Abstract
With recent data demonstrating that lecanemab treatment can slow cognitive and functional decline in early symptomatic Alzheimer disease (AD), it is widely anticipated that this drug and potentially other monoclonal antibody infusions targeting β-amyloid protein will imminently be realistic options for some patients with AD. Given that these new antiamyloid monoclonal antibodies (mAbs) are associated with nontrivial risks and burdens of treatment that are radically different from current mainstays of AD management, effectively and equitably translating their use to real-world clinical care will require systematic and practice-specific modifications to existing workflows and infrastructure. In this Emerging Issues in Neurology article, we provide practical guidance for a wide audience of neurology clinicians on logistic adaptations and decision making around emerging antiamyloid mAbs. Specifically, we briefly summarize the rationale and available evidence supporting antiamyloid mAb use in AD to facilitate appropriate communication with patients and care partners on potential benefits. We also discuss pragmatic approaches to optimizing patient selection and treatment monitoring, with a particular focus on the value of incorporating shared decision making and multidisciplinary collaboration. In addition, we review some of the recognized limitations of current knowledge and highlight areas of future evolution to guide the development of sustainable and flexible models for treatment and follow-up. As the field enters a new era with disease-modifying treatment options for AD, it will be critical for neurology practices to prepare and continually innovate to ensure optimal outcomes for patients.
Introduction
Additional treatment options are needed for Alzheimer disease (AD), which is simultaneously highly prevalent (acknowledging limitations of precise etiologic diagnosis, it is estimated that more than 6 million individuals in the United States are currently affected),1,2 chronic and progressive,3 and devastating to families and society at large.4 Recent data on lecanemab5 and other monoclonal antibody infusions targeting β-amyloid (Aβ) protein6,7 make clear that new agents are highly likely to be part of the toolkit for clinicians caring for patients with AD. In this new reality, it will be critical for neurology practices to sustainably adapt their workflows and infrastructure to optimize the use of these complex, emerging therapeutics.
Scope and Disclaimers
Emerging Issues in Neurology (EIN) articles are published by the American Academy of Neurology and its affiliates. The goal of the EIN series is to provide timely and informal guidance (derived from expert consensus opinion) to neurologists about new or emerging issues that have immediate implications for patient care, but for which a formal evidence base is still evolving. The information in an EIN article (1) should not be considered inclusive of all proper treatments or methods of care, (2) does not represent a statement of the standard of care, (3) is not continually updated, (4) does not mandate any specific course of medical care, (5) is not the result of a systematic review, and (6) is not intended to replace the independent professional judgment of the treating provider. In all cases, decisions about patient care should be considered in the context of treating the individual patient. Use of the information is voluntary.
This EIN article aims to provide practical guidance on emerging antiamyloid monoclonal antibodies (mAbs), including (1) briefly summarizing the rationale and available evidence for their use in AD; (2) discussing pragmatic approaches to patient selection and treatment monitoring, with a focus on lecanemab; and (3) highlighting limitations of current knowledge and anticipated future directions.
A Balanced Rationale for Antiamyloid mAbs as a Component in AD Treatment
Clinically, AD includes a range of syndromic presentations. Biologically, the disease is defined by the presence of Aβ plaques and neurofibrillary tau deposits.8 In addition to being a hallmark neuropathologic finding, abnormal brain amyloidosis is widely proposed as an early event in the AD cascade based on longitudinal aging studies (showing Aβ deposition many years before onset of symptoms)9 and genetics (identifying alterations in genes central to Aβ processing in cases of familial AD).10 This backdrop has formed the basis for therapeutic targeting of Aβ, with the caveat that the disease's underlying mechanisms are likely multifactorial (e.g., amyloid, tau, inflammation, and other processes).
Numerous earlier drug trials targeting Aβ failed to convincingly demonstrate clinical efficacy.11 These included aducanumab, an anti-amyloid mAb which in 2021 received accelerated approval from the US Food and Drug Administration (FDA) amidst much controversy.12 Of note, the FDA approval for aducanumab was based on the drug's ability to lower levels of amyloid plaques measured with PET, on the hypothesis that this would be “reasonably likely to predict a clinical benefit to patients.”13 However, the FDA approval of aducanumab acknowledged that there remained significant uncertainties about the drug's clinical benefits. In particular, the 2 identically designed phase 3 trials of aducanumab (EMERGE and ENGAGE) were terminated early based on findings from an interim futility study, with subsequent analyses on a larger set of acquired data producing conflicting results. The primary end point of both trials was the score on the Clinical Dementia Rating Sum of Boxes (CDR-SB), a standardized scale (range 0–18) of impairment in 6 domains of cognition and function, with scores assigned by trained clinicians after interviews of the patient and a reliable informant.14 Although ENGAGE did not meet its primary end point, participants treated with high-dose aducanumab in EMERGE displayed modestly less decline on the CDR-SB (0.39 points) vs placebo.12,15 In view of these clinical uncertainties, use of aducanumab in real-world practice has been scant overall.
The phase 3 trial of lecanemab (CLARITY-AD) represented a step forward. Lecanemab lowers brain Aβ plaque burden through binding to soluble Aβ protofibrils as well as (to variable extent) other forms of Aβ.16 Among 1,800 older adults in CLARITY-AD with biomarker-supported early AD, lecanemab treatment over 18 months led to slowing of cognitive and functional decline based on the CDR-SB.5 Although the treatment effect (∼0.5-point differential on the CDR-SB) was modest, the approximately 25% reduction in worsening observed would equate to 4–5 months of delay in disease-related clinical progression over 1.5 years. In addition to meeting all primary and secondary outcomes of cognition and function, lecanemab treatment also yielded differences in AD biomarkers (including CSF, plasma, and PET measures of tau), supporting a downstream pathophysiologic effect of Aβ plaque removal.5 Favorable drug effects were also demonstrated on scores from a care partner questionnaire that assessed daily function as well as exploratory indices of quality of life and caregiver burden.17
Other than infusion reactions, the most common side effect of lecanemab treatment was the development of amyloid-related imaging abnormalities (ARIA), a known potential complication of antiamyloid mAbs which is believed to relate to disruptions of vascular integrity.18 Findings of ARIA in CLARITY-AD consisted of ARIA with edema/effusion (ARIA-E) in 12.6% of treated individuals (vs 1.7% with placebo) and ARIA with microhemorrhage/hemosiderosis (ARIA-H) in 17.3% of treated individuals (vs 9.0% with placebo). The overall incidence of ARIA (either ARIA-E or ARIA-H) with lecanemab treatment was 21.3%, which was lower than rates reported for aducanumab (41% at the highest drug dose) in its phase 3 trials.6 Within the confines of the careful patient selection and safety monitoring of CLARITY-AD, nearly 80% of cases with ARIA were asymptomatic, and overall, less than 3% of individuals treated with lecanemab experienced symptomatic ARIA.5
In summary, CLARITY-AD demonstrated that lecanemab (1) can slow decline in early symptomatic AD and (2) is associated with risks beyond those of current mainstays of AD management. The drug is currently available under a traditional FDA approval, potentially alongside other within-class agents in the future. With this backdrop, it stands to reason that some patients with AD will be interested in and potentially qualify for treatment. Effectively supporting their neurologic care will require thoughtful translation of trial experience to real-world clinical practice. As such, the balance of this article will focus on discussion of (1) pragmatic approaches for identifying which patients are most likely to benefit from treatment; (2) innovative workflow considerations to optimize risk counseling, safety monitoring, and practice volume sustainability; and (3) novel aspects of ongoing research to contextualize future directions.
