Abstract
Background and Objectives
Blood biomarkers glial fibrillary acidic protein (GFAP) and ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) have recently been Food and Drug Administration approved as predictors of intracranial lesions on CT after mild traumatic brain injury (mTBI). However, most cases with mTBI are CT negative, and no biomarkers are approved to assist diagnosis in these individuals. In this study, we aimed to determine the optimal combination of blood biomarkers to assist mTBI diagnosis in otherwise healthy adults younger than 50 years presenting to an emergency department within 6 hours of injury. To further understand the utility of biomarkers, we assessed how biological sex, presence or absence of loss of consciousness and/or post-traumatic amnesia (LOC/PTA), and delayed presentation affected classification performance.
Methods
Blood samples, symptom questionnaires, and cognitive tests were prospectively conducted for participants with mTBI recruited from The Alfred Hospital Level 1 Emergency & Trauma Center and uninjured controls. Follow-up testing was conducted at 7 days. Simoa quantified plasma GFAP, UCH-L1, tau, neurofilament light chain (NfL), interleukin (IL)–6, and IL-1β. Area under the receiver operating characteristic (AUC) analysis assessed classification accuracy for diagnosed mTBI, and logistic regression models identified optimal biomarker combinations.
Results
Plasma IL-6 (AUC 0.91, 95% CI 0.86–0.96), GFAP (AUC 0.85, 95% CI 0.78–0.93), and UCH-L1 (AUC 0.79, 95% CI 0.70–0.88) best differentiated mTBI (n = 74) from controls (n = 44) acutely (<6 hours), with NfL (AUC 0.81, 95% CI 0.72–0.90) the only marker to have such utility subacutely (7 days). Biomarker performance was similar between sexes and for participants with and without LOC/PTA, with the exception at 7 days, where GFAP and IL-6 retained some utility in female participants (GFAP: AUC 0.71, 95% CI 0.55–0.88; IL-6: AUC 0.71, 95% CI 0.55–0.87) and in those with LOC/PTA (GFAP: AUC 0.73, 95% CI 0.59–0.86; IL-6: AUC 0.71, 95% CI 0.57–0.84). Acute IL-6 (R2 = 0.50, 95% CI 0.34–0.64) outperformed GFAP and UCH-L1 combined (R2 = 0.35, 95% CI 0.17–0.50), with the best acute model featuring GFAP and IL-6 (R2 = 0.54, 95% CI 0.34–0.68).
Discussion
These findings indicate that adding IL-6 to a panel of brain-specific proteins such as GFAP and UCH-L1 might assist in the acute diagnosis of mTBI in adults younger than 50 years. Multiple markers had high classification accuracy in participants without LOC/PTA. When compared with the best-performing acute markers, subacute measures of plasma NfL resulted in minimal reduction in classification accuracy. Future studies will investigate the optimal time frame over which plasma IL-6 might assist diagnostic decisions and how extracranial trauma affects utility.
Introduction
Timely and accurate diagnosis of mild traumatic brain injury (mTBI) remains challenging, largely due to a lack of objective tests with sufficient diagnostic sensitivity and specificity. Although standardized brief symptom evaluation and computerized neurocognitive tools have been developed to support rapid screening for mTBI,1-3 psychosocial factors complicate their interpretation.4 Neuropsychological testing has identified reduced processing speed and attention as key early markers of mTBI, and therefore characterizing cognitive impairment acutely postinjury may inform evaluation.5 Cognitive assessments, however, are limited in their use acutely due to expertise and time requirements.4 Diagnosis of mTBI remains challenging in settings where expert clinicians are not available, and cases are often missed.6
Blood biomarkers represent promising candidates that might assist mTBI diagnosis by quantification of proteins associated with astroglial (e.g., glial fibrillary acidic protein [GFAP]; S100 calcium-binding protein B, S100B), neuronal (e.g., ubiquitin carboxy-terminal hydrolase L1, UCH-L1), and axonal (e.g., neurofilament light chain, NfL; tau) pathology.7 Measures of S100B (Scandinavia) and GFAP and UCH-L1 combinations (United States) are now approved for clinical use as highly sensitive predictors of intracranial bleeding post-TBI,8 with CENTER-TBI9 and TRACK-TBI10 consortia having demonstrated the added value of day-of-injury biomarkers over existing prognostic models of functional outcome after TBI, albeit primarily in moderate-to-severe TBI. In addition, some classification accuracy with biomarkers has been reported in smaller studies of mTBI in the emergency department (ED) and sport.11
Despite this progress, several key gaps must be addressed before biomarkers can be implemented within clinical practice for mTBI diagnosis. First, findings from other studies may lack generalizability to CT-negative mTBI in the ED. Several hospital-based studies conflate TBI severities or CT-negative and CT-positive mTBI,9,10,12,13 and sport-related concussion (SRC) cohort studies do not capture the heterogeneity in patient demographics and injury mechanisms present at EDs.14 Second, mTBI biomarker candidates have divergent and dynamic temporal profiles.7,11 Thus, findings from studies that collect blood at various time points postinjury may not reflect classification performance in an ED setting where presentations primarily occur within 5 hours.6,15 In addition, although biomarkers with an extended elevation may be more likely to assist with delayed presentations,16,17 it is unknown whether these have comparable classification accuracy with acute biomarkers. Third, while loss of consciousness (LOC; ≤30 minutes) and post-traumatic amnesia (PTA; <24 hours) are strong indicators of mTBI, their association with biomarkers is poorly characterized. Moreover, biomarkers may be more useful to assist mTBI diagnosis in patients who do not present with these overt signs; however, their utility in this subset of patients with mTBI is not established.
Studies have shown that cytokines interleukin (IL)–6 and IL-1β are elevated in the brain and CSF after TBI.18-23 There is some recent evidence that IL-6 may be elevated in blood acutely after concussion in athletes24,25 and military personnel,26 though further work is needed to establish acute classification accuracy for mTBI in the ED. While few studies have investigated peripheral levels of IL-1β after TBI, a new assay using Simoa technology has shown unprecedented sensitivity for detecting IL-1β.27
The aim of this study was to investigate how plasma GFAP, UCH-L1, tau, NfL, IL-6, and IL-1β, each emerging biomarker candidate linked to mTBI pathophysiology, might assist mTBI diagnosis in otherwise healthy patients younger than 50 years who present to an ED within 6 hours, without major extracranial injury, and have no intracranial lesion detected by CT (or no clinical indication for CT). Biomarker classification accuracy was assessed relative to an emergency physician diagnosis of mTBI as per the World Health Organization (WHO) definition.28 We also assessed how biological sex and presence or absence of LOC/PTA affected biomarker performance. By including a 7-day follow-up, we aimed to determine which subacute markers might best assist diagnosis for delayed presentations and how performance compared with the optimal acute stage markers and how biomarker profiles evolve over time. Finally, we included symptom report and cognitive tests to provide insights into their relative classification accuracy. We hypothesized that: (1) GFAP would have the highest classification accuracy when measured at <6 hours and NfL at 7 days, (2) NfL performance at 7 days would be similar to that of GFAP at <6 hours, (3) cytokines at <6 hours would have diagnostic utility and boost classification performance of GFAP and UCH-L1 combined, (4) classification accuracy would be comparatively higher in mTBI with LOC and/or PTA than that without, (5) cognitive and symptom outcomes would differentiate mTBI and controls, and (6) biomarkers would have similar performance in male and female participants.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The Alfred Health Human Research and Ethics Committee (Project ID 105/18) approved this study, and all participants provided written informed consent.
Study Population and Study Design
Individuals with mTBI presenting to the Level 1 Emergency & Trauma Center at The Alfred Hospital servicing the state of Victoria in Australia, who met a priori study criteria, and provided written consent were enrolled. Noninjured individuals responding to hospital and community communications were enrolled as controls.
Study Criteria
Inclusion criteria for the mTBI cohort were as follows: (1) age 18–50 years, (2) observed/reported head strike, (3) mTBI diagnosis as per the hospital-based use of the WHO definition,28 and (4) blood sampling <6 hours and cognitive testing <24 hours postinjury. Exclusion criteria were as follows: (1) previous moderate-to-severe TBI, (2) mTBI within the past year, (3) neurologic, psychiatric, or medical history of significance (i.e., such history acted on for exclusion were as follows: schizophrenia, extensive and acute eating disorder, autism, intellectual disability, and intravenous drug user), (4) medication confounding blood sampling, (5) significant extracranial injury (e.g., open, long bone, or complex fractures), (6) surgery and/or anesthesia requirement, (7) pregnant or breastfeeding status, and (8) English as a second language. Control participants had no observed/reported head strike, were aged 18–50 years, and had the same exclusion criteria.
Participant Information, Symptom Report, and Cognitive Testing
Participant demographics and medical history were gathered with the Acute Concussion Evaluation29 and a basic questionnaire. PTA was ascertained by retrospective self-report of memory loss duration immediately postinjury.
