Abstract
Background and Objectives
Movement disorders (MDs) are underrecognized in the developmental and epileptic encephalopathies (DEEs). There are now more than 800 genes implicated in causing the DEEs; relatively few of these rare genetic diseases are known to be associated with MDs. We identified patients with genetic DEEs who had MDs, classified the nature of their MDs, and asked whether specific patterns correlated with the underlying mechanism.
Methods
We classified the type of MDs associated with specific genetic DEEs in a large international cohort of patients and analyzed whether specific patterns of MDs reflected the underlying biological dysfunction.
Results
Our cohort comprised 77 patients with a genetic DEE with a median age of 9 (range 1–38) years. Stereotypies (37/77, 48%) and dystonia (34/77, 44%) were the most frequent MDs, followed by chorea (18/77, 23%), myoclonus (14/77, 18%), ataxia (9/77, 12%), tremor (7/77, 9%), and hypokinesia (6/77, 8%). In 47% of patients, a combination of MDs was seen. The MDs were first observed at a median age of 18 months (range day 2–35 years). Dystonia was more likely to be observed in nonambulatory patients, while ataxia was less likely. In 46% of patients, therapy was initiated with medication (34/77, 44%), deep brain stimulation (1/77, 1%), or intrathecal baclofen (1/77, 1%). We found that patients with channelopathies or synaptic vesicle trafficking defects were more likely to experience dystonia; whereas, stereotypies were most frequent in individuals with transcriptional defects.
Discussion
MDs are often underrecognized in patients with genetic DEEs, but recognition is critical for the management of these complex neurologic diseases. Distinguishing MDs from epileptic seizures is important in tailoring patient treatment. Understanding which MDs occur with different biological mechanisms will inform early diagnosis and management.
Introduction
Developmental and epileptic encephalopathies (DEEs) are the most severe group of epilepsies, characterized by drug-resistant seizures, epileptiform activity on electroencephalography, and developmental plateauing or regression.1 With advances in next-generation sequencing, the genetic basis is determined in almost 40%–50% of DEEs with more than 800 DEE genes identified.2-4 These genes implicate a wide range of neurobiological processes.5,6
As larger cohorts of patients with a specific genetic DEE are identified, a phenotypic spectrum typically emerges. While there has been a major focus on seizure types, EEG, and MRI findings in individuals with DEEs, less attention has been given to movement disorders (MDs), which may be subtle or missed. Distinguishing MDs from seizures can be challenging, yet critically important for management of both facets of the DEE.
The association between genetically determined epilepsies and MDs has become increasingly apparent. There has been considerable interest in paroxysmal MDs that occur with self-limited epilepsies, such as paroxysmal kinesigenic dyskinesia and self-limited familial infantile epilepsies, which co-occur in the epilepsy syndrome of infantile convulsions choreoathetosis, due to PRRT2 pathogenic variants.7,8 For the DEEs, MDs have been identified in some specific genetic diseases; however, the range of chronic and paroxysmal MDs in genetic DEEs has not been addressed in a large cohort of patients.
A clearer understanding of the range of MDs seen in association with DEE genes also has implications for MD specialists. For some genes usually associated with a DEE phenotype, rare patients with only mild,9 or without,10 epilepsy have been reported. Such patients may present to MD neurologists, so it is important to include DEE genes in the diagnostic workup.
We aimed to understand which MDs are associated with different genetic DEEs. We characterized the pattern of MDs in patients with genetic DEEs.
Methods
Patients with genetic DEEs and a co-existing MD were recruited to the University of Melbourne Epilepsy Genetics Research Program (Melbourne, Australia) and the Department of Neuroscience at the Bambino Gesù Children's Hospital (Rome, Italy). Only patients with an identified molecular diagnosis were included, whereas patients with an acquired or unknown cause were excluded.