Patient Selection: From Overt Indications to Individualized Risk Stratification
The current FDA label specifies that lecanemab is potentially indicated for patients with mild cognitive impairment (MCI) or mild dementia due to AD.19 Patients with MCI display cognitive impairment that is objectively evident (i.e., not representing subjective concerns only) but is not at a severity to affect functioning in instrumental activities of daily living.20 Patients with mild dementia have cognitive impairment that interferes with the ability to function at work or other usual activities, but is not advanced enough to affect functioning in basic activities of daily living (e.g., dressing, grooming).20 As these definitions reflect a continuum of cognitive impairment severity, in CLARITY-AD, a Mini-Mental State Examination (MMSE) score of 22–30 was required to ensure that participants fit the target population of early symptomatic AD.5 Current safety and efficacy data do not support treatment in presymptomatic AD or in moderate/severe AD dementia.
Although the broader inclusion criteria for CLARITY-AD provide additional guideposts for appropriate use, these may not be uniformly applicable toward all clinical contexts. For example, some patients outside of the defined age (50–90 years) or MMSE (22–30) ranges specified in CLARITY-AD could otherwise be considered reasonable candidates for therapy, including younger individuals with sporadic AD who are relatively more likely to have atypical clinical syndromes, which can influence cognitive test performance.21 In addition, strict reliance on the CDR-SB or neuropsychological assessment items could necessitate extra training or resources, which may not be practical for all settings. As such, it will be important for clinicians to consider up front how best to systematically identify patients who are most likely to benefit from treatment while ensuring that drug usage generally adheres to the conditions under which CLARITY-AD provides evidence of clinical efficacy.
Evidence of abnormal brain amyloidosis also needs to be demonstrated before initiation of antiamyloid mAb therapy. In CLARITY-AD, amyloid positivity was determined by either amyloid PET or measurement of CSF biomarkers. Amyloid PET requires specialty scanner and tracer availability as well as neuroradiology expertise and currently is not widely covered by insurance outside of registry-based trials (e.g., the IDEAS study).22 CSF biomarker tests are generally more accessible but require lumbar puncture, proper sample acquisition (including use of special tubes),23 shipment to a qualified laboratory, and expertise in interpretation.24 Although not yet optimized for clinical use, blood-based biomarkers of AD are being actively studied and may revolutionize screening and treatment monitoring in the future.25 However, it is not yet fully clear whether these blood-based biomarkers will have the sensitivity and specificity necessary for use in decision making on treatment initiation, in comparison with use as screening tools.
There is no indication for antiamyloid mAb therapy in patients with primary diagnoses other than AD (e.g., Lewy body disease, frontotemporal dementia, or vascular cognitive impairment) or in individuals with suspected multietiologic cognitive impairment. Particularly given that amyloid positivity is not rare in cognitively unimpaired older adults26 and neurodegenerative copathology is quite common in cognitively impaired individuals,27 comprehensive assessments will remain critical to facilitate individualized counseling on treatment options.
Additional factors influence the risk of side effects from antiamyloid mAbs. A pretreatment brain MRI is necessary to assess the extent of cerebrovascular disease and other relevant structural abnormalities. Microhemorrhages (particularly in a high number or in a cortical location to suggest underlying cerebral amyloid angiopathy28) and superficial siderosis are associated with higher risk of ARIA.18 Patients with a history of cerebral macrohemorrhage, large-territory ischemic stroke, multiple lacunar strokes, or MRI signs of severe small vessel ischemic disease affecting the cerebral white matter were excluded from CLARITY-AD and are presumed to be at elevated risk of side effects from antiamyloid mAb treatment.5,29 Patients with barriers to obtaining MRI would also not be candidates for antiamyloid mAbs because of the need for regular safety monitoring and urgent imaging in case of symptoms.
Anticoagulant medications are commonly prescribed in older adults for various indications, but use of these medications with antiamyloid mAbs is contraindicated because of increased bleeding risk, including the potential for severe macrohemorrhage.29,30 A patient who had received 3 doses of lecanemab and subsequently received an intravenous tissue plasminogen activator (tPA) for acute ischemic stroke syndrome died of extensive, multifocal cerebral hemorrhages.31 Two additional patient deaths have occurred in the open-label extension phase of CLARITY-AD,32 at least one of which involved exposure to anticoagulant medication.33 The effect of antiplatelet medications on ARIA risk is less certain. In CLARITY-AD, participants were allowed to take aspirin while receiving treatment, and there was no evidence of increased risk of ARIA in this setting. Nevertheless, the presence of other possible risk factors of bleeding events in the general population highlights the importance of individualized clinical judgment and counseling regarding the potential for antiplatelet medications to heighten the risk of ARIA-H.