The Rivermead Postconcussion Symptom Questionnaire (RPQ)30 included 16 items self-rated on a 5-point scale that measured symptom frequency (0–16) and symptom severity (0–64) (Cronbach α = 0.94–0.95).31
The Test of Premorbid Functioning32 consists of 70 words of increasingly difficult grapheme-to-phoneme translations (mean = 100, SD = 15) used to estimate premorbid intellectual functioning, with higher age-corrected scale scores indicating greater premorbid functioning.
The Rey Auditory-Verbal Learning Test (RAVLT)33 consists of 5 learning trials, a 30-minute delay recall of 15 words with higher age-corrected34 index scores for total learning (correct trial 1–5 sum score), and delayed recall (correct delayed sum score), indicating greater verbal memory performance. Alternate forms at follow-up were used to reduce practice effects.35
The Digit Span test36 consists of 3 number repetition tasks measuring attention with higher age-corrected total scaled scores (mean = 10, SD = 3) indicating greater performance.
The King-Devick (KD) test37 are rows of numbers (left to right) read aloud with increasingly variable spacing across 3 trials (Cronbach α = 0.92)38 measuring visual scanning speed with lower total speed (seconds) and higher accuracy (omission and commission errors) indicating greater performance.
Cogstate39 is a computerized neurocognitive battery including 3 measures of performance speed: detection (psychomotor speed), identification (attention), and one back (working memory) with higher scores indicating worse performance and 1 measure of accuracy: one card learning (visual learning) with higher scores indicating better performance.
Blood Collection and Analysis
Venous blood was collected into K2EDTA tubes and centrifuged at 1,000–1,100g, with plasma aliquots stored at −80°C. Biomarkers were quantified on a Simoa HD-X Analyzer (Quanterix, Billerica, MA), using commercial kits. Neurology 4-plex B kits were used for GFAP, UCH-L1, NfL and tau, and single-plex Simoa assays were used for IL-6 and IL-1β. All samples were tested in duplicate, with plates balanced between groups. Some samples measured below the lower limit of detection (LLOD) for UCH-L1 (40/168) and IL-1β (3/166; and 2 samples could not be run) and were allocated values equal to the LLOD (i.e., 2.43 and 0.016 pg/mL, respectively). The duplicate coefficient of variation (CoV) of duplicates for GFAP, UCH-L1, NfL, tau, IL-6, and IL-1β was 8%, 27%, 6%, 5%, 4%, and 14%, respectively, and interpolate CoV of controls were 7%, 5%, 17%, 8%, 6%, and 8%, respectively.
Analysis
Statistical analyses were conducted in R, version 4.0.3.28 Unless otherwise stated, all analyses were 2-tailed with significance levels of p < 0.05. Nominal and ordinal variables were evaluated using the Fisher exact test. Biomarkers underwent natural logarithm (Ln) transformation to provide a better approximation of a normal distribution. Cogstate values were transformed as per industry-based recommendations.40 Neuropsychological measures were analyzed in their raw/original format. The R package pROC40 was used to analyze area under the receiver operating characteristic (AUC) curves for plasma, symptom, and cognitive outcomes between groups at <6 hours and 7 days with AUC values classified (<0.5 failed, 0.6–0.7 poor, 0.7–0.8 moderate/acceptable, 0.8–0.9 good, and >0.9 excellent).41,42 Youden index optimal cutoffs were determined for each biomarker with good or excellent classification utility in the overall mTBI vs control comparison for each time point. The pROC package was also used to compare 2 AUC curves and compared unpaired AUC curves using the Delong method with a false discovery rate implemented. In line with our hypotheses, AUC comparisons were made between GFAP and the other biomarkers acutely and between NfL and other biomarkers subacutely. Additional AUC analyses were conducted that adjusted for age and sex using the ROCnReg package.43 Unadjusted AUC were used throughout the manuscript, given age and sex did not result in significant changes. Logistic regression models were developed with different biomarker combinations, assessing model performance with the primary index of Tjur R2, Bayesian Information Criterion (BIC), and Akaike Information Criterion (AIC). Mean and standard deviation data from a study of SRC (acute GFAP in SRC [n = 100] vs contact control [n = 133]; α of 0.05 and desired power of 0.90) were used to determine a required sample size of 43.44
Data Availability
Data sharing requests will be considered by the corresponding author.
Results
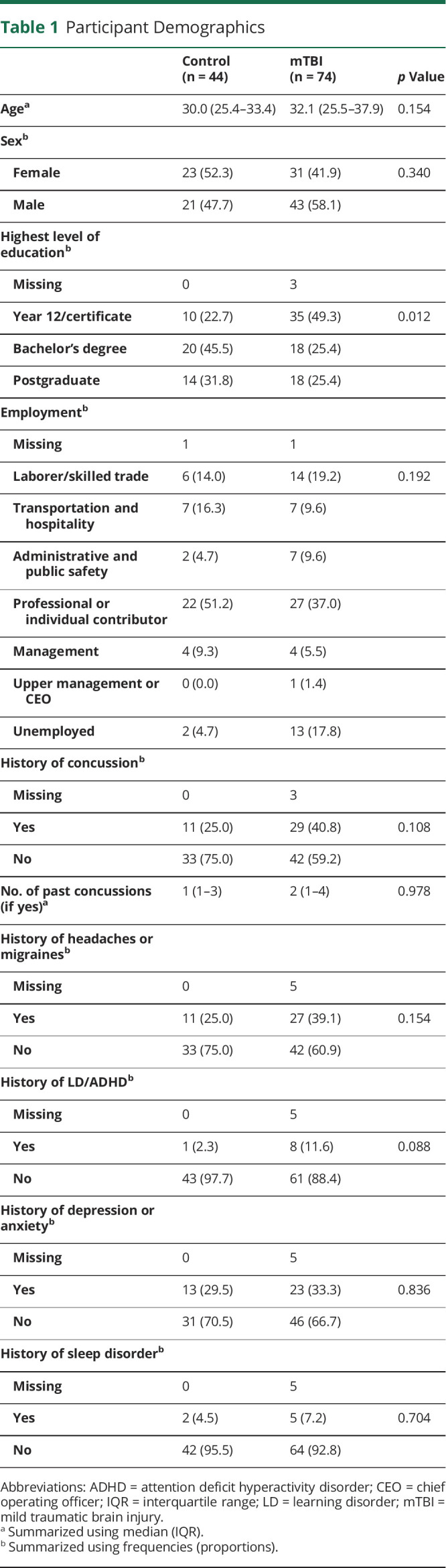
There were 118 participants enrolled, 74 with mTBI and 44 uninjured controls. mTBI follow-ups were completed by 69% (n = 51) at a median of 8 days (interquartile range [IQR] = 7–9, min = 5, max = 15) postinjury. Follow-ups with repeat symptom and cognitive testing were completed by 57% (n = 25) of control participants at a median of 9 days (IQR = 7–10.5, min = 7, max = 15). Comparable demographics and medical histories were observed for participants with mTBI and controls (Table 1), except for higher education among controls.
Table 1.
Participant Demographics
| Control (n = 44) | mTBI (n = 74) | p Value | |
| Agea | 30.0 (25.4–33.4) | 32.1 (25.5–37.9) | 0.154 |
| Sexb | |||
| Female | 23 (52.3) | 31 (41.9) | 0.340 |
| Male | 21 (47.7) | 43 (58.1) | |
| Highest level of educationb | |||
| Missing | 0 | 3 | |
| Year 12/certificate | 10 (22.7) | 35 (49.3) | 0.012 |
| Bachelor's degree | 20 (45.5) | 18 (25.4) | |
| Postgraduate | 14 (31.8) | 18 (25.4) | |
| Employmentb | |||
| Missing | 1 | 1 | |
| Laborer/skilled trade | 6 (14.0) | 14 (19.2) | 0.192 |
| Transportation and hospitality | 7 (16.3) | 7 (9.6) | |
| Administrative and public safety | 2 (4.7) | 7 (9.6) | |
| Professional or individual contributor | 22 (51.2) | 27 (37.0) | |
| Management | 4 (9.3) | 4 (5.5) | |
| Upper management or CEO | 0 (0.0) | 1 (1.4) | |
| Unemployed | 2 (4.7) | 13 (17.8) | |
| History of concussionb | |||
| Missing | 0 | 3 | |
| Yes | 11 (25.0) | 29 (40.8) | 0.108 |
| No | 33 (75.0) | 42 (59.2) | |
| No. of past concussions (if yes)a | 1 (1–3) | 2 (1–4) | 0.978 |
| History of headaches or migrainesb | |||
| Missing | 0 | 5 | |
| Yes | 11 (25.0) | 27 (39.1) | 0.154 |
| No | 33 (75.0) | 42 (60.9) | |
| History of LD/ADHDb | |||
| Missing | 0 | 5 | |
| Yes | 1 (2.3) | 8 (11.6) | 0.088 |
| No | 43 (97.7) | 61 (88.4) | |
| History of depression or anxietyb | |||
| Missing | 0 | 5 | |
| Yes | 13 (29.5) | 23 (33.3) | 0.836 |
| No | 31 (70.5) | 46 (66.7) | |
| History of sleep disorderb | |||
| Missing | 0 | 5 | |
| Yes | 2 (4.5) | 5 (7.2) | 0.704 |
| No | 42 (95.5) | 64 (92.8) |
Abbreviations: ADHD = attention deficit hyperactivity disorder; CEO = chief operating officer; IQR = interquartile range; LD = learning disorder; mTBI = mild traumatic brain injury.
