Table 1.
Demographic, Clinical, and Paraclinical Characteristics of the Cohort (N = 178)a
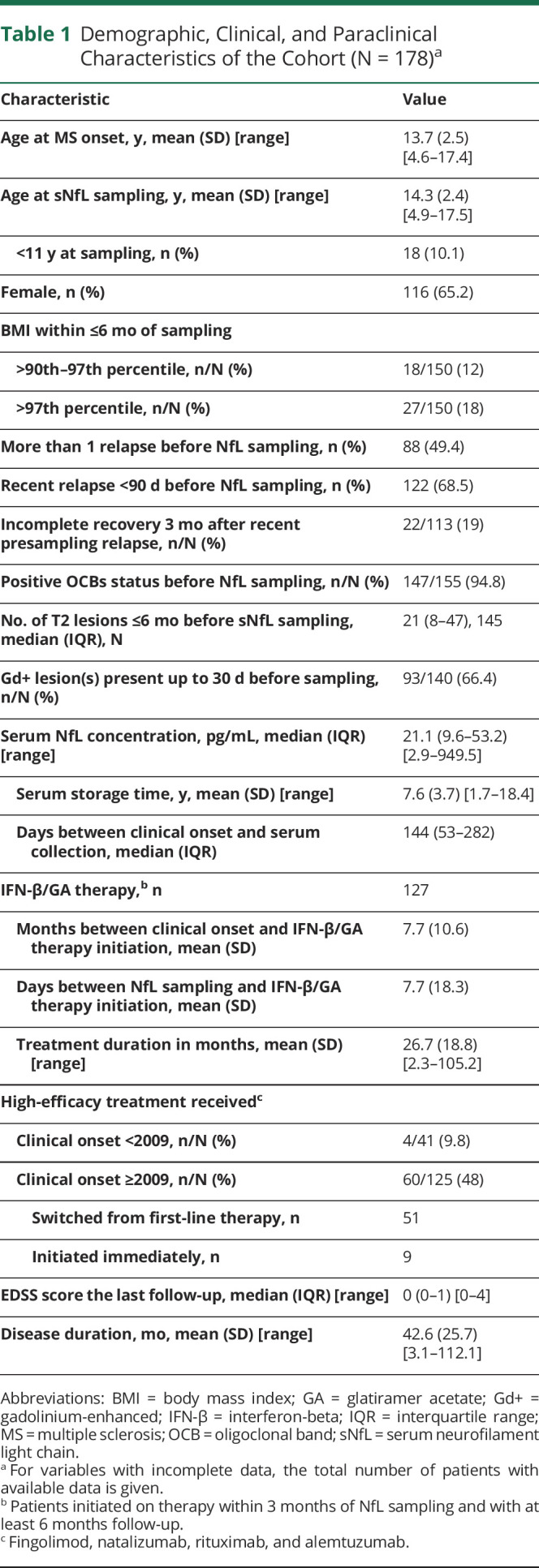
| Characteristic | Value |
| Age at MS onset, y, mean (SD) [range] | 13.7 (2.5) [4.6–17.4] |
| Age at sNfL sampling, y, mean (SD) [range] | 14.3 (2.4) [4.9–17.5] |
| <11 y at sampling, n (%) | 18 (10.1) |
| Female, n (%) | 116 (65.2) |
| BMI within ≤6 mo of sampling | |
| >90th–97th percentile, n/N (%) | 18/150 (12) |
| >97th percentile, n/N (%) | 27/150 (18) |
| More than 1 relapse before NfL sampling, n (%) | 88 (49.4) |
| Recent relapse <90 d before NfL sampling, n (%) | 122 (68.5) |
| Incomplete recovery 3 mo after recent presampling relapse, n/N (%) | 22/113 (19) |
| Positive OCBs status before NfL sampling, n/N (%) | 147/155 (94.8) |
| No. of T2 lesions ≤6 mo before sNfL sampling, median (IQR), N | 21 (8–47), 145 |
| Gd+ lesion(s) present up to 30 d before sampling, n/N (%) | 93/140 (66.4) |
| Serum NfL concentration, pg/mL, median (IQR) [range] | 21.1 (9.6–53.2) [2.9–949.5] |
| Serum storage time, y, mean (SD) [range] | 7.6 (3.7) [1.7–18.4] |
| Days between clinical onset and serum collection, median (IQR) | 144 (53–282) |
| IFN-β/GA therapy,b n | 127 |
| Months between clinical onset and IFN-β/GA therapy initiation, mean (SD) | 7.7 (10.6) |
| Days between NfL sampling and IFN-β/GA therapy initiation, mean (SD) | 7.7 (18.3) |
| Treatment duration in months, mean (SD) [range] | 26.7 (18.8) [2.3–105.2] |
| High-efficacy treatment receivedc | |
| Clinical onset <2009, n/N (%) | 4/41 (9.8) |
| Clinical onset ≥2009, n/N (%) | 60/125 (48) |
| Switched from first-line therapy, n | 51 |
| Initiated immediately, n | 9 |
| EDSS score the last follow-up, median (IQR) [range] | 0 (0–1) [0–4] |
| Disease duration, mo, mean (SD) [range] | 42.6 (25.7) [3.1–112.1] |
Abbreviations: BMI = body mass index; GA = glatiramer acetate; Gd+ = gadolinium-enhanced; IFN-β = interferon-beta; IQR = interquartile range; MS = multiple sclerosis; OCB = oligoclonal band; sNfL = serum neurofilament light chain.
For variables with incomplete data, the total number of patients with available data is given.
Patients initiated on therapy within 3 months of NfL sampling and with at least 6 months follow-up.
Fingolimod, natalizumab, rituximab, and alemtuzumab.