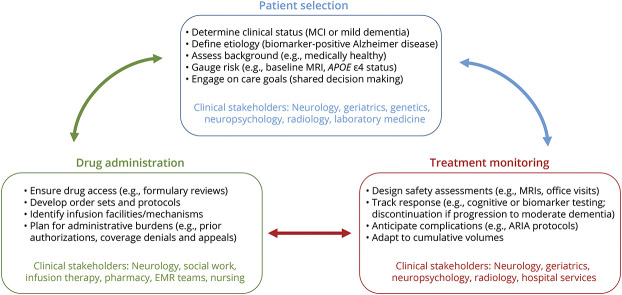
The APOE ε4 allele is the strongest known genetic risk factor of sporadic (i.e., nonfamilial) AD,34 and it is associated with higher rates of ARIA with antiamyloid mAb therapy, particularly among APOE ε4 homozygotes.5,18 As a result, it is widely felt that APOE ε4 allele testing should be offered before antiamyloid mAb treatment so that risks of ARIA can be appropriately considered.29,30 It will be important for clinicians to develop processes for pre-test and post-test genetic counseling regarding the implications of this testing for patients and families, particularly given that there are no other current indications for obtaining APOE allele testing in clinical neurology practice.34 Other major neurologic and systemic medical conditions that may influence benefits and risks of therapy (e.g., seizure disorders, cancers, hematologic or immunologic disorders, exposure to other monoclonal antibody therapies) will also require thoughtful decision making at practice and patient levels (Figure 1).
Figure 1. A Pragmatic Model for Clinical Implementation of Antiamyloid Monoclonal Antibody Therapies for Alzheimer Disease.
Comprehensive advanced planning, multidisciplinary stakeholder engagement, and iterative adaptations are all critical for neurology practices to sustainably incorporate antiamyloid monoclonal antibody therapies into viable options for qualifying patients with Alzheimer disease. Practice-specific choices on appropriate patient selection, drug administration protocols, and processes for treatment monitoring all have the potential to affect response to treatment for patients and risks of complications from treatment for patients and health systems. This clinical decision making will benefit from remaining dynamic to changes at the practice level (e.g., capacity for safety MRIs), research level (e.g., identification of new risk factors for ARIA), and patient level (e.g., clinical progression or other factors precluding further therapy). ARIA = amyloid-related imaging abnormalities; EMR = electronic medical record; MCI = mild cognitive impairment.
For interested readers, a working group recently composed recommendations on appropriate use of lecanemab.29 In particular, the publication includes a table summarizing key inclusion and exclusion criteria for CLARITY-AD (which extend to discussion of non-AD neurologic and medical conditions) alongside recommendations from the working group for appropriate clinical use.29 Guidance on clinical use6 and appropriate use recommendations30 were also published for aducanumab.
Shared Decision Making: Alignment of Treatment Options to Individualized Care Goals
When treatment with antiamyloid mAbs may be a viable option, a shared deliberative process among clinicians, patients, and caregivers will be critical to ensure alignment of expectations, benefits, risks, and goals of care.6 Given that patients with symptomatic AD may have or develop impairments in a decision-making capacity, it is critical that advanced directives and/or authorizations (e.g., for a designated care partner to make health care decisions on the patient's behalf) are within the medical record before considering treatment. Almost all individuals with AD will have initial interest in a therapy that can potentially slow progression. However, it is important that patients understand that the goal of treatment is slowing of decline rather than stabilization or improvement in cognitive functioning. In addition, some patients may be less enthusiastic when they weigh the possible side effects (e.g., infusion reactions, ARIA) and personal financial costs and logistic burdens associated with treatment. Financial costs of treatment to patients extend beyond medication copays to include recurring infusion center fees, clinic evaluations, and MRI scans and other diagnostic tests. The degree to which insurers will cover these costs is currently unknown. Regular (biweekly or monthly) infusions may also be logistically challenging or negatively affect quality of life for some individuals, particularly based on geographic or transportation factors. Here, social work resources could be particularly valuable in navigating paperwork and other needs.
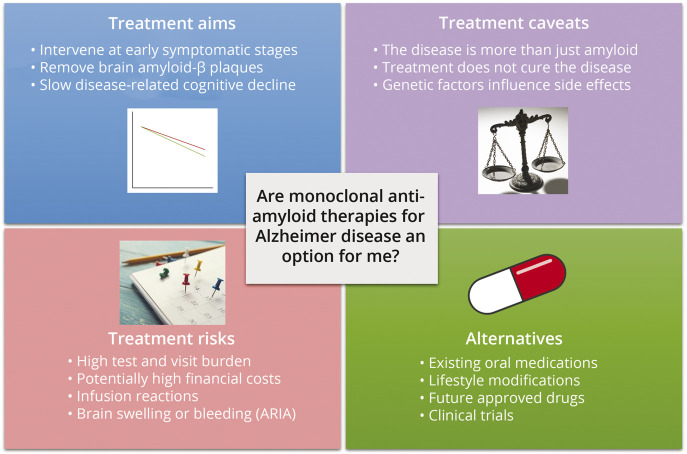
More broadly, shared decision making in this space will need to be individualized and accepting of areas of uncertainty, including risk factors of ARIA and other side effects, limitations of existing trial data (e.g., for minority populations and patients with clinically atypical AD), and ideal treatment duration amidst unknowns about longer term (beyond 18 months) sequelae of drug therapy. Overall, it will be vital for neurology clinicians, patients, and caregivers to engage in nuanced discussions that acknowledge that antiamyloid mAbs are not a cure for AD but could be a reasonable option for seeking additional slowing of disease progression in some patients with early disease and low likelihood of side effects (Figure 2).
Figure 2. Potential Template for Patient Education on Antiamyloid Monoclonal Antibody Therapies for Alzheimer Disease.
Almost all patients with Alzheimer disease will have initial interest in a new treatment option for a progressive, devastating disorder. Patient-friendly educational tools may assist with automated handling of initial inquiries, setting appropriate expectations, and transitioning discussions beyond simple drug availability and toward a broader consideration of treatment aims, risks, burdens, and alternatives.
Treatment Administration: Drug Infusions, Safety Monitoring, and Contingencies
Current frameworks of AD care include relatively infrequent use of office visits and advanced diagnostic testing. To adapt these for administration of antiamyloid mAbs, neurology practices will benefit from multidisciplinary engagement to ensure balanced volumes and access throughout the treatment course (Figure 1). Lecanemab is administered every 2 weeks intravenously over approximately 1 hour.19 Along with facilitating appropriate patient selection and arranging recurring infusions, clinicians must be prepared to respond to ARIA and other potential complications of treatment. This includes having pathways for urgent evaluation in case of symptoms and liaising with local radiology and emergency medicine teams to ensure awareness around new indications and orders.