Summarized using median (IQR).
Summarized using frequencies (proportions).
Injury Characteristics
Causes of mTBI were most commonly falls (23%), sport-related injuries (19%), motor vehicle accident (16%), “other” blunt trauma (16%), and cycling (14%) (eTable 1, links.lww.com/WNL/D130). Of 74 participants with mTBI, 63 (85%) underwent a CT brain scan, and all revealed no intracranial pathology. A CT scan was not performed on 11 participants, as determined by the treating clinician. mTBIs were regarded isolated injury in 65 (88%) participants or mTBI with minor extracranial injuries in 9 (12%) participants (eTable 2).
Symptoms
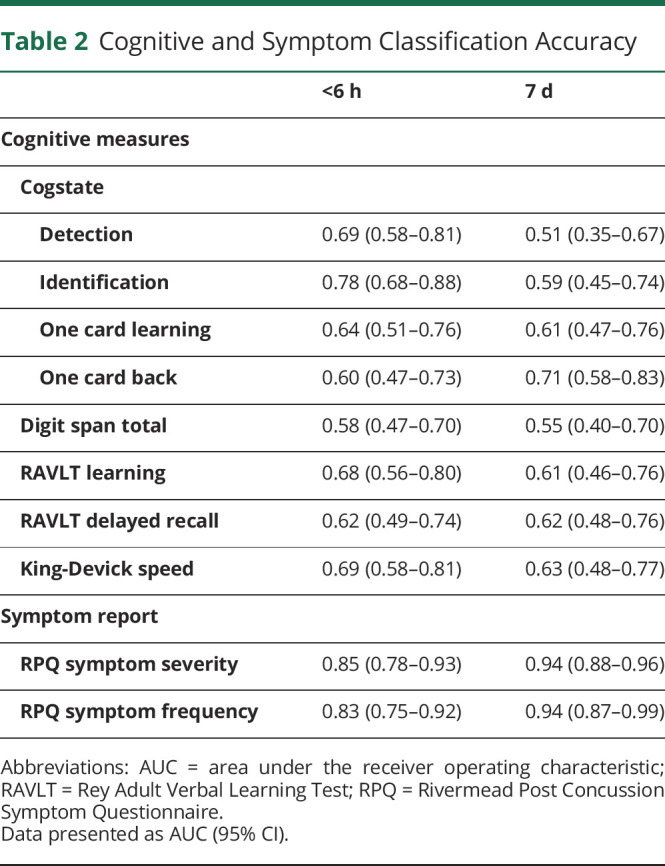
Participants with mTBI endorsed greater RPQ symptom frequency and severity than controls at both time points (eFigure 1, links.lww.com/WNL/D130), with AUC analysis demonstrating good (<6 hours) to excellent (7 days) mTBI classification accuracy (Table 2).
Table 2.
Cognitive and Symptom Classification Accuracy
| <6 h | 7 d | |
| Cognitive measures | ||
| Cogstate | ||
| Detection | 0.69 (0.58–0.81) | 0.51 (0.35–0.67) |
| Identification | 0.78 (0.68–0.88) | 0.59 (0.45–0.74) |
| One card learning | 0.64 (0.51–0.76) | 0.61 (0.47–0.76) |
| One card back | 0.60 (0.47–0.73) | 0.71 (0.58–0.83) |
| Digit span total | 0.58 (0.47–0.70) | 0.55 (0.40–0.70) |
| RAVLT learning | 0.68 (0.56–0.80) | 0.61 (0.46–0.76) |
| RAVLT delayed recall | 0.62 (0.49–0.74) | 0.62 (0.48–0.76) |
| King-Devick speed | 0.69 (0.58–0.81) | 0.63 (0.48–0.77) |
| Symptom report | ||
| RPQ symptom severity | 0.85 (0.78–0.93) | 0.94 (0.88–0.96) |
| RPQ symptom frequency | 0.83 (0.75–0.92) | 0.94 (0.87–0.99) |
Abbreviations: AUC = area under the receiver operating characteristic; RAVLT = Rey Adult Verbal Learning Test; RPQ = Rivermead Post Concussion Symptom Questionnaire.
Data presented as AUC (95% CI).
Cognitive Performance
All control participants completed all testing; however, 31 (41.9%) participants with mTBI did not complete <6 hours Cogstate (primarily due to cervical collar use) and 7 (9.5%) in total were unable to complete other individual tasks. Visual scanning speed (KD) revealed slower performance among those with mTBI when compared with that among controls at <6 hours (t(105.8) = −4.41, p < 0.001) and 7 days (t(64.3) = −2.78, p = 0.007) (eFigure 2, links.lww.com/WNL/D130), with AUC values of 0.69 and 0.63, respectively (Table 2). Digit span performance was worse in participants with mTBI at <6 hours (t(83.1) = 2.32, p = 0.023) though not at 7 days (t(40.2) = 1.28, p = 0.208); however, classification accuracy was poor at both time points. RAVLT Total Learning and Delayed Recall performance was worse in participants with mTBI at <6 hours (Total Learning: t(107.4) = 5.13, p < 0.001; Delayed Recall: t(107.1) = 3.54, p < 0.001), though not at 7 days (Total Learning: t(43.2) = 2.0, p = 0.052; Delayed Recall: t(48.2) = 1.76, p = 0.085). Cogstate identification [attention] at <6 hours was poorer in participants with mTBI (t(73.8) = −4.31, p < 0.001) and had most utility with moderate/acceptable classification accuracy (AUC = 0.78). Cogstate detection [speed] (t(52.2) = −3.24, p = 0.002), one card learning [visual learning] (t(74.6) = 3.31, p = 0.001), and one back [working memory] (t(77.3) = −2.21, p = 0.03) tasks at <6 hours were poorer in participants with mTBI. Cogstate one back [working memory] at 7 days was poorer in participants with mTBI (t(66.9) = −3.59, p < 0.001) and had most utility with moderate/acceptable classification accuracy (AUC = 0.71). Classification accuracy for cognitive measures was largely unchanged after controlling for premorbid functioning (eTable 3).
Biomarker Temporal Profile
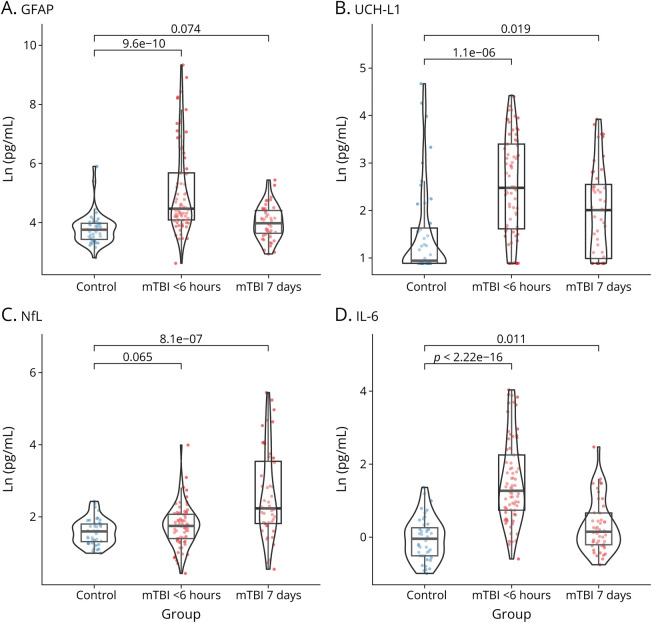
Multiple differences in Ln-transformed concentrations were observed between controls and participants with mTBI (Figure 1). Untransformed concentrations are shown in eFigure 3 (links.lww.com/WNL/D130). One <6 hours mTBI sample was unsuitable for analysis, and samples from 3 participants with mTBI were missing at 7 days. When compared with controls, GFAP was elevated in mTBI at <6 hours (t(98.8) = +6.76, p < 0.001) but not at 7 days (t(89.9) = +1.81, p = 0.074). Participants with mTBI had higher UCH-L1 levels at <6 hours (t(94.8) = +5.22, p < 0.001) and 7 days (t(88.6) = +2.40, p = 0.019). NfL levels in those with mTBI were comparable with those in controls at <6 hours (t(114.8) = +1.86, p = 0.065), though higher at 7 days (t(55.6) = +5.55, p < 0.001). For IL-6, levels were higher for those with mTBI than for controls at <6 hours (t(112.5) = +9.71, p < 0.001) and at 7 days (t(88.8) = +2.61, p = 0.011). No differences in tau and IL-1β were found (eFigure 4). eTable 4 summarizes that <6 hours biomarker and symptom profiles were comparable between participants with mTBI who did and did not return at 7 days.
