4) | 37.0 (10.2) | 36.9 (10.1) |
| Age group, n (%) | ||||
| <30 y | 859 (12.5) | 4,081 (12.8) | 232 (24.0) | 1,082 (23.9) |
| 30–39 y | 1,690 (24.6) | 7,936 (24.9) | 351 (36.3) | 1,677 (37.0) |
| 40–49 y | 2,137 (31.1) | 9,962 (31.3) | 288 (29.8) | 1,315 (29.0) |
| ≥50 y | 2,177 (31.7) | 9,886 (31.0) | 95 (9.8) | 460 (10.1) |
| Socioeconomic status, n (%) | ||||
| 1 (most deprived) | 1,184 (17.2) | 5,476 (17.2) | 145 (15.0) | 763 (16.8) |
| 2 | 1,286 (18.7) | 5,950 (18.7) | 180 (18.6) | 812 (17.9) |
| 3 or missingb | 1,563 (22.7) | 7,022 (22.0) | 237 (24.5) | 1,045 (23.0) |
| 4 | 1,465 (21.4) | 6,826 (21.4) | 194 (20.1) | 962 (21.2) |
| 5 (most affluent) | 1,365 (19.9) | 6,591 (20.7) | 210 (21.7) | 952 (21.0) |
| Index year, n (%) | ||||
| 1996–2000 | 2,001 (29.2) | 9,216 (28.9) | 589 (61.0) | 2,764 (61.0) |
| 2001–2005 | 2,149 (31.3) | 9,914 (31.1) | 344 (35.6) | 1,618 (35.7) |
| 2006–2010 | 2,078 (30.3) | 9,736 (30.6) | 33 (3.4) | 152 (3.4) |
| 2011–2013 | 635 (9.3) | 2,999 (9.4) | — | — |
| MS disease course, n (%) | ||||
| Relapsing-onset | N/A | — | 904 (93.6) | — |
| Primary progressive | N/A | — | 62 (6.4) | — |
Abbreviations: MS = multiple sclerosis; N/A = not available.
There were 24 (2.5%) cases identified in the clinical cohort that were not identified in the administrative cohort.
Information was missing for 150 (2.2%) patients with MS and 693 (2.2%) controls in the administrative cohort and 33 (3.4%) patients with MS and 150 (3.3%) controls in the clinical cohort.
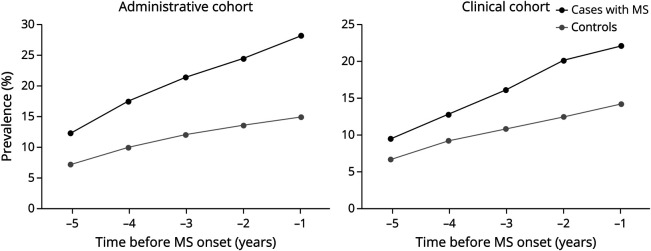
In both cohorts, the prevalence of psychiatric morbidity for patients with MS was higher than that for controls in each of the 5 years before the index dates. Moreover, these differences increased steadily as the index date approached (Figure 1). Over the entire 5-year period, 28.0% (1,920/6,863) of patients with MS and 14.9% (4,738/31,865) of controls in the administrative cohort had a psychiatric morbidity, as did 22.0% (213/966) patients with MS and 14.1% (638/4,534) of controls in the clinical cohort.
Figure 1. Prevalence of Psychiatric Morbidity Among Patients With MS and Controls in the Administrative and Clinical Cohorts in the 5 Years Before MS Onset.
In the administrative cohort, an individual's first demyelinating disease-related claim represented MS onset. In the clinical cohort, MS onset was defined as the date of MS symptom onset, as recorded by an MS neurologist. The period represents the 5-year period before MS onset (e.g., −5 reflects the fifth year before MS onset and −1 the year before MS onset). MS = multiple sclerosis.
In the administrative cohort, the prevalence ratio of psychiatric morbidity in the 5 years before the first demyelinating event among patients with MS was 91% higher than that among controls (prevalence ratio 1.91; 95% CI 1.83–2.00). For the clinical cohort, the prevalence ratio was similarly high for patients with MS (prevalence ratio 1.58; 95% CI 1.38–1.81) in the 5 years before MS symptom onset.