Common symptoms of ARIA include dizziness, headache, visual disturbances, and increased confusion.18 However, most cases of radiologic ARIA in CLARITY-AD were asymptomatic,5 which creates a risk of worsening without sufficient monitoring. The FDA label for lecanemab specifies obtaining safety MRI scans before the 5th, 7th, and 14th infusions.19 This reflects that in CLARITY-AD, ARIA (particularly ARIA-E) were most frequent in the first 4 months of therapy.5 However, additional scans may be warranted in individuals at higher risk of ARIA, including in patients with APOE ε4 who display initial episodes of ARIA-E beyond this early time window.5 Among consensus recommendations,18 MRI scans for ARIA detection should at minimum include T2–fluid-attenuated inversion recovery, T2* gradient recalled echo, and diffusion-weighted imaging sequences, and benefit from standardization of scanner types and protocols across time points. Given that some ARIA findings can be subtle, with treatment ramifications based on gradation, neuroradiology expertise (through existing local providers or remote partnerships) will be important.
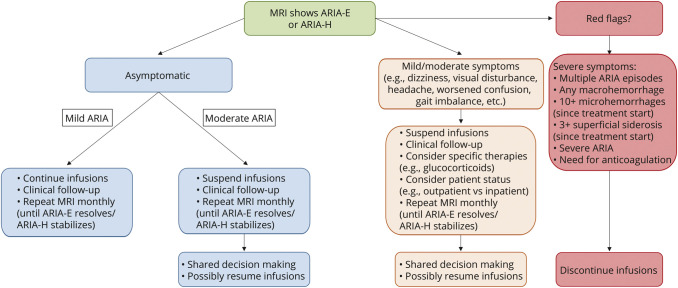
A framework for management based on radiologic severity of ARIA (provided in the FDA label for lecanemab)19 and clinical symptoms is presented in Figure 3. In CLARITY-AD, most cases of ARIA (more than 80%) resolved within 4 months on discontinuation of lecanemab infusions.5 For some patients with symptomatic or radiologically severe ARIA, providers may consider early initiation of high-dose steroids (e.g., intravenous methylprednisolone 1 g daily for 3–5 days, followed by an oral steroid taper over several weeks). Other goal-directed therapies (e.g., antiseizure medications), diagnostic tests, or hospital admission may be required on a patient-specific basis. These contingencies imply that clinical practices offering antiamyloid mAb treatments will need to ensure that there is sufficient access to general floor and intensive care unit beds, inpatient MRI and EEG, and other hospital resources. Furthermore, patients and clinicians should be aware that treatments indicated for non-AD medical conditions (e.g., intravenous tPA for acute ischemic stroke) could substantially influence the risk of brain macrohemorrhage and, therefore, may be precluded in the setting of antiamyloid mAb treatment.31,35
Figure 3. Suggested Management Based on ARIA Radiologic Severity and Clinical Condition.
A suggested framework for managing imaging findings of ARIA is displayed, with differentiations by clinical and radiologic severity noted. Grading of radiographic ARIA is based on the US Food and Drug Administration prescribing label for lecanemab as follows: (ARIA-E) mild—one site of sulcal or cortical/subcortical FLAIR hyperintensity measuring <5 cm; moderate—one site of FLAIR hyperintensity measuring 5–10 cm or more than 1 site of FLAIR hyperintensity each measuring <10 cm; and severe—any site of FLAIR hyperintensity measuring >10 cm; (ARIA-H) mild—≤4 treatment-emergent microhemorrhages or 1 focal area of superficial siderosis; moderate—5–9 treatment-emergent microhemorrhages or 2 focal areas of superficial siderosis; and severe—10 or more treatment-emergent microhemorrhages or 3 or more focal areas of superficial siderosis. Importantly, practice and provider-specific clinical decision making that is appropriately individualized to the patient will be inherent to optimizing management of complications of therapy with antiamyloid monoclonal antibodies for Alzheimer disease. ARIA = amyloid-related imaging abnormalities; ARIA-E = ARIA with edema/effusion; ARIA-H = ARIA with microhemorrhage/hemosiderosis; FLAIR = fluid-attenuated inversion recovery.
Practice Infrastructure: Sustainably Adapting to New Demands
The emergence of antiamyloid mAbs occurs at a time when communities have variable access to general neurologists, cognitive-subspecialist neurologists, other qualified clinicians, and dementia care resources.6,36 In this setting, collaborative care efforts (through formal and informal relationships) and practice-specific adaptations will be critical to ensure financially and logistically sustainable efforts that meet patients' needs.37 Presumably, it would be most practical for a large proportion of the community-based population to receive initial evaluations by nonspecialist clinicians, for which cognitive screening may be a reimbursable service with appropriate downstream yield (e.g., Medicare annual wellness visit). Training non–dementia-specialist clinicians in improved conceptual awareness and examination skills may help them serve as a dedicated “front line” for communities. Triage models that could identify patients with high risk and/or complexity would likely also have major effects on appointment access and clinician and patient experience.
In addition, heightened requests for information from patients and caregivers, which will vary by demographic and other factors, can be anticipated. Efforts to improve efficiency of request response rates will help to conserve practice resources. One potential strategy to mitigate excess demand involves developing a dedicated website for patient and caregiver education. Phone call or electronic medical record response duration may be improved by providing template responses to frequently asked questions. For some practices, designing a consultation service, through which the specialist communicates with nonspecialist clinicians to support and triage appropriate patients38 and through which the health system addresses any limitations of payer reimbursement for current diagnostic codes, may also mitigate demand.
Ultimately, clinicians offering antiamyloid mAbs for AD will depend on reimbursements through direct pay, pharmaceutical company support, or a third-party payer. If drug therapy is covered by a payer, policies may vary between traditional Medicare vs Medicare Advantage plans. In addition, within traditional Medicare, coverage determination may be standardized across the United States (i.e., through a national coverage determination) or the coverage decision may be determined on a regional basis (i.e., local coverage determination). Clinicians managing the administration of antiamyloid mAbs should anticipate and plan for the administrative burden related to drug prior authorization and potential denials and appeals. Overall, neurology practices considering these treatments must be cognizant of the patient's payer and policies, both of which may be dynamic.