Figure 1. Distribution of Plasma Biomarkers (Ln Transformed) Among Participants With mTBI at <6 Hours and 7 Days Postinjury in Comparison With Control Participants.
Compared to uninjured controls, plasma levels of GFAP (A), UCH-L1 (B), and IL-6 (D) were significantly elevated in mTBI <6 hours, while NfL levels (D) were elevated at 7 days. GFAP = glial fibrillary acidic protein; IL = interleukin; mTBI = mild traumatic brain injury; NfL = neurofilament light chain; UCH-L1 = ubiquitin carboxy-terminal hydrolase L1.
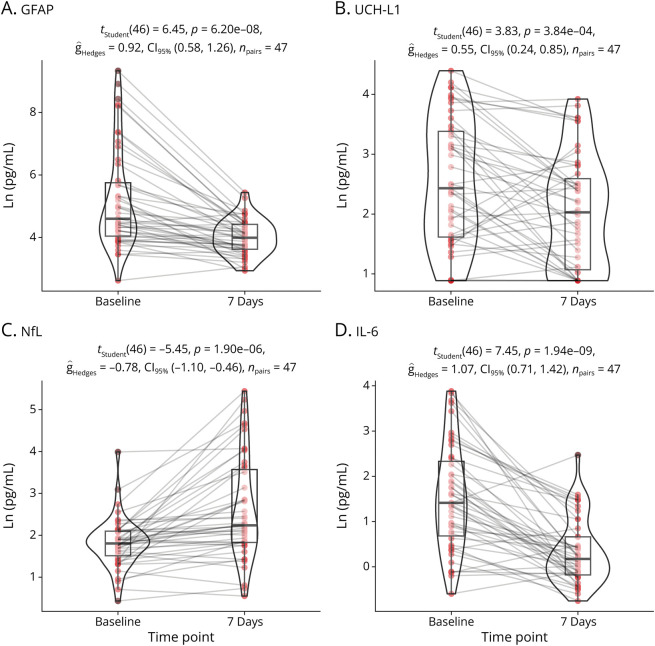
Biomarkers were compared across time for participants with mTBI who completed testing at both <6 hours and 7 days (Figure 2). Lower concentrations were observed at 7 days for GFAP (t(46) = −6.45, p < 0.001), UCH-L1 (t(46) = −3.83, p < 0.001), tau (t(46) = −2.65, p = 0.01), and IL-6 (t(46) = −7.45, p < 0.001). Conversely, plasma NfL values were higher at 7 days (t(46) = +5.45, p < 0.001).
Figure 2. Temporal Profile of Plasma Biomarkers in 48 Participants With mTBI Who Completed Sampling at Both <6 Hours and 7 Days.
Analyzing within subjects, comparing baseline (<6 hours) and 7 day testing, we observed significantly elevated levels of GFAP (A), UCH-L1 (B), and IL-6 at 6 hours. Conversely, NfL (C) exhibited an increase at 7 days. GFAP = glial fibrillary acidic protein; IL = interleukin; mTBI = mild traumatic brain injury; NfL = neurofilament light chain; UCH-L1 = ubiquitin carboxy-terminal hydrolase L1.
Biomarker AUC Analysis
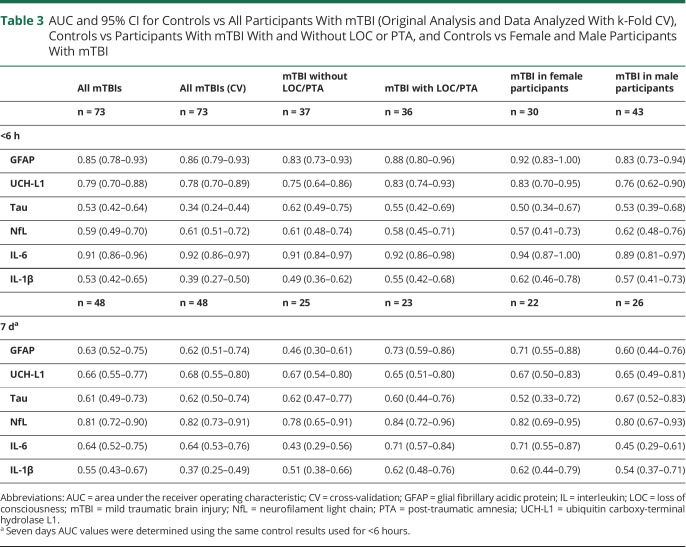
Table 3 details biomarker classification performance. Age and sex were found to have a minimal influence on AUCs; adjusted values are summarized in eTable 5 (links.lww.com/WNL/D130). Figure 3, A and B shows the relative AUC for the overall controls vs participants with mTBI, and Figure 3, C and D shows the AUC comparisons between biomarkers.
Table 3.
AUC and 95% CI for Controls vs All Participants With mTBI (Original Analysis and Data Analyzed With k-Fold CV), Controls vs Participants With mTBI With and Without LOC or PTA, and Controls vs Female and Male Participants With mTBI
| All mTBIs | All mTBIs (CV) | mTBI without LOC/PTA | mTBI with LOC/PTA | mTBI in female participants | mTBI in male participants | |
| n = 73 | n = 73 | n = 37 | n = 36 | n = 30 | n = 43 | |
| <6 h | ||||||
| GFAP | 0.85 (0.78–0.93) | 0.86 (0.79–0.93) | 0.83 (0.73–0.93) | 0.88 (0.80–0.96) | 0.92 (0.83–1.00) | 0.83 (0.73–0.94) |
| UCH-L1 | 0.79 (0.70–0.88) | 0.78 (0.70–0.89) | 0.75 (0.64–0.86) | 0.83 (0.74–0.93) | 0.83 (0.70–0.95) | 0.76 (0.62–0.90) |
| Tau | 0.53 (0.42–0.64) | 0.34 (0.24–0.44) | 0.62 (0.49–0.75) | 0.55 (0.42–0.69) | 0.50 (0.34–0.67) | 0.53 (0.39–0.68) |
| NfL | 0.59 (0.49–0.70) | 0.61 (0.51–0.72) | 0.61 (0.48–0.74) | 0.58 (0.45–0.71) | 0.57 (0.41–0.73) | 0.62 (0.48–0.76) |
| IL-6 | 0.91 (0.86–0.96) | 0.92 (0.86–0.97) | 0.91 (0.84–0.97) | 0.92 (0.86–0.98) | 0.94 (0.87–1.00) | 0.89 (0.81–0.97) |
| IL-1β | 0.53 (0.42–0.65) | 0.39 (0.27–0.50) | 0.49 (0.36–0.62) | 0.55 (0.42–0.68) | 0.62 (0.46–0.78) | 0.57 (0.41–0.73) |
| n = 48 | n = 48 | n = 25 | n = 23 | n = 22 | n = 26 | |
| 7 da | ||||||
| GFAP | 0.63 (0.52–0.75) | 0.62 (0.51–0.74) | 0.46 (0.30–0.61) | 0.73 (0.59–0.86) | 0.71 (0.55–0.88) | 0.60 (0.44–0.76) |
| UCH-L1 | 0.66 (0.55–0.77) | 0.68 (0.55–0.80) | 0.67 (0.54–0.80) | 0.65 (0.51–0.80) | 0.67 (0.50–0.83) | 0.65 (0.49–0.81) |
| Tau | 0.61 (0.49–0.73) | 0.62 (0.50–0.74) | 0.62 (0.47–0.77) | 0.60 (0.44–0.76) | 0.52 (0.33–0.72) | 0.67 (0.52–0.83) |
| NfL | 0.81 (0.72–0.90) | 0.82 (0.73–0.91) | 0.78 (0.65–0.91) | 0.84 (0.72–0.96) | 0.82 (0.69–0.95) | 0.80 (0.67–0.93) |
| IL-6 | 0.64 (0.52–0.75) | 0.64 (0.53–0.76) | 0.43 (0.29–0.56) | 0.71 (0.57–0.84) | 0.71 (0.55–0.87) | 0.45 (0.29–0.61) |
| IL-1β | 0.55 (0.43–0.67) | 0.37 (0.25–0.49) | 0.51 (0.38–0.66) | 0.62 (0.48–0.76) | 0.62 (0.44–0.79) | 0.54 (0.37–0.71) |
Abbreviations: AUC = area under the receiver operating characteristic; CV = cross-validation; GFAP = glial fibrillary acidic protein; IL = interleukin; LOC = loss of consciousness; mTBI = mild traumatic brain injury; NfL = neurofilament light chain; PTA = post-traumatic amnesia; UCH-L1 = ubiquitin carboxy-terminal hydrolase L1.