Patients with DEEs were included in the study when we identified a MD in the setting of a known genetic etiology. Video recordings of each patient's MD were reviewed by MD neurologists, and the nature of the MD was classified for each patient. Videos were obtained from video-EEG recordings, video recordings by the physician, or home video provided by family members or carers. A small number of patients were included where their neurologist described the MD(s) but a video was not available. We classified MDs into hyperkinetic MD phenotypes of stereotypies, dystonia, chorea, myoclonus, and tremor in addition to hypokinetic and ataxic MDs.11
We determined the age at onset at which the abnormal movements were first noticed by parents or carers or documented in the medical record, whichever was earlier. We reviewed the clinical course of the MD and patients' seizure types, severity of intellectual disability (ID), impairment in gait and speech, and abnormalities on brain MRI. The severity of ID was determined by IQ scores (where available) or information on the level of functioning in accordance with the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.12 An epilepsy syndrome diagnosis was made where possible.13
Data were analyzed in MATLAB R2022a (MathWorks, Natick, MA) with descriptive analyses for clinical characteristics. Comparison of categorical data among groups was performed using a χ2 test to determine the level of significance (p < 0.05).
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the Austin Health Human Research Ethics Committee. Written informed consent was obtained for research participation and video recording of the MD from all patients or their parents or legal guardians in the case of minors or adults with ID.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
We identified 77 patients (41 female, 53%) with a genetic DEE and a co-existing MD. They had a median age of 9 years (range 1–38 years); 3 (4%) patients were deceased at ages 3, 3, and 12 years, respectively.
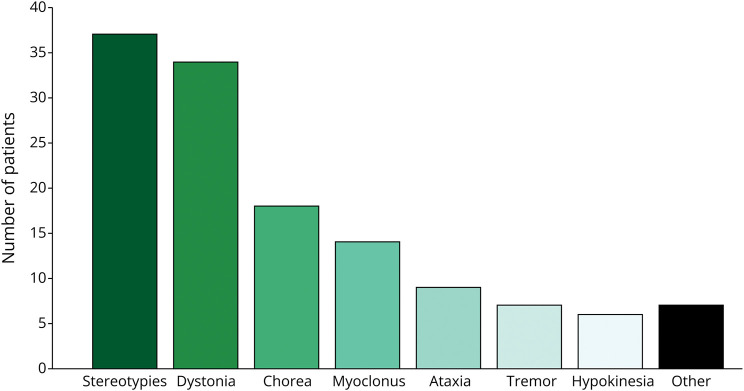
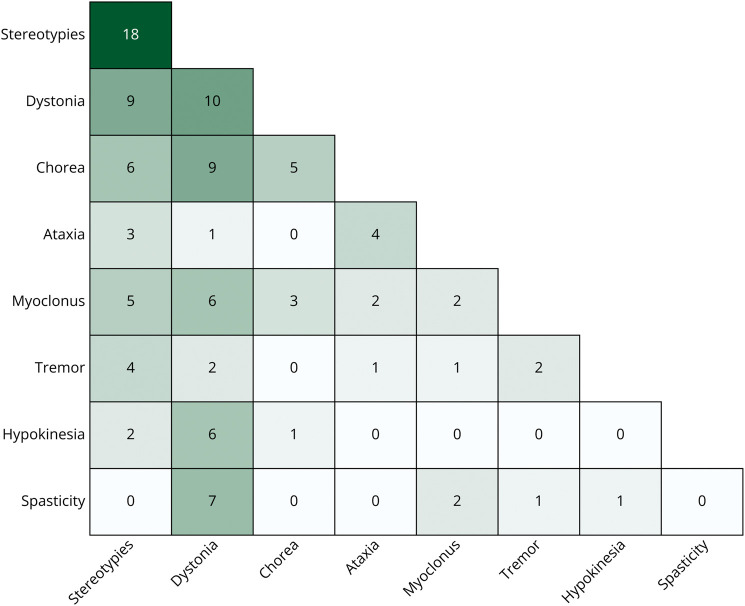
The most common MDs were stereotypies occurring in 37 (48%) and dystonia in 34 (44%) patients, followed by chorea in 18 (23%), myoclonus in 14 (18%), ataxia in 9 (12%), tremor in 7 (9%), and hypokinesia in 6 (8%) patients (Figure 1). In patients with chorea, the movements were predominantly choreoathetosis in 11 and ballistic in 7 patients. In 41 (53%) patients, a single MD was observed, with stereotypies being most common (18 patients) (Figure 2). In the remaining 36 (47%) patients, more than 1 MD was observed of which chorea with dystonia, hypokinetic rigidity syndrome with dystonia, and dystonia with spasticity were most frequent. While stereotypies were the most common finding, they co-occurred at times with all the other MDs apart from spasticity (Figure 2), without a distinctive association emerging. The MDs were paroxysmal in 10/71 (14%), with dystonia (8/10), ataxia (1/10), and tremor (1/10), while showing chronic persistence in the remainder of patients.