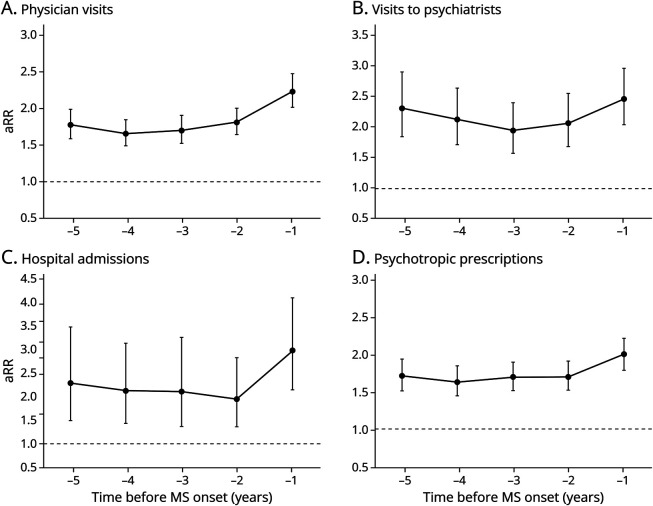
In the administrative cohort, people with MS, regardless of whether they fulfilled the algorithm for psychiatric morbidity, had more all-physician visits for psychiatric morbidity, visits to psychiatrists, psychiatric-related hospital admissions, and “psychotropic” prescriptions during each of the 5 years before their first demyelinating event, compared with the matched controls (Figure 2). For example, the rate of psychiatric-related physician visits was 78% higher in people with MS than in controls in the fifth year before the index date (aRR 1.78; 95% CI 1.59–1.98), increasing to 124% higher in the year before (aRR 2.24; 95% CI 2.03–2.49; Figure 2A). Visits to psychiatrists were 132% higher in the fifth year before the index date (aRR 2.32; 95% CI 1.85–2.92) and 146% higher in the year before (aRR 2.46; 95% CI 2.04–2.98; Figure 2B). Psychiatric-related hospital admissions ranged from 129% to 197% higher in the fifth to the first year preindex date (aRR 2.29; 95% CI 1.50–3.50 to aRR 2.97; 95% CI 2.15–4.10; Figure 2C), and the rate of psychotropic prescriptions ranged from 72% to 100% higher over the same periods preindex date in people with MS than in controls (aRR 1.72; 95% CI 1.54–1.93 to aRR 2.00; 95% CI 1.81–2.22; Figure 2D).
Figure 2. Rate Ratios of Physician Visits for Psychiatric Morbidity, Visits to Psychiatrists, Psychiatric-Related Hospital Admissions, and “Psychotropic” Prescriptions in the 5 Years Before MS Onset for Patients With MS vs Matched Controls in the Administrative Cohort.
In the administrative cohort, an individual's first demyelinating disease-related claim represented MS onset. In the clinical cohort, MS onset was defined as the date of MS symptom onset, as recorded by an MS neurologist. The period represents the 5-year period before MS onset (e.g., −5 reflects the fifth year before MS onset and −1 the year before MS onset). An adjusted rate ratio greater than 1 indicates a higher rate of visits, admissions, or prescriptions filled in people with MS relative to matched controls. The bars represent 95% CIs. Models were adjusted for age at index date (continuous), sex (categorical), socioeconomic status (quintiles), and index year (continuous). aRR = adjusted rate ratio; MS = multiple sclerosis.
While the direction of findings was broadly similar in the clinical cohort, the magnitude of the associations was modest; few reached statistical significance in this smaller population, and the 95% CIs were often wide (eTable 4, links.lww.com/WNL/D118). See eTables 5 and 6 for the results of the covariates included in the models.
Discussion
In this large population population-based study using administrative and clinical data, the prevalence of psychiatric morbidity was higher in people with MS than in matched general population controls in each of the 5 years preceding the first demyelinating event or MS symptom onset. Moreover, these differences increased steadily as MS onset date approached. Over the entire 5-year period before MS onset, the prevalence ratio of psychiatric morbidity was higher for patients with MS than that for controls by 58% before MS symptom onset in the clinical cohort and by 91% before a first demyelinating event in the administrative cohort. When each of the different types of health service use was evaluated, we found that in the administrative cohort, the yearly number of psychiatric-related physician visits and hospital admissions and visits to psychiatrists and psychotropic prescription dispensations increased in magnitude among individuals with MS vs controls across each of the 5 years before the first demyelinating event. Together, these findings suggest that psychiatric morbidity constitutes a significant burden very early in the MS disease course and may be a feature of the MS prodrome.