Seven days AUC values were determined using the same control results used for <6 hours.
Figure 3. Comparison of Blood Biomarker mTBI Classification Accuracy (AUC).
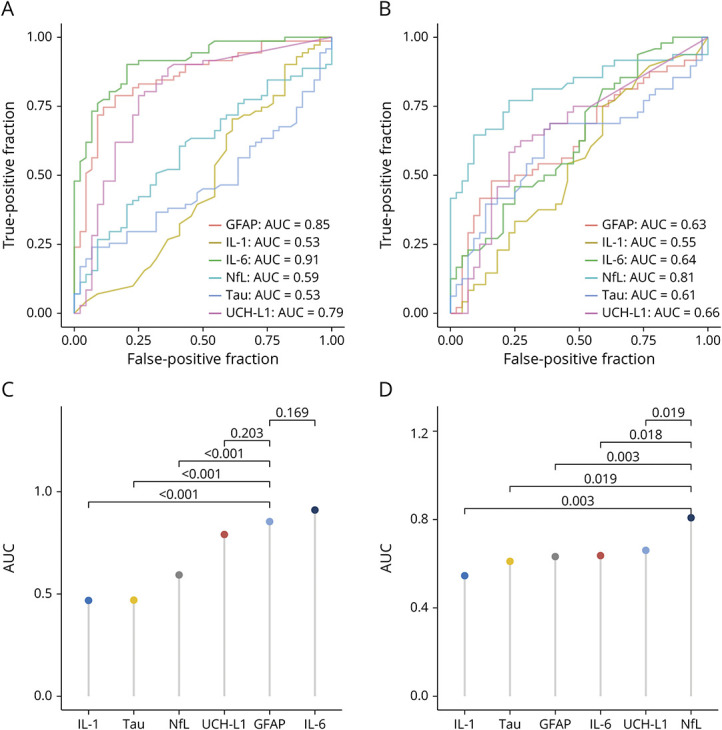
Panels A and C present findings of biomarker performance within 6 hours after injury. Panels B and D present findings for biomarker performance at the 7-day follow-up. In Panels C and D, classification accuracy was compared between our hypothesized leading biomarker candidate at each time point and other biomarkers, with a false discovery rate correction for multiple comparisons applied. AUC = area under the receiver operating characteristic; GFAP = glial fibrillary acidic protein; IL = interleukin; mTBI = mild traumatic brain injury; NfL = neurofilament light chain; UCH-L1 = ubiquitin carboxy-terminal hydrolase L1.
eTable 6 (links.lww.com/WNL/D130) summarizes the AUCs for the entire mTBI cohort with blood samples (n = 73) compared with the same cohort with 11 participants excluded because of lack of CT scans, as determined by the treating clinician. The results showed negligible differences in AUC between the 2 cohorts; thus, considering the clinical rationale for not performing CT, the larger cohort is used and classified as CT negative.
GFAP
Acute plasma GFAP demonstrated good accuracy in distinguishing participants with mTBI from controls (AUC 0.85, 95% CI 0.78–0.93), with comparable AUC values in participants with (AUC 0.88, 95% CI 0.80–0.96) and without (AUC 0.83, 95% CI 0.73–0.93) LOC/PTA and for male (AUC 0.83, 95% CI 0.73–0.94) and female (AUC 0.92, 95% CI 0.89–0.97) participants with mTBI and control counterparts. At 7 days, moderate/acceptable GFAP performance was observed between controls and participants with mTBI with LOC/PTA (AUC 0.74, 95% CI 0.59–0.86), and between female controls and female participants with mTBI (AUC 0.71, 95% CI 0.55–0.88), but not for participants without LOC/PTA or among male participants.
UCH-L1
Plasma UCH-L1 had moderate/acceptable accuracy for distinguishing mTBI and control cohorts at <6 hours (AUC 0.79, 95% CI 0.70–0.88). UCH-L1 measured at <6 hours had comparable AUC values in participants with (AUC 0.83, 95% CI 0.74–0.93) and without (AUC 0.75, 95% CI 0.64–0.86) LOC/PTA and for male (AUC 0.76, 95% CI 0.62–0.90) and female (AUC 0.83, 95% CI 0.70–0.95) participants. At 7 days, moderate/acceptable UCH-L1 utility was observed between controls and participants with mTBI with LOC/PTA (AUC 0.74, 95% CI 0.59–0.86) and between female controls and female participants with mTBI (AUC 0.71, 95% CI 0.55–0.88); however, this 7 days utility was reduced when controlling for age (within female participants) or age and sex (LOC/PTA vs controls) (eTable 5, links.lww.com/WNL/D130).
NfL
Poor or no utility was seen for all <6 hours plasma NfL; however, NfL had a good ability to distinguish overall controls from all participants with mTBI at 7 days (AUC 0.81, 95% CI 0.72–0.90). Classification accuracy at 7 days was comparable for female (AUC 0.82, 95% CI 0.62–0.95) and male (AUC 0.80, 95% CI 0.67–0.93) participants and those with (AUC 0.84, 95% CI 0.72–0.96) and without (AUC 0.78, 95% CI 0.65–0.91) LOC/PTA.
IL-6
Excellent utility was found for distinguishing those with mTBI from controls at <6 hours (AUC 0.91, 95% CI 0.86–0.96). While performance among the mTBI subgroup (9/74) with minor extracranial injury were higher than among those with isolated mTBI (eTable 2, links.lww.com/WNL/D130), their omission resulted in highly comparable IL-6 utility (AUC 0.90, 95% CI 0.84–0.96). Acute IL-6 had highly comparable AUC values in participants with (AUC 0.91, 95% CI 0.84–0.97) and without (AUC 0.92, 95% CI 0.86–0.98) LOC/PTA and for male (AUC 0.89, 95% CI 0.81–0.97) and female (AUC 0.94, 95% CI 0.84–1.00) participants. By 7 days, utility was restricted to female participants (AUC 0.71, 95% CI 0.55–0.87) and those with LOC/PTA (AUC 0.71, 95% CI 0.57–0.84); however, these 7 days differences were not present after controlling for age (within female participants) or age and sex (LOC/PTA vs controls) (eTable 5).
Optimal Biomarker Exploratory Cutoffs
At <6 hours, a GFAP cutoff of 63.0 pg/mL produced a sensitivity of 0.74 and specificity of 0.89. For UCH-L1 at <6 hours, a cutoff of 4.96 pg/mL had a sensitivity of 0.79 and specificity of 0.75. For acute IL-6, a cutoff of 1.44 pg/mL had a sensitivity of 0.90 and specificity of 0.80. For 7 days NfL, a cutoff of 7.40 pg/mL produced a sensitivity of 0.65 and specificity of 0.91.
AUC Comparison Between Biomarkers
Classification accuracy was compared between our hypothesized leading biomarker candidate at each time point and other biomarkers, that is, GFAP and other biomarkers at <6 hours (Figure 3C) and NfL and other biomarkers at 7 days (Figure 3D). At <6 hours, GFAP performed better than other all biomarkers except UCH-L1 (z = 1.27, p = 0.203) and IL-6 (z = −1.49, p = 0.169). At 7 days, NfL outperformed all biomarkers. When comparing AUC values across time points (i.e., mTBI vs control for participants with mTBI who had biomarker results at both <6 hours and 7 days), <6 hours GFAP was comparable with 7 days NfL (z = 0.836, p = 0.403), as was <6 hours UCH-L1 and 7 days NfL (z = 0.258, p = 0.796); however, <6 hours IL-6 did outperform 7 days NfL (z = 2.13, p = 0.033).
Examining Added Utility of Acute Biomarker Combinations
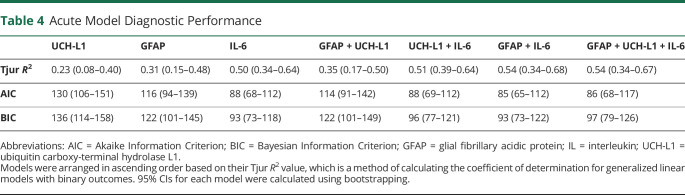
Biomarkers with AUC >0.70 were investigated in various combinations. As summarized in Table 4, IL-6 alone (R2 = 0.50) outperformed a GFAP and UCH-L1 combination (R2 = 0.35; p = 0.046), and that combination was improved by the addition of IL-6 (R2 = 0.54; p < 0.001, AUC = 0.92). Overall, AIC and BIC postestimation assessment supported these conclusions.
Table 4.