Figure 1. Frequency of Movement Disorders in a Cohort of 77 Patients With Genetic DEEs.
The “other” box refers to movement disorders such as dyskinetic exacerbations (n = 3), dyskinetic eye blinking (n = 2), orofacial dyskinesias (n = 1), and an exaggerated startle reflex (n = 1). DEE = developmental and epileptic encephalopathy.
Figure 2. Co-occurrence of Different Types of Movement Disorders and Spasticity in 77 Patients With Genetic DEEs.
DEE = developmental and epileptic encephalopathy.
The age at onset of the MD was known in 67/77 patients and began at a median age of 18 months (range day 2–35 years). In the patients who only had a single MD, the age at onset for stereotypies was 21 months (range 6 months–14 years), dystonia 14.5 months (range 6 months–13 years), and chorea 12 months (range 4 months–13 years).
The median age at onset of epileptic seizures in our cohort was 4 months (range day 1–11 years). The most common epileptic seizure types were epileptic spasms (36/75), tonic seizures (34/75), myoclonic seizures (30/75), and tonic-clonic seizures (30/75). Thirty-seven of 77 patients had an early infantile DEE with onset by 3 months of age,13 while 30/77 had an infantile-onset DEE by age 2 years, with 10/77 having later onset. Epilepsy syndrome diagnoses in 25/77 (32%) patients included Lennox-Gastaut syndrome (8/77), Dravet syndrome (5/77), infantile epileptic spasms syndrome (4/77), epilepsy in infancy with migrating focal seizures (4/77), epilepsy with myoclonic-atonic seizures (3/77), and progressive myoclonus epilepsy (1/77).
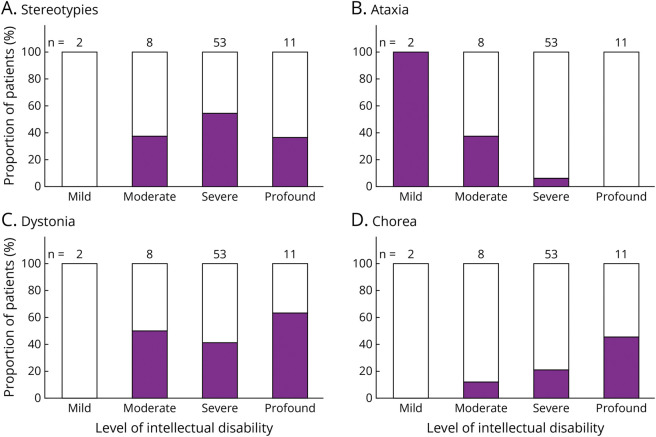
ID was severe in 53/74 (72%) patients, profound in 11/74 (15%), with a smaller proportion having moderate (8/74) or mild (2/74) ID. In 3 patients, the severity of their ID was not available. The correlation between type of MD and severity of ID was examined and found that ataxia was less frequently associated with severe ID (χ2 = 24.96, p < 0.0001, Figure 3). By contrast, stereotypies and dystonia were observed evenly across different severity levels of ID.