Few studies have comprehensively examined psychiatric morbidity in the years leading up to MS onset. For example, a prior Canada-based study from our group found that from 14 morbidities examined in the 5 years before a first demyelinating claim, the prevalence of mood/anxiety disorders exhibited one of the highest differences between patients with MS and controls (present in 24% of patients with MS and 15% of controls, p < 0.001).5 A UK study exploring records of general practitioners also found that up to 5–10 years before a first diagnostic (Read code) for MS or clinically isolated syndrome, individuals with MS were more likely than controls to have a Read code for anxiety and depression (e.g., 11.4% of patients with MS and 9.8% of controls had depression in the 5–10 years before; OR 1.26; 95% CI 1.07–1.47).10 Our current psychiatric-focused study substantially advances these previous observations by demonstrating differences among patients with MS and controls after applying a validated case definition to identify psychiatric morbidity and exploring psychiatric-related health care use (i.e., physician visits, hospital admissions, and prescription dispensations) for the entire population regardless as to whether individuals fulfilled this algorithm.
Another population-based study in Manitoba, Canada, observed that the incidence of psychiatric disorders was higher up to 5–10 years before a diagnosis of an immune-mediated inflammatory disease (MS, inflammatory bowel disease, or rheumatoid arthritis) vs controls, with an incidence rate ratio for psychiatric comorbidity of 1.60 five years before diagnosis rising to 2.92 the year before for individuals with any of these inflammatory conditions.4 Together, these findings suggest that psychiatric morbidity are both a relevant feature of the MS prodrome and common in the prodromal phase of other inflammatory conditions. These observations are intriguing and could relate to a number of different factors. For example, psychosocial stress may be directly caused by the other unexplained ongoing prodromal symptoms or the process of navigating the health care system.5 Other alternatives include that individuals with MS and psychiatric morbidity may have common genetic determinants28 or share similar risk factors, such as adverse childhood experiences or obesity.29,30 Furthermore, common etiopathologic pathways, such as low-grade inflammation, could be behind both MS and the psychiatric phenomenon,31 with evidence supporting the role of inflammation driving the development not only of depression but also of other psychiatric disorders including bipolar disorder, anxiety, and schizophrenia.32,33 Finally, there is a possibility of confounding. Our results showed that the difference in the prevalence of psychiatric morbidity became substantially more evident as MS onset (the index date) approached, raising the possibility that a progressive increase in inflammation may be sufficient for psychiatric morbidity to manifest but not yet for MS to become apparent.
A better characterization of the MS prodrome may allow for earlier recognition, diagnosis, and management of MS.34,35 Our findings further advance our understanding of this early stage of the disease by providing a comprehensive assessment of the frequency and relative burden of psychiatric morbidity during the MS prodrome. How best to harness these observations to benefit individuals seeking an explanation for their prodrome-related signs and symptoms, which are also common in the general population, requires further work and careful consideration. Examples could include judicious raising of awareness of the characteristics of the MS prodrome among physician specialties that are often sought by such individuals, including psychiatrists. Such specialties may play a valuable role in the identification of early MS. In addition, because psychiatric comorbidity negatively affects quality of life15 and MS disability progression,16 their prompt management may benefit both short-term and longer-term health outcomes in MS.
Study limitations included the potential underestimation or overestimation of the true burden of psychiatric morbidity during the MS prodrome. Underestimation could occur, given that case definitions used to identify psychiatric disorders in administrative data may experience low sensitivity.26 Reasons for this may include avoidance of care by people experiencing mental health problems due to social stigma and inability to capture self-managed symptomatology. Undercoding of psychiatric disorders by physicians in the presence of other competing conditions is also possible.36 In addition, administrative data will not capture encounters with nonphysician providers (e.g., counselors, therapists, psychologists, etc). While these limitations would be expected to affect both patients with MS and controls, it remains possible that the true psychiatric burden during the MS prodrome is much higher than estimated. Equally, the psychiatric burden could be an overestimate due to surveillance bias because people in the prodromal phase interact more frequently with the health care system7 and therefore may present more opportunities to be diagnosed with a psychiatric disorder.