Donanemab: A Potential Addition to the Treatment Framework
Donanemab is another high-potency antiamyloid mAb that is infused intravenously every 4 weeks. Recently, top-line results were announced for the phase 3 trial of donanemab (TRAILBLAZER-ALZ 2). Compared with placebo, donanemab treatment over 18 months resulted in slowing of cognitive and functional decline by approximately 35% in the primary target population studied.39 ARIA-E and ARIA-H occurred in 24.0% and 31.4% of treated individuals, respectively.39 TRAILBLAZER-ALZ 2 included some unique aspects of study design that limit direct comparisons with CLARITY-AD. Under a “treat-to-clear” model, serial amyloid PET scans were used to determine treatment duration, with 52% of treated participants converting to amyloid PET–negative status by 12 months.39 In addition, tau PET was obtained at baseline to stratify participants, resulting in the study sample being relatively enriched with individuals having intermediate (vs high) levels of tau burden who were hypothesized to be at earlier disease stages with greater likelihood of treatment response. Further details on TRAILBLAZER-ALZ 2 are anticipated to be released later in 2023, and an application for traditional FDA approval is pending a decision over the next year. In the event of donanemab being available clinically in the future, many facets of a framework for implementing lecanemab would be anticipated to translate to donanemab. However, the different trial designs for these in-class agents indicate that additional drug-specific adaptations to workflows will be necessary. In addition, clinicians will need to engage with patients who are eligible for treatment to weigh available options, which may be influenced by differential insurance coverage and copays, infusion and scan frequency, clinical efficacy, and side effect rates. Models for this approach can be adapted from other neurologic subspecialties (such as multiple sclerosis and neuro-oncology), in which frequently there are multiple options for disease-modifying therapy in any individual patient.
Antiamyloid mAbs in AD: Current Foundation and Future Advances
The collection of late-phase clinical trials of antiamyloid mAbs represents merely the initial forays of updates to neurologic care for AD in the era ahead. As with other complex diseases for which treatment options have advanced iteratively (e.g., HIV and AIDS), patient selection, clinical decision making, and safety monitoring should all be anticipated to evolve with additional real-world experience and research extensions. In particular, most CLARITY-AD participants had sporadic, clinically typical (i.e., amnestic multidomain dementia syndrome) presentations of AD. There is limited information on how the trial results may extend to clinically atypical AD. Furthermore, individuals with autosomal dominant AD or Down syndrome are currently recommended to be excluded from antiamyloid mAb treatment because of increased rates of cerebral amyloid angiopathy.29 Additional data may inform changes to these principles. Given the complexity of antiamyloid mAbs, provider-enrolled patient registries, such as ALZ-NET,40 may provide an avenue for modest flexibility on appropriate use at an individual patient level while facilitating data-driven practice changes for the broader field.
Drug-specific characteristics may inform future developments. Existing highly potent antiamyloid mAbs target various steps in the cascade leading to Aβ plaque deposition, and it remains possible that subtle mechanistic and pharmacokinetic differences among these agents could influence efficacy, side effects, and optimal contexts for use,41 similar to what is observed with newer generation therapies for multiple sclerosis. In particular, the magnitude and rate of Aβ plaque reduction may differentially regulate downstream pathophysiologic consequences of therapy and resulting clinical outcomes,42 which could affect patient candidacy and drug selection. Compared with placebo, treatment with several antiamyloid mAbs has also been associated with lower brain volumes,43 and further research is needed to determine the underlying mechanisms and any longer term implications of these findings. In addition, there is no current consensus on the optimal duration of antiamyloid mAb therapy. The longer term burdens on patients and economic feasibility of treatment may differ widely depending on whether infusions are to continue indefinitely until progression to a moderate dementia, for a defined period (e.g., 18 months), or until reaching amyloid PET–negative status (as with the model used for TRAILBLAZER-ALZ 2).7,39
The timing of therapy, relative to disease stage, may also evolve in the future. Given that high rates of Aβ deposition largely precede, in some cases by many years, the development of significant clinical impairment,44 the value of earlier intervention in preclinical and asymptomatic AD remains of high interest. Unfortunately, results of recent trials of solanezumab, in the Anti-Amyloid Treatment in Asymptomatic Alzheimer's Disease study,45 and crenezumab, in a cohort of cognitively unimpaired PSEN1 E280A mutation carriers,46 were not positive. However, as lecanemab was the first antiamyloid mAb to convincingly demonstrate a clinical benefit in mildly symptomatic AD and displays higher potency of cerebral Aβ reduction than previous drugs tested in this setting, results of the AHEAD 3–45 study testing this agent in preclinical AD are eagerly anticipated.47
Developments around antiamyloid mAbs are occurring on the backdrop of nuanced debate over what degree and character of clinical change (1) could be reasonably expected within AD trials and (2) would be considered meaningful by patients and care partners. In the early symptomatic stages of AD, it is conceivable that a 0.5-point differential in the CDR-SB could manifest with substantive effects on daily life, for example, if distinctions (e.g., between mild forgetfulness and more moderate memory loss affecting daily activities) affect the ability to maintain employment or remain in a long-standing shared home.17 Post hoc survival analyses in CLARITY-AD also suggested that by 18 months, a smaller proportion of patients treated with lecanemab (24%) had declined by a global CDR score (0.5 = MCI, 1.0 = mild dementia, 2.0 = moderate dementia) compared with placebo (32%).5 However, patients will need to be aware that it is possible that this modest reduction in the rate of decline may not be overtly evident to them or their care partners. Longer term follow-up observations on treated patients from the open-label extension will also be needed to clarify whether nonlinear courses of benefit are a reasonable expectation.48 Part of the emphasis on shared decision making around antiamyloid mAbs is grounded in the idea that there will not be a “one-size-fits-all” formula for judging whether these treatments are right for an individual patient. Here, it will be prudent to draw upon experience from other areas of neurology that routinely weigh evidence-based data and uncertainties among treatment options.