Figure 3. Proportion of 77 Patients With Genetic DEEs With the 4 Most Common Movement Disorders According to Severity of Intellectual Disability.
DEE = developmental and epileptic encephalopathy.
Fifty-three of 77 (70%) patients were nonambulant. The inability to walk was more likely in patients with dystonia (30/34, χ2 = 9.98, p = 0.002) while, understandably, ataxia was much less likely to be observed in nonambulatory individuals (2/9, χ2 = 10.92, p ≤ 0.001). Chorea, myoclonus, and stereotypies were not associated with severity of motor impairment.
Fewer than half (34/74, 46%) of the patients were treated with medication for their MD. In general, drugs were initiated for dystonia, chorea, and myoclonus, but not for stereotypies, tremor, and hypokinesia. They received trihexyphenidyl (15/34), tetrabenazine (12/34), clonazepam (7/34), clonidine (6/34), baclofen (6/34), gabapentin (4/34), levodopa (3/34), botulinum toxin (3/34), tizanidine (1/34), and propranolol (1/34). Limited information on drug responsiveness was available in 16/34 patients. Levodopa was ineffective for dystonia in 3/3 patients. Tetrabenazine improved dystonia and chorea in patients with pathogenic variants in GNAO1 and UGDH, but was ineffective in 3 patients with different sodium channel DEEs (SCN1A, SCN2A and SCN8A). Paroxysmal dystonia was treated in 3/10 patients; 2 patients showed mild improvement on treatment with tetrabenazine and combination therapy (baclofen, clonazepam, and lorazepam) in another. Invasive therapies were performed in 2 patients, both with symptom improvement: deep brain stimulation (DBS) of the bilateral globus pallidus internus in a patient with GNAO1-DEE causing chorea and dystonia14 and an intrathecal baclofen pump in a patient with an SCN2A early infantile DEE causing dystonia.
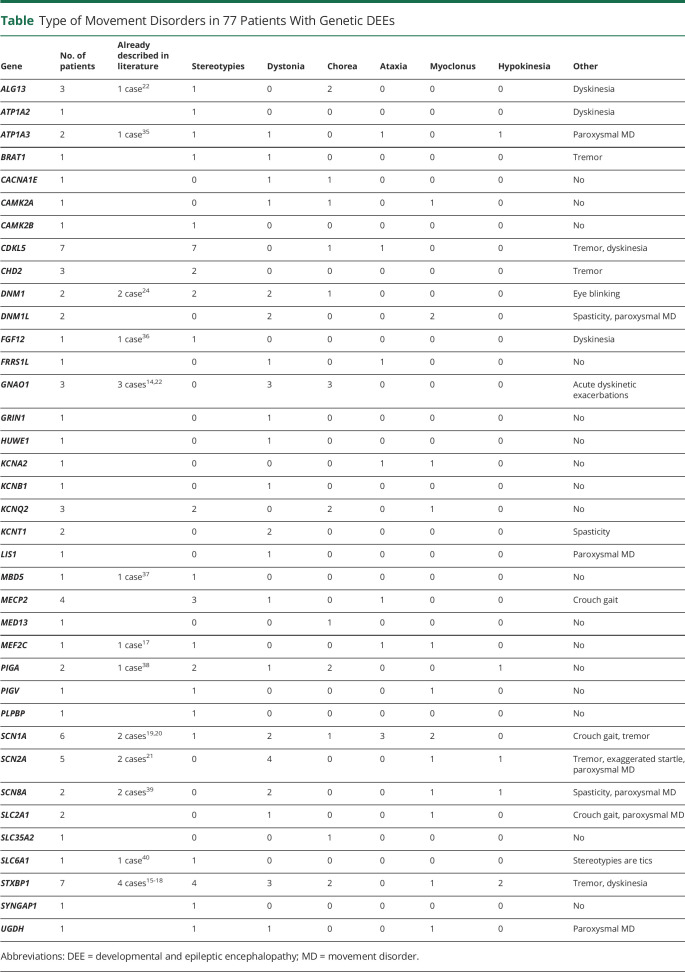
Our patient cohort had 38 different genetic diseases, with STXBP115-18 and CDKL5 most frequently seen (7 patients each), followed by SCN1A (6/77),19,20 SCN2A (5/77),21 MECP2 (4/77), KCNQ2 (3/77), GNAO1 (3/77),14,22 CHD2 (3/77), and ALG13 (3/77).22 The remaining 29 genes were identified in 2 patients (7/38) or single (22/38) cases. All 7 patients with CDKL5-DEE had stereotypies, and 3/3 patients with GNAO1-DEE had chorea and acute dyskinetic exacerbations. We looked for consistent MD patterns in 3 or more cases with a specific genetic disease (Table). We found that 4/5 patients with SCN2A-DEE had dystonia, 3/6 with SCN1A-DEE had ataxia, 4/7 with STXBP1-DEE had stereotypies, and 3/7 had dystonia.