Acute Model Diagnostic Performance
| UCH-L1 | GFAP | IL-6 | GFAP + UCH-L1 | UCH-L1 + IL-6 | GFAP + IL-6 | GFAP + UCH-L1 + IL-6 | |
| Tjur R2 | 0.23 (0.08–0.40) | 0.31 (0.15–0.48) | 0.50 (0.34–0.64) | 0.35 (0.17–0.50) | 0.51 (0.39–0.64) | 0.54 (0.34–0.68) | 0.54 (0.34–0.67) |
| AIC | 130 (106–151) | 116 (94–139) | 88 (68–112) | 114 (91–142) | 88 (69–112) | 85 (65–112) | 86 (68–117) |
| BIC | 136 (114–158) | 122 (101–145) | 93 (73–118) | 122 (101–149) | 96 (77–121) | 93 (73–122) | 97 (79–126) |
Abbreviations: AIC = Akaike Information Criterion; BIC = Bayesian Information Criterion; GFAP = glial fibrillary acidic protein; IL = interleukin; UCH-L1 = ubiquitin carboxy-terminal hydrolase L1.
Models were arranged in ascending order based on their Tjur R2 value, which is a method of calculating the coefficient of determination for generalized linear models with binary outcomes. 95% CIs for each model were calculated using bootstrapping.
Discussion
This study primarily found the following: (1) plasma IL-6 measured within 6 hours of mTBI had an excellent ability to correctly discriminate CT-negative participants with isolated mTBI from controls; (2) IL-6 significantly outperformed the combination of GFAP and UCH-L1 in differentiating participants with mTBI and controls, with the overall best performing acute stage classification model featuring the combination of GFAP and IL-6; (3) NfL at 7 days had a comparable classification accuracy with that of acute GFAP and UCH-L1; and (4) biomarker performance was largely comparable between sexes and for participants with and without LOC/PTA. While there are some important considerations, particularly regarding extracerebral sources of IL-6, these findings indicate that plasma biomarkers may not only assist with the prediction of an intracranial bleed8 but also provide an objective measure of mTBI in CT-negative individuals.
A profound elevation in plasma IL-6 was seen in most of the participants with mTBI at <6 hours, when compared with control and 7 days mTBI levels. Elevated blood IL-6 at similar acute time points, and moderate/acceptable to good classification accuracy, has been reported for concussed military cadets (AUC 0.81)26 and athletes (AUC 0.78)24,25; however, the excellent classification accuracy (AUC 0.91) in our predominately isolated mTBI ED cohort was an unexpected finding and is likely reflective of a significant neuroinflammatory response induced by brain trauma. The lack of brain specificity of IL-6 does create some potential limitations in clinical practice, for example, it may produce false positives in the presence of extracranial trauma.45 While IL-6 levels were increased in 9 participants with mTBI with minor extracranial trauma when compared with 65 participants with isolated mTBI, this subgroup did not skew biomarker performance. Nonetheless, given extracerebral sources are also a factor for established biomarkers such as S100B, which is used for CT screening, it is possible that in a similar manner to S100B, IL-6 should only be considered as a marker for cases without extracranial trauma.
Acute levels of GFAP and UCH-L1 were significantly elevated after mTBI, when compared with controls and 7 days mTBI, with these biomarkers showing good and moderate/acceptable classification accuracy, respectively. Although previous studies of mTBI and SRC have revealed acute elevations in these biomarkers, wide-ranging time points and a focus on prediction of CT positivity as a primary outcome mean that our study provides some novelty in the context of screening for mTBI within the first few hours after head impact. Notably, when IL-6 was added to the classification model of GFAP and UCH-L1, accuracy was increased. We hypothesize that it is biologically plausible for a neuroinflammatory response to be triggered by mTBI in the absence of appreciable neuronal and glial pathology, and as such, the combination of these markers may serve as an ideal panel with which to assess mTBI acutely. Given that UCH-L1 and GFAP have some prognostic value for functional outcome after TBI (although reduced in mTBI),46 and inflammation has been postulated as a mechanism underpinning prolonged symptoms after mTBI, future studies should investigate how these acute measures may have prognostic utility.
Like other recent studies in SRC,16,17 we found elevated plasma NfL and good classification accuracy in our ED mTBI cohort at 7 days. Notably, this subacute NfL classification accuracy (AUC 0.81) was comparable with acute GFAP (AUC 0.85), the best-performing acute biomarker with high brain specificity. Analysis of optimal cutoff concentrations of <6 hours GFAP (63.0 pg/mL) and 7 days NfL (7.4 pg/mL) revealed both biomarkers had approximately 70% sensitivity and 90% specificity, suggesting some false negatives but few false positives. Although representing different aspects of pathology, this comparable performance provides further evidence that NfL may assist diagnosis for delayed presentations. Multiple studies have shown that blood NfL levels can remain elevated and, in some cases, peak weeks after TBI. Therefore, the window for NfL to assist mTBI diagnosis is likely to be greater than 7 days. Considering recent evidence that blood NfL levels may indicate vulnerability to repeated mTBI47 and that NfL trajectories can differ between cases with mTBI,16,17 it seems likely that subacute NfL measures might assist retrospectively grading the significance of an mTBI and for helping with return to play/work decisions.
Although the presence and duration of LOC or PTA after mTBI are used as diagnostic indicators, we did not see a substantial drop in classification accuracy of acute GFAP, UCH-L1, and IL-6 acutely, or subacute NfL, in participants without these clinical signs. This finding might seem to be somewhat in contrast to a recent CARE consortium study of SRC44; however, it is important to consider that our study featured only individuals who presented to the ED. As such, while it is likely that classification accuracy may be slightly reduced in SRC that does not feature LOC and/or PTA, our findings support the utility of biomarkers in the ED for acute screening of patients without these overt clinical signs. At 7 days, GFAP retained a moderate/acceptable classification accuracy for participants with mTBI with LOC and/or PTA only, suggesting that astroglial pathology may persist for longer in these patients.
Participants with mTBI performed more poorly, compared with controls, on all cognitive tasks assessed. The classification accuracy of blood biomarkers, however, outperformed cognitive measures. It is important to consider that a primary strength of cognitive measures is to assess an individual's needs to inform the process of rehabilitation. Furthermore, while cognitive tests represent a more objective measure than symptom reporting, performance remains subject to confounds including psychosocial factors such as mood, pain, and the effects of medication.4 Considered together, blood biomarker quantification could form a part of the initial screening of mTBI alongside evaluation of neurologic signs and symptoms, with subsequent tests of psychomotor speed and attention (acutely) and working memory (subacutely) used to further characterize mTBI phenotype and potentially inform prognosis.
It is important to recognize that it is not possible for any combination of biomarkers to improve classification accuracy beyond a clinical evaluation when this remains the gold standard reference. Our findings indicate highly comparable classification accuracy between biomarkers and clinical evaluation. Future research is needed to validate the findings against a population with possible mTBI to establish whether their use can increase the number of patients diagnosed with mTBI. Biomarkers might be of greatest utility when the gold standard reference is not possible or difficult. This might include some settings outside of the ED, when neurologic signs or symptoms might be attributed to something other than brain trauma, if a patient is unwilling or unable to communicate symptoms or if symptoms are subtle or absent but mTBI is suspected due to the nature of the incident. Nonetheless, given that the optimal biomarker concentration cutoffs had high specificity but relatively lower sensitivity, it seems likely that high biomarker levels might be used to help confirm mTBI diagnosis; however, concentrations below these cutoffs should not be used to exclude mTBI.
This study has limitations. First, investigation of biomarkers in a separate and larger cohort is required to validate the findings and establish precise classification accuracies. In addition, as with all CT-negative mTBI research, clinical diagnosis remains the gold standard, but imperfect comparator and, as such, recruitment exclusion of true positives or inclusion of false positives may have affected classification accuracy. The clinical diagnosis of mTBI may be prone to subjectivity, partly due to the heterogeneity of nonspecific signs and symptoms associated with mTBI. While this study relied on expert physicians, inter-rater reliability was not assessed. There was an observable trend toward a higher proportion of participants with mTBI reporting a history of mTBI compared with controls. While studies have demonstrated that our exclusion period for previous mTBI (i.e., 12 months) is generally sufficient for resolution of GFAP, NfL, tau, and UCH-L1 levels to control values in most participants,17,48 cytokine profiles are less well understood, and possibly, ongoing neurobiological changes resulting from a prior mTBI could potentially affect biomarker levels and thereby affect classification accuracy. The lack of a trauma control group prohibited insights on the influence of extracranial trauma on IL-6 levels. Significant peripheral injuries can increase blood IL-6 levels,45 and as such, IL-6 utility in mTBI is likely restricted to isolated mTBI. In addition, differences in education levels between controls and participants with mTBI may have affected cognitive assessment results. While the Level 1 trauma center from which the study recruited participants has a wide catchment, this study setting and a priori study exclusion criteria of population cohorts limits generalizability.