As trials of antiamyloid mAbs in AD continue to develop, steps must also be taken to ensure that these studies include a diverse range of participants reflective of real-world clinical neurology. This lack of diversity has been a clear shortcoming in AD clinical trials to date, which potentially undermines the generalizability of results.49 In the phase 3 aducanumab and lecanemab trials,5,15 only 0.6% and 2.5%, respectively, of participants were Black and just 1.5% and 12.4%, respectively, were Hispanic. Under-representation in clinical trials is particularly problematic when paired with the findings that the incidence and prevalence of dementia is dramatically higher in these populations compared with White populations.50,51 Although a detailed consideration of the causes of these disparities is beyond the scope of this article, relevant factors include lack of awareness and access, cultural and language barriers, provider and investigator bias, financial burdens, and distrust sowed through inappropriate medical care and research practices with minoritized persons.52 To sustainably diversify clinical trial recruitment for the betterment of patient care, it will be critical for research centers to build infrastructure for effective outreach, develop supportive and long-term relationships with minoritized communities that expand awareness around issues related to cognition and aging, and enhance diversity of personnel involved in AD clinical trials.53
With emerging therapeutics comes the need to close long-standing imbalances between the prevalence of AD and the number of qualified clinicians supporting its care. In comparison with the nearly 7 million people in the United States with AD,1 fewer than 600 physicians are certified by the United Council for Neurologic Subspecialties in Behavioral Neurology and Neuropsychiatry. Although this figure does not include other clinician colleagues who directly diagnose and treat AD (and thus underestimates the true size of the AD clinician workforce), it, nonetheless, underscores that effectively implementing new treatments will require significantly expanding expertise in and access to those therapies. Collaboration of subspecialists with general neurologists, advanced practice providers, geriatricians, and others will thus be at a premium. In this effort, care must be taken to avoid exacerbating preexisting severe racial and ethnic disparities in diagnosis and treatment.54
Conclusions
Recent developments with antiamyloid mAbs include nuance and limitations but represent a step in the right direction for patients with AD. As with developments over time in disease-modifying therapies for other complex disorders (e.g., HIV and AIDS, hypertension, multiple sclerosis), there remains reason for optimism that antiamyloid mAbs may facilitate slowing of the disease process in some patients with AD and may contribute to precision-based patient selection and combination regimens in the future. In this new era, ensuring a breadth and depth of expertise in these therapeutic options and effective patient selection, administration, and monitoring protocols across neurology practices will be crucial to optimize patient outcomes and enable future advances.
Acknowledgment
The following individuals participated in the development of this Emerging Issues in Neurology article as members of the AAN Quality Committee: Sarah M. Benish, MD (University of Minnesota Physicians); Calli L. Cook, DNP, FNP-C (Emory Healthcare); Gregory S. Day, MD, MSc, FAAN (Mayo Clinic); Tiffany L. Fisher, MD, PhD (Penn State Health); Lyell K. Jones, Jr., MD (Mayo Clinic); Charles Kassardjian, MD (St. Michael's Hospital); Vita Kesner, MD, PhD, FAAN (Emory University); Emily Klatte, MD (OhioHealth Neurological Physicians); Kathryn Kvam, MD (Stanford Medicine); Julius Latorre, MD, FAAN; Elisabeth B. Marsh, MD (Johns Hopkins University School of Medicine); Lidia Maria Veras Rocha Moura, MD, PhD, MPH, FAAN (Massachusetts General Hospital); Anup D. Patel, MD (Nationwide Children's Hospital); Michael Phipps, MD, MHS (University of Maryland School of Medicine); Alexander D. Rae-Grant, MD (Cleveland Clinic); Jose G. Romano, MD (University of Miami Miller School of Medicine); and Adam Webb, MD (Emory University School of Medicine).
Glossary
- Aβ
β-amyloid
- AD
Alzheimer disease
- ARIA
amyloid-related imaging abnormalities
- ARIA-E
ARIA with edema/effusion
- ARIA-H
ARIA with microhemorrhage/hemosiderosis
- CDR-SB
Clinical Dementia Rating Sum of Boxes
- EIN
Emerging Issues in Neurology
- FDA
US Food and Drug Administration
- mAb
monoclonal antibody
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- tPA
tissue plasminogen activator
Appendix. Authors
| Name | Location | Contribution |
| Vijay K. Ramanan, MD, PhD | Department of Neurology, Mayo Clinic, Rochester, MN | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Melissa J. Armstrong, MD, MSc | Department of Neurology, University of Florida College of Medicine; Norman Fixel Institute for Neurologic Diseases, University of Florida, Gainesville | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Parichita Choudhury, MD | Cleo Roberts Center, Banner Sun Health Research Institute, Sun City, AZ | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Katherine A. Coerver, MD, PhD | Rocky Mountain Neurology, Lone Tree, CO | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Roy H. Hamilton, MD, MS | Department of Neurology, Department of Physical Medicine and Rehabilitation, and Department of Psychiatry, University of Pennsylvania Perelman School of Medicine, Philadelphia | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Brad C. Klein, MD, MBA | Abington Neurological Associates, Ltd., Abington, PA | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| David A. Wolk, MD | Department of Neurology, University of Pennsylvania Perelman School of Medicine, Philadelphia | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Scott R. Wessels, MPS, ELS | American Academy of Neurology, Minneapolis, MN | Drafting/revision of the manuscript for content, including medical writing for content |
| Lyell K. Jones, Jr., MD | Department of Neurology, Mayo Clinic, Rochester, MN | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Study Funding
No targeted funding reported.
Disclosure
V.K. Ramanan is a site clinician in the Eisai-supported AHEAD 3-45 trial which is testing lecanemab in patients with preclinical Alzheimer disease, is the co-PI for a clinical trial sponsored by the Alzheimer's Association, is a site clinician for clinical trials supported by the Alzheimer's Treatment and Research Institute at USC and Transposon Therapeutics, Inc., reports research funding from the NIH and the Mangurian Foundation for Lewy Body disease research all unrelated to this work, and has also provided educational content for Medscape. M.J. Armstrong is employed by the University of Florida; receives research support from the NIH (R01AG068128, P30AG047266, R01NS121099, R44AG062072), the Florida Department of Health (grant 20A08), and as the local PI of a Lewy Body Dementia Association Research Center of Excellence; serves on the DSMBs for the Alzheimer's Therapeutic Research Institute/Alzheimer's Clinical Trial Consortium and the Alzheimer's Disease Cooperative Study; and has provided educational content for Medscape and Vindico. P. Choudhury reports research support from the Arizona Alzheimer's Research Consortium, is a site clinician for the Eisai-sponsored AHEAD3-45 and CLARITY-AD open-label extension trials, is a site clinician for trials supported by Alzheimer's Disease Cooperative Study (ADCS), Alzheimer's Treatment and Research Institute at USC, Cassava Sciences, Inc., Biogen, Cognition Therapeutics, Novo Nordisk A/S, and the Michael J. Fox Foundation, and is PI for a clinical trial sponsored by Cognito Therapeutics. K.A. Coerver receives salary support from CenExel and research support from Eli Lilly, Sage Therapeutics, Annovis, CND Life and Spark Neuro Inc., and Athira. R.H. Hamilton reports research funding from the NIH, the Department of Defense, the Chan Zuckerberg Initiative, and private philanthropic giving all unrelated to this work and has received funding as a speaker on topics related to diversity and inclusion by Alexion Pharmaceuticals and Starfish Neurosciences. B.C. Klein has received honoraria for speaking at American Academy of Neurology courses; serves on the speaker's bureau of Abbvie, Biohaven, Eli Lilly, Impel, Theranica, and Lundbeck; has served as consultant for Abbvie, Biohaven, Eli Lilly, Lundbeck, and Pfizer; has served as an advisor for Ipsen; has received (via his practice) commercial research support from Abbvie; has equity interest in Abington Neurological Associates, Ltd. and AppsByDocs, LLC, makers of p-cog; and is a member of the American Academy of Neurology Board of Directors. D.A. Wolk has received personal fees from Eli Lilly, GE Healthcare, Qynapse, and Functional Neuromodulation and grants from Biogen, the NIH, and the Alzheimer's Association outside of the submitted work. S.R. Wessels has reported no conflicts of interest. L.K. Jones serves in a voluntary capacity on boards of directors of the Mayo Clinic ACO and the American Academy of Neurology Institute and is editor-in-chief of Continuum. Go to Neurology.org/N for full disclosures.