Table.
Type of Movement Disorders in 77 Patients With Genetic DEEs
| Gene | No. of patients | Already described in literature | Stereotypies | Dystonia | Chorea | Ataxia | Myoclonus | Hypokinesia | Other |
| ALG13 | 3 | 1 case22 | 1 | 0 | 2 | 0 | 0 | 0 | Dyskinesia |
| ATP1A2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | Dyskinesia | |
| ATP1A3 | 2 | 1 case35 | 1 | 1 | 0 | 1 | 0 | 1 | Paroxysmal MD |
| BRAT1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | Tremor | |
| CACNA1E | 1 | 0 | 1 | 1 | 0 | 0 | 0 | No | |
| CAMK2A | 1 | 0 | 1 | 1 | 0 | 1 | 0 | No | |
| CAMK2B | 1 | 1 | 0 | 0 | 0 | 0 | 0 | No | |
| CDKL5 | 7 | 7 | 0 | 1 | 1 | 0 | 0 | Tremor, dyskinesia | |
| CHD2 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | Tremor | |
| DNM1 | 2 | 2 case24 | 2 | 2 | 1 | 0 | 0 | 0 | Eye blinking |
| DNM1L | 2 | 0 | 2 | 0 | 0 | 2 | 0 | Spasticity, paroxysmal MD | |
| FGF12 | 1 | 1 case36 | 1 | 0 | 0 | 0 | 0 | 0 | Dyskinesia |
| FRRS1L | 1 | 0 | 1 | 0 | 1 | 0 | 0 | No | |
| GNAO1 | 3 | 3 cases14,22 | 0 | 3 | 3 | 0 | 0 | 0 | Acute dyskinetic exacerbations |
| GRIN1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | No | |
| HUWE1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | No | |
| KCNA2 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | No | |
| KCNB1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | No | |
| KCNQ2 | 3 | 2 | 0 | 2 | 0 | 1 | 0 | No | |
| KCNT1 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | Spasticity | |
| LIS1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | Paroxysmal MD | |
| MBD5 | 1 | 1 case37 | 1 | 0 | 0 | 0 | 0 | 0 | No |
| MECP2 | 4 | 3 | 1 | 0 | 1 | 0 | 0 | Crouch gait | |
| MED13 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | No | |
| MEF2C | 1 | 1 case17 | 1 | 0 | 0 | 1 | 1 | 0 | No |
| PIGA | 2 | 1 case38 | 2 | 1 | 2 | 0 | 0 | 1 | No |
| PIGV | 1 | 1 | 0 | 0 | 0 | 1 | 0 | No | |
| PLPBP | 1 | 1 | 0 | 0 | 0 | 0 | 0 | No | |
| SCN1A | 6 | 2 cases19,20 | 1 | 2 | 1 | 3 | 2 | 0 | Crouch gait, tremor |
| SCN2A | 5 | 2 cases21 | 0 | 4 | 0 | 0 | 1 | 1 | Tremor, exaggerated startle, paroxysmal MD |
| SCN8A | 2 | 2 cases39 | 0 | 2 | 0 | 0 | 1 | 1 | Spasticity, paroxysmal MD |
| SLC2A1 | 2 | 0 | 1 | 0 | 0 | 1 | 0 | Crouch gait, paroxysmal MD | |
| SLC35A2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | No | |
| SLC6A1 | 1 | 1 case40 | 1 | 0 | 0 | 0 | 0 | 0 | Stereotypies are tics |
| STXBP1 | 7 | 4 cases15-18 | 4 | 3 | 2 | 0 | 1 | 2 | Tremor, dyskinesia |
| SYNGAP1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | No | |
| UGDH | 1 | 1 | 1 | 0 | 0 | 1 | 0 | Paroxysmal MD |
Abbreviations: DEE = developmental and epileptic encephalopathy; MD = movement disorder.