Plasma IL-6 measured within 6 hours showed excellent discriminatory ability, alongside the more established plasma GFAP and UCH-L1, in distinguishing CT-negative participants with mTBI from controls in otherwise healthy adults younger than 50 years. Moreover, acute GFAP, UCH-L1, and IL-6 demonstrated comparable utility even among mTBI without overt clinical features of LOC and/or PTA, and NfL demonstrated value for mTBI screening after delayed presentation. Given the frequency of CT-negative mTBI and the diagnostic challenges of this condition, our findings provisionally support the use of biomarkers as a standard component of the immediate mTBI workup, acknowledging that assessment of neurologic signs and symptoms is paramount to mTBI diagnosis and management. Further research should focus on the utility of these biomarkers in settings where clinical assessment may be limited or whether biomarkers can improve diagnostic rates of mTBI.
Glossary
- AIC
Akaike Information Criterion
- AUC
area under the receiver operating characteristic
- BIC
Bayesian Information Criterion
- CoV
coefficient of variation
- ED
emergency department
- GFAP
glial fibrillary acidic protein
- IL
interleukin
- IQR
interquartile range
- KD
King-Devick
- LLOD
lower limit of detection
- LOC
loss of consciousness
- mTBI
mild traumatic brain injury
- NfL
neurofilament light chain
- PTA
post-traumatic amnesia
- RAVLT
Rey Auditory-Verbal Learning Test
- RPQ
Rivermead Postconcussion Symptom Questionnaire
- S100B
S100 calcium-binding protein B
- SRC
sport-related concussion
- UCH-L1
ubiquitin carboxy-terminal hydrolase L1
- WHO
World Health Organization
Appendix. Authors
| Name | Location | Contribution |
| Jonathan Reyes, DPsych | Department of Neuroscience, Monash University; School of Psychological Sciences, Monash University; Monash-Epworth Rehabilitation Research Centre, Epworth Hospital, Melbourne, Australia | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Gershon Spitz, PhD | Department of Neuroscience, Monash University; School of Psychological Sciences, Monash University; Monash-Epworth Rehabilitation Research Centre, Epworth Hospital, Melbourne, Australia | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Brendan P. Major, PhD | Department of Neuroscience, Monash University, Melbourne, Australia | Major role in the acquisition of data; study concept or design |
| William T. O'Brien, PhD | Department of Neuroscience, Monash University, Melbourne, Australia | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Lauren P. Giesler, BA | Department of Neuroscience, Monash University, Melbourne, Australia | Major role in the acquisition of data |
| Jesse W.P. Bain, BSc | Department of Neuroscience, Monash University, Melbourne, Australia | Major role in the acquisition of data |
| Becca Xie, MSc | Department of Neuroscience, Monash University, Melbourne, Australia | Analysis or interpretation of data |
| Jeffrey V. Rosenfeld, MD | Department of Neurosurgery, The Alfred Hospital; Department of Surgery, Monash University, Melbourne, Australia | Study concept or design; analysis or interpretation of data |
| Meng Law, MD | Department of Neuroscience, Monash University; Department of Radiology, The Alfred Hospital; Department of Electrical and Computer Systems Engineering, Monash University, Melbourne, Australia | Major role in the acquisition of data; study concept or design |
| Jennie L. Ponsford, PhD | Department of Neuroscience, School of Psychological Sciences, Monash University; Monash-Epworth Rehabilitation Research Centre, Epworth Hospital, Melbourne, Australia | Drafting/revision of the article for content, including medical writing for content; study concept or design |
| Terence J. O'Brien, MD | Department of Neuroscience, Monash University; Department of Neurology, The Alfred Hospital, Melbourne; Department of Medicine, Royal Melbourne Hospital, The University of Melbourne, Parkville, Australia | Drafting/revision of the article for content, including medical writing for content; study concept or design |
| Sandy R. Shultz, PhD | Department of Neuroscience, Monash University; Department of Neurology, The Alfred Hospital, Melbourne; Department of Medicine, Royal Melbourne Hospital, The University of Melbourne, Parkville, Australia; Health Sciences, Vancouver Island University, Nanaimo, British Columbia, Canada | Drafting/revision of the article for content, including medical writing for content; study concept or design; and analysis or interpretation of data |
| Catherine Willmott, PhD | School of Psychological Sciences, Monash University; Monash-Epworth Rehabilitation Research Centre, Epworth Hospital; Australian Football League (AFL), Melbourne, Australia | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Biswadev Mitra, PhD | Emergency & Trauma Centre, The Alfred Hospital; School of Public Health & Preventive Medicine, Monash University, Melbourne, Australia | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Stuart J. McDonald, PhD | Department of Neuroscience, Monash University; Department of Neurology, The Alfred Hospital, Melbourne, Australia | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
Footnotes
CME Course: NPub.org/cmelist
Study Funding
This study was funded by a grant (2020/GNT2002689) from the Australian National Health and Medical Research Council (NHMRC).
Disclosure
J. Reyes is the Concussion Research Lead for the Australian Football League, with this appointment being made within the course of the study data collection and having no influence on study funding, design, or outcomes. G. Spitz, B.P. Major, W.T. O'Brien, L.P. Giesler, J.W.P. Bain, B. Xie, J.V. Rosenfeld, M. Law, J.L. Ponsford, T.J. O'Brien, and S.R. Shultz report no disclosures relevant to the manuscript. C. Willmott is the Head of Concussion Innovation and Research for the Australian Football League, with this appointment being made within the course of the study data collection and having no influence on study funding, design, or outcomes. B. Mitra and S.J. McDonald report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Louey AG, Cromer JA, Schembri AJ, et al. Detecting cognitive impairment after concussion: sensitivity of change from baseline and normative data methods using the CogSport/Axon cognitive test battery. Arch Clin Neuropsychol. 2014;29(5):432-441. doi: 10.1093/arclin/acu020 [DOI] [PubMed] [Google Scholar]
- 2.Nelson LD, LaRoche AA, Pfaller AY, et al. Prospective, head-to-head study of three computerized neurocognitive assessment tools (CNTs): reliability and validity for the assessment of sport-related concussion. J Int Neuropsychol Soc. 2016;22(1):24-37. doi: 10.1017/s1355617715001101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Echemendia RJ, Meeuwisse W, McCrory P, et al. The Sport Concussion Assessment Tool 5th Edition (SCAT5): background and rationale. Br J Sports Med. 2017;51(11):848-850. doi: 10.1136/bjsports-2017-097506 [DOI] [PubMed] [Google Scholar]
- 4.McGrath N, Eloi J. The role of neuropsychology in the evaluation of concussion. Semin Pediatr Neurol. 2019;30:83-95. doi: 10.1016/j.spen.2019.03.013 [DOI] [PubMed] [Google Scholar]
- 5.Ponsford J, Willmott C, Rothwell A, et al. Factors influencing outcome following mild traumatic brain injury in adults. J Int Neuropsychol Soc. 2000;6(5):568-579. doi: 10.1017/s1355617700655066 [DOI] [PubMed] [Google Scholar]
- 6.Pozzato I, Meares S, Kifley A, et al. Challenges in the acute identification of mild traumatic brain injuries: results from an emergency department surveillance study. BMJ Open. 2020;10(2):e034494. doi: 10.1136/bmjopen-2019-034494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald SJ, Shultz SR, Agoston DV. The known unknowns: an overview of the state of blood-based protein biomarkers of mild traumatic brain injury. J Neurotrauma. 2021;38(19):2652-2666. doi: 10.1089/neu.2021.0011 [DOI] [PubMed] [Google Scholar]
- 8.Mondello S, Sorinola A, Czeiter E, et al. Blood-based protein biomarkers for the management of traumatic brain injuries in adults presenting to emergency departments with mild brain injury: a living systematic review and meta-analysis. J Neurotrauma. 2021;38(8):1086-1106. doi: 10.1089/neu.2017.5182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helmrich I, Czeiter E, Amrein K, et al. Incremental prognostic value of acute serum biomarkers for functional outcome after traumatic brain injury (CENTER-TBI): an observational cohort study. Lancet Neurol. 2022;21(9):792-802. doi: 10.1016/s1474-4422(22)00218-6 [DOI] [PubMed] [Google Scholar]