References
- 1.Alzheimer's Association. Alzheimer's Disease Facts and Figures. Alzheimer's Association; 2023. [Google Scholar]
- 2.Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA. Population estimate of people with clinical Alzheimer's disease and mild cognitive impairment in the United States (2020-2060). Alzheimers Dement. 2021;17(12):1966-1975. doi: 10.1002/alz.12362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knopman DS, Amieva H, Petersen RC, et al. Alzheimer disease. Nat Rev Dis Primers. 2021;7(1):33. doi: 10.1038/s41572-021-00269-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326-1334. doi: 10.1056/nejmsa1204629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388(1):9-21. doi: 10.1056/NEJMoa2212948 [DOI] [PubMed] [Google Scholar]
- 6.Day GS, Scarmeas N, Dubinsky R, et al. Aducanumab use in symptomatic Alzheimer disease evidence in focus: a report of the AAN Guidelines Subcommittee. Neurology. 2022;98(15):619-631. doi: 10.1212/wnl.0000000000200176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in early Alzheimer's disease. N Engl J Med. 2021;384(18):1691-1704. doi: 10.1056/nejmoa2100708 [DOI] [PubMed] [Google Scholar]
- 8.Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535-562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jack CR Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207-216. doi: 10.1016/s1474-4422(12)70291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120(4):545-555. doi: 10.1016/j.cell.2005.02.008 [DOI] [PubMed] [Google Scholar]
- 11.Karran E, De Strooper B. The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nat Rev Drug Discov. 2022;21(4):306-318. doi: 10.1038/s41573-022-00391-w [DOI] [PubMed] [Google Scholar]
- 12.Knopman DS, Jones DT, Greicius MD. Failure to demonstrate efficacy of aducanumab: an analysis of the EMERGE and ENGAGE trials as reported by Biogen, December 2019. Alzheimers Dement. 2021;17(4):696-701. doi: 10.1002/alz.12213 [DOI] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration. FDA's decision to approve new treatment for Alzheimer's disease [online]. Accessed May 25, 2023. fda.gov/drugs/news-events-human-drugs/fdas-decision-approve-new-treatment-alzheimers-disease.
- 14.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412.2-2412-a. doi: 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- 15.Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer's disease. J Prev Alzheimers Dis. 2022;9(2):197-210. doi: 10.14283/jpad.2022.30 [DOI] [PubMed] [Google Scholar]
- 16.Soderberg L, Johannesson M, Nygren P, et al. Lecanemab, aducanumab, and gantenerumab: binding profiles to different forms of amyloid-beta might explain efficacy and side effects in clinical trials for Alzheimer's disease. Neurotherapeutics. 2022;20(1):195-206. doi: 10.1007/s13311-022-01308-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolk DA, Rabinovici GD, Dickerson BC. A step forward in the fight against dementia: are we there yet? JAMA Neurol. 2023;80(5):429-430. doi: 10.1001/jamaneurol.2023.0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cogswell PM, Barakos JA, Barkhof F, et al. Amyloid-related imaging abnormalities with emerging Alzheimer disease therapeutics: detection and reporting recommendations for clinical practice. AJNR Am J Neuroradiol. 2022;43(9):E19-E35. doi: 10.3174/ajnr.a7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US Food and Drug Administration. FDA approved drugs: lecanemab (reference ID 5105416) [online]. Accessed May 25, 2023. accessdata.fda.gov/drugsatfda_docs/label/2023/761269s000lbl.pdf.
- 20.Knopman DS, Petersen RC. Mild cognitive impairment and mild dementia: a clinical perspective. Mayo Clin Proc. 2014;89(10):1452-1459. doi: 10.1016/j.mayocp.2014.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graff-Radford J, Yong KXX, Apostolova LG, et al. New insights into atypical Alzheimer's disease in the era of biomarkers. Lancet Neurol. 2021;20(3):222-234. doi: 10.1016/s1474-4422(20)30440-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabinovici GD, Gatsonis C, Apgar C, et al. Association of amyloid positron emission tomography with subsequent change in clinical management among Medicare beneficiaries with mild cognitive impairment or dementia. JAMA. 2019;321(13):1286-1294. doi: 10.1001/jama.2019.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansson O, Mikulskis A, Fagan AM, et al. The impact of preanalytical variables on measuring cerebrospinal fluid biomarkers for Alzheimer's disease diagnosis: a review. Alzheimers Dement. 2018;14(10):1313-1333. doi: 10.1016/j.jalz.2018.05.008 [DOI] [PubMed] [Google Scholar]
- 24.Graff-Radford J, Jones DT, Wiste HJ, et al. Cerebrospinal fluid dynamics and discordant amyloid biomarkers. Neurobiol Aging. 2022;110:27-36. doi: 10.1016/j.neurobiolaging.2021.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansson O, Edelmayer RM, Boxer AL, et al. The Alzheimer's Association appropriate use recommendations for blood biomarkers in Alzheimer's disease. Alzheimers Dement. 2022;18(12):2669-2686. doi: 10.1002/alz.12756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Therriault J, Pascoal TA, Benedet AL, et al. Frequency of biologically defined Alzheimer disease in relation to age, sex, APOE ε4, and cognitive impairment. Neurology. 2021;96(7):e975-e985. doi: 10.1212/WNL.0000000000011416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson JL, Lee EB, Xie SX, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. 2018;141(7):2181-2193. doi: 10.1093/brain/awy146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, Pruzin J, Sperling R, van Veluw SJ. Cerebral amyloid angiopathy and Alzheimer disease: one peptide, two pathways. Nat Rev Neurol. 2020;16(1):30-42. doi: 10.1038/s41582-019-0281-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cummings J, Apostolova L, Rabinovici GD, et al. Lecanemab: appropriate use recommendations. J Prev Alzheimers Dis. 2023;10(3):362-377. doi: 10.14283/jpad.2023.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cummings J, Rabinovici GD, Atri A, et al. Aducanumab: appropriate use recommendations update. J Prev Alzheimers Dis. 2022;9(2):221-230. doi: 10.14283/jpad.2022.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reish NJ, Jamshidi P, Stamm B, et al. Multiple cerebral hemorrhages in a patient receiving lecanemab and treated with t-PA for stroke. N Engl J Med. 2023;388(5):478-479. doi: 10.1056/nejmc2215148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Science. Scientists tie third clinical trial death to experimental Alzheimer's drug [online]. Accessed May 25, 2023. science.org/content/article/scientists-tie-third-clinical-trial-death-experimental-alzheimer-s-drug.