Discussion
The presence of MDs is often not appreciated in patients with DEEs because they can be subtle with the major clinical focus being on drug-resistant seizures.1 In this study, our international collaborative effort ascertained 77 patients with a genetic DEE who had a concomitant MD. Stereotypies and dystonia were most common, followed by chorea, myoclonus, ataxia, tremor, and hypokinesia. MDs can be observed from as early as the second day of life or as late as the fourth decade and are associated with a wide range of genetic diseases. Approximately half of our patients required treatment for their MDs, especially those with dystonia or chorea, with limited effect.
MDs are classically divided into paroxysmal, where they occur episodically and may be triggered by a specific task, such as initiation of movement or exercise, or constant, which may still be intermittent in nature, as seen with stereotypies or dystonia. In both instances, a differential diagnosis of an epileptic seizure may need to be considered. For example, all 3 patients with GNAO1-DEE had paroxysmal dyskinetic exacerbations that need to be distinguished from motor seizures.23 In patients with hyperkinetic MDs, such as DNM1-DEE,24 distinction from myoclonic seizures can be challenging; whereas stereotypies are frequent in CDKL5-DEE and may be hard to distinguish from focal motor seizures.25 With the widespread availability of smart phones, multiple videos of an event of concern can be illuminating. Several videos of an event allow the clinician to take into account the context of the abnormal movements, including any potential triggers, and whether the episodes are stereotyped. Where distinction is challenging, video-EEG monitoring, preferably with EMG recording, can make a definitive diagnosis and guide management.
Dystonia occurred in isolation or in combination with chorea, myoclonus, a hypokinetic rigidity syndrome, or spasticity. Of interest, the 4/34 patients who were ambulatory did not receive therapy for dystonia, whereas, 19/30 nonambulatory patients had a trial of medical or surgical therapy. A wide range of pharmacologic drugs was trialed for dystonia, including trihexyphenidyl, tetrabenazine, baclofen, gabapentin, levodopa, and botulinum toxin. Two of our patients received invasive therapies with improvement of symptoms: DBS of the bilateral globus pallidus internus for a boy with GNAO1-DEE and an intrathecal baclofen pump for a girl with SCN2A early infantile DEE. While DBS has been shown to be a safe and effective treatment for dystonia, clinical outcomes vary according to different genetic pathologies.26 DBS is most effective for TOR1A disease, which causes dystonia but not epilepsy; lower efficacy has been reported for genetic diseases due to ATP1A3 and ADCY5 pathogenic variants.27 In GNAO1-related dyskinesias, DBS has been shown to be effective, especially for preventing severe hyperkinetic exacerbations and status dystonicus; however, the long-term benefit on the baseline MD requires further study.28 Thus, understanding the genetic etiology is crucial when considering whether DBS would be suitable for a child with a DEE and dystonia, although large cohorts of each genetic DEE are needed to draw definitive conclusions. Intrathecal baclofen is well established for severe secondary generalized dystonia but has rarely been reported in pediatric neurodegenerative diseases.29,30 The marked clinical benefit of intrathecal baclofen for extreme distress associated with dystonia in our patient with SCN2A early infantile DEE led to significant improvement in quality of life for the patient and her family because it alleviated her extremely severe irritability through the first 18 months of life.