Strengths of our study include the use of administrative health data within a universal health care setting, which allowed us to capture all physician visits, hospital admissions, and prescription dispensations across nearly the entire province, regardless of the resident's ability to pay, minimizing selection bias. In addition, prospective capture of health-related data virtually eliminates recall bias. Our ability to access both administrative and clinical cohorts was a study strength as well. Differences in the index date definition for both cohorts explain the higher average age at the index date for the administrative cohort relative to the clinical cohort. Nonetheless, our 2 cohorts were similar for the other sociodemographic factors, including sex and SES. Arguably, the period before MS symptom onset, the index date of the clinical cohort, is more representative of the “true” MS prodrome because not every symptom likely attributable to early MS necessarily leads to health care system utilization and/or may not lead to a demyelinating disease-related issue being recognized and recorded. However, our clinical cohort was also rather modest in size, such that while findings were in a similar direction to those observed in the larger administrative cohort, many did not reach significance. We do not expect that age differences between cohorts played a role in this given that the prevalence of psychiatric disorders has not been shown to increase with age in the population with MS.37-39 Finally, the use of a validated algorithm to identify both MS and psychiatric morbidity was also a study strength.
Psychiatric morbidity represents a contributor to increased health care use by individuals with MS in the 5 years before MS onset. Findings suggest that psychiatric morbidity constitutes a significant burden very early in the MS disease course and may be a feature of the MS prodrome.
Acknowledgment
Access to data provided by the Data Steward(s) is subject to approval, but can be requested for research projects through the Data Steward(s) or their designated service providers. All inferences, opinions, and conclusions drawn in this publication are those of the author(s) and do not reflect the opinions or policies of the Data Steward(s). The authors thank the British Columbia Multiple Sclerosis Clinic neurologists who contributed to the study through patient examination (current/active members during data extraction listed here by primary clinic): UBC MS Clinic: V. Devonshire, MD, FRCPC; A.-L. Sayao, MD, FRCPC; A. Traboulsee, MD, FRCPC; S. Hashimoto, MD, FRCPC (UBC and Victoria MS Clinics); J. Hooge, MD, FRCPC (UBC and Prince George MS Clinic); L. Kastrukoff, MD, FRCPC (UBC and Prince George MS Clinic); J. Oger, MD, FRCPC. Kelowna MS Clinic: D. Adams, MD, FRCPC; D. Craig, MD, FRCPC; S. Meckling, MD, FRCPC. Prince George MS Clinic: L. Daly, MD, FRCPC. Victoria MS Clinic: O. Hrebicek, MD, FRCPC; D. Parton, MD, FRCPC; K Atwell-Pope, MD, FRCPC. The views expressed in this study do not necessarily reflect the views of each individual acknowledged.
Glossary
- aRR
adjusted rate ratio
- ATC
Anatomical Therapeutic Chemical
- BC
British Columbia
- ICD
International Classification of Diseases
- MS
multiple sclerosis
- MSP
Medical Services Plan
- OR
odds ratio
- SES
socioeconomic status
Appendix. Authors

| Name | Location | Contribution |
| Anibal S. Chertcoff, MD | Faculty of Medicine (Neurology), University of British Columbia and The Djavad Mowafaghian Center for Brain Health, Vancouver, Canada | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Fardowsa L.A. Yusuf, MSc | Faculty of Medicine (Neurology), University of British Columbia and The Djavad Mowafaghian Center for Brain Health; School of Population and Public Health, University of British Columbia, Vancouver, Canada | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Feng Zhu, MSc | Faculty of Medicine (Neurology), University of British Columbia and The Djavad Mowafaghian Center for Brain Health, Vancouver, Canada | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Charity Evans, PhD | College of Pharmacy and Nutrition, University of Saskatchewan, Saskatoon, Canada | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| John D. Fisk, PhD | Nova Scotia Health and the Departments of Psychiatry, Psychology & Neuroscience, and Medicine, Dalhousie University, Halifax, Canada | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Yinshan Zhao, PhD | Faculty of Medicine (Neurology), University of British Columbia and The Djavad Mowafaghian Center for Brain Health, Vancouver, Canada | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Ruth Ann Marrie, MD, PhD | Departments of Internal Medicine and Community Health Sciences, Health Sciences Center, Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, Canada | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Helen Tremlett, PhD | Faculty of Medicine (Neurology), University of British Columbia and The Djavad Mowafaghian Center for Brain Health, Vancouver, Canada | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
Footnotes
Editorial, page 873
Study Funding
This work was supported by the National Multiple Sclerosis Society and MS Society of Canada (RG5063A4/1; RFA-2103-37392; EGID: P002; PI: Helen Tremlett), an MS Society of Canada's endMS Postdoctoral Fellowship, and the Michael Smith Foundation for Health Research Trainee Award (awardee: Chertcoff).