- 10.Korley FK, Jain S, Sun X, et al. Prognostic value of day-of-injury plasma GFAP and UCH-L1 concentrations for predicting functional recovery after traumatic brain injury in patients from the US TRACK-TBI cohort: an observational cohort study. Lancet Neurol. 2022;21(9):803-813. doi: 10.1016/s1474-4422(22)00256-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hier DB, Obafemi-Ajayi T, Thimgan MS, et al. Blood biomarkers for mild traumatic brain injury: a selective review of unresolved issues. Biomarker Res. 2021;9(1):70. doi: 10.1186/s40364-021-00325-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papa L, Brophy GM, Welch RD, et al. Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH-L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA Neurol. 2016;73(5):551-560. doi: 10.1001/jamaneurol.2016.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papa L, Zonfrillo MR, Welch RD, et al. Evaluating glial and neuronal blood biomarkers GFAP and UCH-L1 as gradients of brain injury in concussive, subconcussive and non-concussive trauma: a prospective cohort study. BMJ Paediatr Open. 2019;3(1):e000473. doi: 10.1136/bmjpo-2019-000473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mercier E, Tardif P-A, Emond M, et al. Characteristics of patients included and enrolled in studies on the prognostic value of serum biomarkers for prediction of postconcussion symptoms following a mild traumatic brain injury: a systematic review. BMJ Open. 2017;7(9):e017848. doi: 10.1136/bmjopen-2017-017848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowe BH, Eliyahu L, Lowes J, et al. Concussion diagnoses among adults presenting to three Canadian emergency departments: missed opportunities. Am J Emerg Med. 2018;36(12):2144-2151. doi: 10.1016/j.ajem.2018.03.040 [DOI] [PubMed] [Google Scholar]
- 16.McDonald SJ, O'Brien WT, Symons GF, et al. Prolonged elevation of serum neurofilament light after concussion in male Australian football players. Biomark Res. 2021;9(1):4. doi: 10.1186/s40364-020-00256-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald SJ, Piantella S, O'Brien WT, et al. Clinical and blood biomarker trajectories after concussion: new insights from a longitudinal pilot study of professional flat-track jockeys. J Neurotrauma. 2023;40(1-2):52-62. doi: 10.1089/neu.2022.0169 [DOI] [PubMed] [Google Scholar]
- 18.Simon DW, McGeachy MJ, Bayir H, Clark RSB, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. 2017;13(3):171-191. doi: 10.1038/nrneurol.2017.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winter CD, Pringle AK, Clough GF, Church MK. Raised parenchymal interleukin-6 levels correlate with improved outcome after traumatic brain injury. Brain. 2004;127(2):315-320. doi: 10.1093/brain/awh039 [DOI] [PubMed] [Google Scholar]
- 20.Woodcock T, Morganti-Kossmann MC. The role of markers of inflammation in traumatic brain injury. Front Neurol. 2013;4:18. doi: 10.3389/fneur.2013.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar RG, Diamond ML, Boles JA, et al. Acute CSF interleukin-6 trajectories after TBI: associations with neuroinflammation, polytrauma, and outcome. Brain Behav Immun. 2015;45:253-262. doi: 10.1016/j.bbi.2014.12.021 [DOI] [PubMed] [Google Scholar]
- 22.Juengst SB, Kumar RG, Failla MD, Goyal A, Wagner AK. Acute inflammatory biomarker profiles predict depression risk following moderate to severe traumatic brain injury. J Head Trauma Rehabil. 2015;30(3):207-218. doi: 10.1097/htr.0000000000000031 [DOI] [PubMed] [Google Scholar]
- 23.Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012;8(9):1254-1266. doi: 10.7150/ijbs.4679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meier TB, Huber DL, Bohorquez-Montoya L, et al. A prospective study of acute blood-based biomarkers for sport-related concussion. Ann Neurol. 2020;87(6):907-920. doi: 10.1002/ana.25725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nitta ME, Savitz J, Nelson LD, et al. Acute elevation of serum inflammatory markers predicts symptom recovery after concussion. Neurology. 2019;93(5):e497-e507. doi: 10.1212/wnl.0000000000007864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards KA, Gill JM, Pattinson CL, et al. Interleukin-6 is associated with acute concussion in military combat personnel. BMC Neurol. 2020;20(1):209. doi: 10.1186/s12883-020-01760-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Brien WT, Symons GF, Bain J, et al. Elevated serum interleukin-1β levels in male, but not female, collision sport athletes with a concussion history. J Neurotrauma. 2021;38(10):1350-1357. doi: 10.1089/neu.2020.7479 [DOI] [PubMed] [Google Scholar]
- 28.Holm L, Cassidy JD, Carroll LJ, Borg J; Neurotrauma Task Force on Mild Traumatic Brain Injury of the WHOCC. Summary of the WHO Collaborating Centre for Neurotrauma Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2005;37(3):137-141. doi: 10.1080/16501970510027321 [DOI] [PubMed] [Google Scholar]
- 29.Gioia G, Collins M. Acute Concussion Evaluation (ACE): Physician/Clinician Office Version [online]. Accessed January 10, 2023. cdc.gov/headsup/pdfs/providers/ace_v2-a.pdf. [Google Scholar]
- 30.King NS, Crawford S, Wenden FJ, Moss NEG, Wade DT. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242(9):587-592. doi: 10.1007/bf00868811 [DOI] [PubMed] [Google Scholar]
- 31.Medvedev ON, Theadom A, Barker-Collo S, Feigin V, Group BR. Distinguishing between enduring and dynamic concussion symptoms: applying Generalisability Theory to the Rivermead Post Concussion Symptoms Questionnaire (RPQ). PeerJ. 2018;6:e5676. doi: 10.7717/peerj.5676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wechsler D. The Test of Premorbid Functioning: UK Version (TOPF UK) Manual. Psychological Corporation; 2011. [Google Scholar]
- 33.Rey A. Clinical Tests in Psychology [in French]. Presses Universitaires de France; 1964. [Google Scholar]
- 34.Schmidt M. Rey Auditory Verbal Learning Test: A Handbook. Western Psychological Services; 1996. [Google Scholar]
- 35.Ryan JJ, Geisser ME, Randall DM, Georgemiller RJ. Alternate form reliability and equivalency of the rey auditory verbal learning test. J Clin Exp Neuropsychol. 1986;8(5):611-616. doi: 10.1080/01688638608405179 [DOI] [PubMed] [Google Scholar]
- 36.Wechsler D. Wechsler Adult Intelligence Scale, 4th ed. Pearson; 2008. [Google Scholar]
- 37.King AT, Devick S. The Proposed King-Devick Test and Its Relation to the Pierce Saccade Test and Reading Levels. Illinois College of Optometry; 1976. [Google Scholar]
- 38.Moran RN, Covassin T. King-Devick test normative reference values and internal consistency in youth football and soccer athletes. Scand J Med Sci Sports. 2018;28(12):2686-2690. doi: 10.1111/sms.13286 [DOI] [PubMed] [Google Scholar]
- 39.Collie A, Maruff P, Makdissi M, McCrory P, McStephen M, Darby D. CogSport: reliability and correlation with conventional cognitive tests used in postconcussion medical evaluations. Clin J Sport Med. 2003;13(1):28-32. doi: 10.1097/00042752-200301000-00006 [DOI] [PubMed] [Google Scholar]
- 40.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12(1):77. doi: 10.1186/1471-2105-12-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5(9):1315-1316. doi: 10.1097/jto.0b013e3181ec173d [DOI] [PubMed] [Google Scholar]
- 42.Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8(4):283-298. doi: 10.1016/s0001-2998(78)80014-2 [DOI] [PubMed] [Google Scholar]
- 43.Rodríguez-Álvarez MX, Inácio V. ROCnReg: an R package for receiver operating characteristic curve inference with and without covariates. R J. 2021;13(1):525. doi: 10.32614/rj-2021-066 [DOI] [Google Scholar]
- 44.McCrea M, Broglio SP, McAllister TW, et al. Association of blood biomarkers with acute sport-related concussion in collegiate athletes: findings from the NCAA and Department of Defense CARE Consortium. JAMA Netw Open. 2020;3(1):e1919771. doi: 10.1001/jamanetworkopen.2019.19771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gebhard F, Pfetsch H, Steinbach G, Strecker W, Kinzl L, Bruckner UB. Is interleukin 6 an early marker of injury severity following major trauma in humans? Arch Surg. 2000;135(3):291-295. doi: 10.1001/archsurg.135.3.291 [DOI] [PubMed] [Google Scholar]
- 46.McDonald SJ, O'Brien TJ, Shultz SR. Biomarkers add value to traumatic brain injury prognosis. Lancet Neurol. 2022;21(9):761-763. doi: 10.1016/s1474-4422(22)00306-4 [DOI] [PubMed] [Google Scholar]
- 47.O'Brien WT, Wright DK, van Emmerik A, et al. Serum neurofilament light as a biomarker of vulnerability to a second mild traumatic brain injury. Transl Res. 2023;255:77-84. doi: 10.1016/j.trsl.2022.11.008 [DOI] [PubMed] [Google Scholar]
- 48.Clarke GJB, Skandsen T, Zetterberg H, et al. One-year prospective study of plasma biomarkers from CNS in patients with mild traumatic brain injury. Front Neurol. 2021;12:643743. doi: 10.3389/fneur.2021.643743 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing requests will be considered by the corresponding author.