Ataxia occurred more frequently in ambulatory patients with mild to moderate ID. Because dysmetria or intention tremor require goal-directed movements, it may not be possible to identify these features in patients with more severe impairment. Similarly, dysarthria is difficult to determine in patients who only speak a few words or are nonverbal. Therefore, detection of cerebellar features is much more likely in those with milder disability and may be present, but not assessable, in severely impaired individuals.
Differentiation between types of MDs can be challenging, especially in complex neurologic disorders with features of spasticity, rigidity, hypotonia, or cognitive impairment.31 The blanket terms “hyperkinetic” and “dyskinetic” are often applied to several types of MDs. In this study, we distill MDs into MD phenotypes of stereotypies, dystonia, chorea, myoclonus, and tremors.11,31
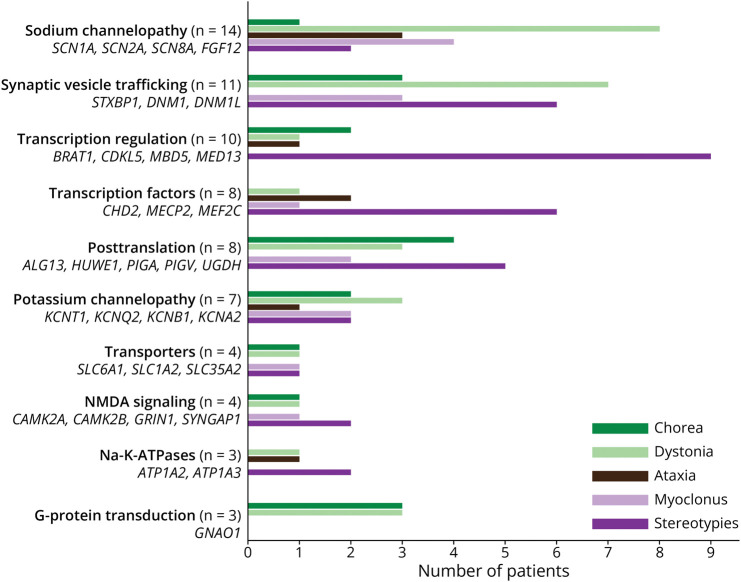
A broad range of neurobiological mechanisms has been implicated in the DEEs associated with MDs.32-34 These include channelopathies, synaptic defects, transcriptional dysregulation, impairment in posttranslational modifications, transporter and signaling dysfunction, and metabolic disorders.33 We found that patients with sodium (SCN1A, SCN2A, and SCN8A), potassium (KCNQ2), and calcium (CACNA1E) channelopathies were more likely to experience dystonia, as were patients with synaptic vesicle trafficking disorders (STXBP1, DNM1, and DNM1L) (Figure 4). While stereotypies were also frequently seen with synaptic vesicle trafficking disorders (STXBP1, DNM1, and DNM1L), stereotypies were the most frequent MD in our cohort with transcriptional defects (CHD2, MECP2, MEF2C, BRAT1, CDKL5, and MBD5). More mixed pictures were observed in patients with posttranslational defects and transporter and signaling disorders.
Figure 4. Patterns of the 5 Most Common Movement Disorders Grouped According to the Biological Mechanisms Causing Genetic DEEs (in 3 or More Individuals).
DEE = developmental and epileptic encephalopathy.
The cross-sectional nature of this study prevents us from analyzing the evolution of MDs over time in individual patients. Furthermore, distinctive patterns for specific genetic diseases did not emerge because the phenotype varied between patients and each genetic cohort was relatively small. A prospective natural history study of specific genetic DEEs may identify consistent patterns over time, together with determining the efficacy of therapeutic approaches.
Recognition of MDs is critical in managing patients with complex neurologic conditions because seizures need targeted management that differs from treatment of MDs. MDs may severely affect quality of life. We analyzed the types of MDs in individuals with genetic DEEs to identify patterns associated with different biological mechanisms. Recognition of DEE-MD patterns will aid in identification of specific genetic etiologies. With the promise of precision medicine for genetic DEEs, MDs will need to be addressed in concert with drug-resistant seizures and developmental impairment.