Disclosure
A.S. Chertcoff receives funding from the MS Society of Canada's endMS Postdoctoral Fellowship and the Michael Smith Foundation for Health Research Trainee Award. F.L.A. Yusuf is funded by a Fredrick Banting and Charles Best Canada Graduate Scholarship from the Canadian Institutes of Health Research (CIHR). F. Zhu, C. Evans, and Y. Zhao have no disclosures. J.D. Fisk received research grant support from the Canadian Institutes of Health Research, the National Multiple Sclerosis Society, the Multiple Sclerosis Society of Canada, Crohn's and Colitis Canada and Research Nova Scotia, and consultation and distribution royalties from MAPI Research Trust. R.A. Marrie receives research funding from CIHR, Research Manitoba, Multiple Sclerosis Society of Canada, Multiple Sclerosis Scientific Foundation, Crohn's and Colitis Canada, National Multiple Sclerosis Society, CMSC, the Arthritis Society and the US Department of Defense and is a coinvestigator on studies receiving funding from Biogen Idec and Roche Canada. H. Tremlett has, in the past 5 years, received research support from the Canada Research Chair Program, the National Multiple Sclerosis Society, the Canadian Institutes of Health Research, the Multiple Sclerosis Society of Canada, the Multiple Sclerosis Scientific Research Foundation, and the EDMUS Foundation (“Fondation EDMUS contre la sclérose en plaques”); in addition, in the past 5 years, has had travel expenses or registration fees prepaid or reimbursed to present at CME conferences from the Consortium of MS Centers (2018, 2023), National MS Society (2018, 2022), ECTRIMS/ACTRIMS (2017–2022), American Academy of Neurology (2019). Speaker honoraria are either declined or donated to an MS charity or to an unrestricted grant for use by HT's research group. Go to Neurology.org/N for full disclosures.
References
- 1.Marrie RA, Fisk JD, Tremlett H, et al. Differences in the burden of psychiatric comorbidity in MS vs the general population. Neurology. 2015;85(22):1972-1979. doi: 10.1212/wnl.0000000000002174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305-317. doi: 10.1177/1352458514564487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrie RA, O'Mahony J, Maxwell C, et al. Increased mental health care use by mothers of children with multiple sclerosis. Neurology. 2020;94(10):e1040-e1050. doi: 10.1212/wnl.0000000000008871 [DOI] [PubMed] [Google Scholar]
- 4.Marrie RA, Walld R, Bolton JM, et al. Rising incidence of psychiatric disorders before diagnosis of immune-mediated inflammatory disease. Epidemiol Psychiatr Sci. 2019;28(3):333-342. doi: 10.1017/s2045796017000579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wijnands JM, Zhu F, Kingwell E, et al. Five years before multiple sclerosis onset: phenotyping the prodrome. Mult Scler. 2019;25(8):1092-1101. doi: 10.1177/1352458518783662 [DOI] [PubMed] [Google Scholar]
- 6.Yusuf FLA, Ng B, Wijnands J, Kingwell E, Marrie RA, Tremlett H. A systematic review of morbidities suggestive of the multiple sclerosis prodrome. Expert Rev Neurother. 2020;20(8):799-819. doi: 10.1080/14737175.2020.1746645 [DOI] [PubMed] [Google Scholar]
- 7.Wijnands JMA, Kingwell E, Zhu F, et al. Health-care use before a first demyelinating event suggestive of a multiple sclerosis prodrome: a matched cohort study. Lancet Neurol. 2017;16(6):445-451. doi: 10.1016/s1474-4422(17)30076-5 [DOI] [PubMed] [Google Scholar]
- 8.Tremlett H, Marrie RA. The multiple sclerosis prodrome: emerging evidence, challenges, and opportunities. Mult Scler. 2021;27(1):6-12. doi: 10.1177/1352458520914844 [DOI] [PubMed] [Google Scholar]