- 33.Stata News. Death of patient in closely watched Alzheimer's trial raises concern about risk for some groups [online]. Accessed May 25, 2023. statnews.com/2022/10/28/patient-death-lecanemab-alzheimers-trial/.
- 34.Choudhury P, Ramanan VK, Boeve BF. APOE allele testing and alzheimer disease—reply. JAMA. 2021;325(21):2211. doi: 10.1001/jama.2021.4925 [DOI] [PubMed] [Google Scholar]
- 35.Sabbagh M, van Dyck CH. Response to: multiple cerebral hemorrhages in a patient receiving lecanemab and treated with t-PA for stroke. N Engl J Med. 2023;388(5):480. doi: 10.1056/NEJMc2215907 [DOI] [PubMed] [Google Scholar]
- 36.Lin CC, Callaghan BC, Burke JF, et al. Geographic variation in neurologist density and neurologic care in the United States. Neurology. 2021;96(3):e309-e321. doi: 10.1212/wnl.0000000000011276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galvin JE, Aisen P, Langbaum JB, et al. Early stages of Alzheimer's disease: evolving the care team for optimal patient management. Front Neurol. 2020;11:592302. doi: 10.3389/fneur.2020.592302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vimalananda VG, Gupte G, Seraj SM, et al. Electronic consultations (e-consults) to improve access to specialty care: a systematic review and narrative synthesis. J Telemed Telecare. 2015;21(6):323-330. doi: 10.1177/1357633x15582108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eli Lilly and Company. Lilly's donanemab significantly slowed cognitive and functional decline in phase 3 study of early Alzheimer's disease [online]. Accessed May 25, 2023. investor.lilly.com/news-releases/news-release-details/lillys-donanemab-significantly-slowed-cognitive-and-functional.
- 40.Alzheimer's Association launches ALZ-NET. A long-term data collection and sharing network for new treatments. Alzheimers Dement. 2022;18:1694-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panza F, Lozupone M, Logroscino G, Imbimbo BP. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat Rev Neurol 2019;15(2):73-88. doi: 10.1038/s41582-018-0116-6 [DOI] [PubMed] [Google Scholar]
- 42.Shcherbinin S, Evans CD, Lu M, et al. Association of amyloid reduction after donanemab treatment with tau pathology and clinical outcomes: the TRAILBLAZER-ALZ randomized clinical trial. JAMA Neurol. 2022;79(10):1015-1024. doi: 10.1001/jamaneurol.2022.2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swanson CJ, Zhang Y, Dhadda S, et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer's disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimers Res Ther. 2021;13(1):80. doi: 10.1186/s13195-021-00813-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jack CR Jr, Wiste HJ, Lesnick TG, et al. Brain beta-amyloid load approaches a plateau. Neurology. 2013;80(10):890-896. doi: 10.1212/wnl.0b013e3182840bbe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Press Release. A4 study results: investigational anti-amyloid treatment started before Alzheimer's symptoms did not slow memory loss [online]. Accessed May 25, 2023. brighamandwomens.org/about-bwh/newsroom/press-releases-detail?id=4384.
- 46.Sink KM, Doody RS, Ríos-Romenets S, Tariot PN, Reiman EM, Langbaum JB. API ADAD Colombia Trial initial findings: a randomized, double-blind, placebo-controlled, parallel-group study in cognitively unimpaired PSEN1 E280A mutation carriers evaluating efficacy and safety of crenezumab. Alzheimers Dement. 2022;18(S10):e069724. doi: 10.1002/alz.069724 [DOI] [Google Scholar]
- 47.Rafii MS, Sperling RA, Donohue MC, et al. The AHEAD 3-45 Study: design of a prevention trial for Alzheimer's disease. Alzheimers Dement. 2022;19(4):1227-1233. doi: 10.1002/alz.12748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petersen RC, Aisen PS, Andrews JS, et al. Expectations and clinical meaningfulness of randomized controlled trials. Alzheimers Dement. 2023;19(6):2730-2736. doi: 10.1002/alz.12959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grill J, Molina-Henry DP, Sperling RA, et al. Diversity in phase 2 and phase 3 placebo-controlled, double-blind, lecanemab and elenbecestat early Alzheimer's disease studies. Alzheimers Dement. 2022;18(S10):e069198. doi: 10.1002/alz.069198 [DOI] [Google Scholar]
- 50.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12(3):216-224. doi: 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mehta KM, Yeo GW. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimers Dement. 2017;13(1):72-83. doi: 10.1016/j.jalz.2016.06.2360 [DOI] [PubMed] [Google Scholar]
- 52.Bae AS. Key barriers against racial and ethnic minority participation in U.S. clinical trials. Int J Clin Trials. 2022;9(3):227-233. doi: 10.18203/2349-3259.ijct20221876 [DOI] [Google Scholar]
- 53.Raman R, Aisen PS, Carillo MC, et al. Tackling a major deficiency of diversity in Alzheimer's disease therapeutic trials: an CTAD task force report. J Prev Alzheimers Dis 2022;9(3):388-392. doi: 10.14283/jpad.2022.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsoy E, Kiekhofer RE, Guterman EL, et al. Assessment of racial/ethnic disparities in timeliness and comprehensiveness of dementia diagnosis in California. JAMA Neurol. 2021;78(6):657-665. doi: 10.1001/jamaneurol.2021.0399 [DOI] [PMC free article] [PubMed] [Google Scholar]