- 9.Wijnands JMA, Zhu F, Kingwell E, et al. Prodrome in relapsing-remitting and primary progressive multiple sclerosis. Eur J Neurol. 2019;26(7):1032-1036. doi: 10.1111/ene.13925 [DOI] [PubMed] [Google Scholar]
- 10.Disanto G, Zecca C, MacLachlan S, et al. Prodromal symptoms of multiple sclerosis in primary care. Ann Neurol. 2018;83(6):1162-1173. doi: 10.1002/ana.25247 [DOI] [PubMed] [Google Scholar]
- 11.Högg T, Wijnands JMA, Kingwell E, et al. Mining healthcare data for markers of the multiple sclerosis prodrome. Mult Scler Relat Disord. 2018;25:232-240. doi: 10.1016/j.msard.2018.08.007 [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Wijnands J, Högg T, et al. Interrogation of the multiple sclerosis prodrome using high dimensional health data. Neuroepidemiology. 2020;54(2):140-147. doi: 10.1159/000505331 [DOI] [PubMed] [Google Scholar]
- 13.Yusuf F, Wijnands J, Kingwell E, et al. Fatigue, sleep disorders, anaemia and pain in the multiple sclerosis prodrome. Mult Scler. 2021;27(2):290-302. doi: 10.1177/1352458520908163 [DOI] [PubMed] [Google Scholar]
- 14.Bjornevik K, Munger KL, Cortese M, et al. Serum neurofilament light chain levels in patients with presymptomatic multiple sclerosis. JAMA Neurol. 2020;77(1):58-64. doi: 10.1001/jamaneurol.2019.3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marrie RA, Patten SB, Berrigan LI, et al. ; CIHR Team in the Epidemiology and Impact of Comorbidity on Multiple Sclerosis (ECoMS). Diagnoses of depression and anxiety versus current symptoms and quality of life in multiple sclerosis. Int J MS Care. 2018;20(2):76-84. doi: 10.7224/1537-2073.2016-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKay KA, Tremlett H, Fisk JD, et al. ; CIHR Team in the Epidemiology and Impact of Comorbidity on Multiple Sclerosis. Psychiatric comorbidity is associated with disability progression in multiple sclerosis. Neurology. 2018;90(15):e1316-e1323. doi: 10.1212/wnl.0000000000005302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrie RA, Elliott L, Marriott J, et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology. 2015;85(3):240-247. doi: 10.1212/wnl.0000000000001718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.British Columbia Ministry of Health. Medical Services Plan (MSP) Payment Information File. V2. Population Data BC. Data Extract. MOH; 2017. Accessed June 1, 2023. popdata.bc.ca/data. [Google Scholar]
- 19.Canadian Institute for Health Information. Discharge Abstract Database (Hospital Separations). V2. Population Data BC. Data Extract. MOH; 2017. Accessed June 1, 2023. popdata.bc.ca/data. [Google Scholar]
- 20.British Columbia Ministry of Health. PharmaNet. V2. BC Ministry of Health. Data Extract. Data Stewardship Committee; 2017. Accessed June 1, 2023. popdata.bc.ca/data. [Google Scholar]
- 21.British Columbia Ministry of Health. Consolidation File (MSP Registration & Premium Billing). V2. Population Data BC. Data Extract. MOH; 2017. Accessed June 1, 2023. popdata.bc.ca/data. [Google Scholar]
- 22.BC Vital Statistics Agency. Vital Statistics Deaths. V2. Population Data BC. Data Extract. BC Vital Statistics Agency; 2016. Accessed June 1, 2023. popdata.bc.ca/data. [Google Scholar]
- 23.Canadian Institute for Health Information and Statistics Canada. Health Indicators 2013. CIHI, 2013. Accessed June 1, 2023. cihi.ca/sites/default/files/document/health-indicators-2013-en.pdf. [Google Scholar]
- 24.Ng HS, Zhu F, Kingwell E, et al. Characteristics of a population-based multiple sclerosis cohort treated with disease-modifying drugs in a universal healthcare setting. Expert Rev Neurother. 2021;21(1):131-140. doi: 10.1080/14737175.2021.1847085 [DOI] [PubMed] [Google Scholar]
- 25.Al-Sakran LH, Marrie RA, Blackburn DF, Knox KB, Evans CD. Establishing the incidence and prevalence of multiple sclerosis in Saskatchewan. Can J Neurol Sci. 2018;45(3):295-303. doi: 10.1017/cjn.2017.301 [DOI] [PubMed] [Google Scholar]
- 26.Marrie RA, Fisk JD, Yu BN, et al. Mental comorbidity and multiple sclerosis: validating administrative data to support population-based surveillance. BMC Neurol. 2013;13:16. doi: 10.1186/1471-2377-13-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690-1691. doi: 10.1001/jama.280.19.1690 [DOI] [PubMed] [Google Scholar]
- 28.Kowalec K, Fitzgerald KC, Salter A, et al. Polygenicity of comorbid depression in multiple sclerosis. Neurology. 2023;101(5):e522-e532. doi: 10.1212/WNL.0000000000207457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan A, Bernstein CN, Graff LA, et al. Childhood maltreatment and psychiatric comorbidity in immune-mediated inflammatory disorders. Psychosom Med. 2022;84(1):10-19. doi: 10.1097/psy.0000000000001025 [DOI] [PubMed] [Google Scholar]
- 30.Harroud A, Marrie RA, Fitzgerald KC, et al. Mendelian randomization provides no evidence for a causal role in the bidirectional relationship between depression and multiple sclerosis. Mult Scler. 2021;27(13):2077-2084. doi: 10.1177/1352458521993075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossi S, Studer V, Motta C, et al. Neuroinflammation drives anxiety and depression in relapsing-remitting multiple sclerosis. Neurology. 2017;89(13):1338-1347. doi: 10.1212/wnl.0000000000004411 [DOI] [PubMed] [Google Scholar]
- 32.Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39(1):125-137. doi: 10.1016/j.psc.2015.09.006 [DOI] [PubMed] [Google Scholar]
- 33.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22-34. doi: 10.1038/nri.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tremlett H, Munger KL, Makhani N. The multiple sclerosis prodrome: evidence to action. Front Neurol. 2022;12:761408. doi: 10.3389/fneur.2021.761408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marrie RA, Allegretta M, Barcellos LF, et al. From the prodromal stage of multiple sclerosis to disease prevention. Nat Rev Neurol. 2022;18(9):559-572. doi: 10.1038/s41582-022-00686-x [DOI] [PubMed] [Google Scholar]
- 36.Marrie RA, McKay K. Administrative data for observational research in multiple sclerosis: opportunities and challenges. Mult Scler. 2022;28(1):3-6. doi: 10.1177/13524585211055787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan CK, Tian F, Pimentel Maldonado D, Mowry EM, Fitzgerald KC. Depression in multiple sclerosis across the adult lifespan. Mult Scler. 2021;27(11):1771-1780. doi: 10.1177/1352458520979304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia J, Finlayson M. Mental health and mental health service use among people aged 45+ with multiple sclerosis. Can J Commun Ment Health. 2009;24(2):9-22. doi: 10.7870/cjcmh-2005-0011 [DOI] [PubMed] [Google Scholar]
- 39.Patten SB, Beck CA, Williams JV, Barbui C, Metz L. Major depression in multiple sclerosis: a population-based perspective. Neurology. 2003;61(11):1524-1527. doi: 10.1212/01.wnl.0000095964.34294.b4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Because we are not the data custodians, we are not authorized to make these data available. With the appropriate approvals, data may be accessed through the Population Data British Columbia. For this study, data access was permitted through until end/February 2023 to perform the following: present findings at the Americas Committee for Treatment and Research in Multiple Sclerosis Forum, San Diego 2023 (selected for a platform presentation); complete this study; and submit for peer-review publication. This was in accordance with the BC Ministry of Health requirements surrounding data access. No further analyses or data linkages are permitted.