Acknowledgment
The authors thank the patients and their families for participating in our research program.
Glossary
- DBS
deep brain stimulation
- DEE
developmental and epileptic encephalopathy
- MD
movement disorder
Appendix. Authors
| Name | Location | Contribution |
| Sterre van der Veen, MD | University Medical Center Groningen, the Netherlands | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
| Gabrielle T.W. Tse, MD | Austin Health, Melbourne, Australia | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Alessandro Ferretti, MD | Bambino Gesù Children's Hospital, Rome, Italy | Major role in the acquisition of data |
| Giacomo Garone, MD | Bambino Gesù Children's Hospital, Tor Vergata University, Rome, Italy | Major role in the acquisition of data |
| Bart Post, MD, PhD | Radboud UMC, Nijmegen, the Netherlands | Major role in the acquisition of data |
| Nicola Specchio, MD, PhD | Ospedale Pediatrico Bambino Gesù, Rome, Italy | Major role in the acquisition of data |
| Victor S.C. Fung, MBBS, PhD | Westmead Hospital, Australia | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and study concept or design |
| Marina Trivisano, MD, PhD | Bambino Gesù Children's Hospital, Rome, Italy | Major role in the acquisition of data |
| Ingrid E. Scheffer, MBBS, PhD | University of Melbourne, Australia | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
Footnotes
Editorial, page 815
Study Funding
No targeted funding reported.
Disclosure
S. van der Veen received funding from “Stichting Beatrixoord Noord-Nederland.” N. Specchio has served on scientific advisory boards for GW Pharma, BioMarin, Arvelle, Marinus, and Takeda, has received speaker honoraria from Eisai, Biomarin, Livanova, and Sanofi, and has served as an investigator for Zogenix, Marinus, Biomarin, UCB, and Roche. V.S.C. Fung receives a salary from NSW Health, has received unrestricted research grants from the Michael J. Fox Foundation, Abbvie, and Merz, has been on Advisory Boards for Abbvie, Allergan, Ipsen, Merz, Praxis, Seqirus, Stada, Teva, and UCB, and receives royalties from Health Press Ltd. I.E. Scheffer has served on scientific advisory boards for BioMarin, Chiesi, Eisai, Encoded Therapeutics, Knopp Biosciences, Takeda Pharmaceuticals, UCB, and Xenon Pharmaceuticals, has received speaker honoraria from UCB, BioMarin, Biocodex, Chiesi, Liva Nova, Zuellig Pharma, and Eisai, has received funding for travel from UCB, Biocodex, GlaxoSmithKline, Biomarin, Encoded Therapeutics, and Eisai, has served as an investigator for Anavex Life Sciences, Cerecin Inc, Cerevel Therapeutics, Eisai, Encoded Therapeutics, EpiMinder Inc, Epygenyx, ES-Therapeutics, GW Pharma, Marinus, Neurocrine BioSciences, Ovid Therapeutics, Takeda Pharmaceuticals, UCB, Ultragenyx, Xenon Pharmaceuticals, Zogenix, and Zynerba, has consulted for Care Beyond Diagnosis, Epilepsy Consortium, Atheneum Partners, Ovid Therapeutics, UCB, Zynerba Pharmaceuticals, BioMarin, Encoded Therapeutics and Biohaven Pharmaceuticals, and is a Nonexecutive Director of Bellberry Ltd and a Director of the Australian Academy of Health and Medical Sciences and the Australian Council of Learned Academies Limited. She received support from the Australian Epilepsy Research Foundation grant, Australian National Health and Medical Research Council (NHMRC) Center for Research Excellence Grant (GNT2006841), Synergy Grant (GNT2010562), and Senior Investigator Fellowship (GNT1172897). She may accrue future revenue on pending patent WO61/010176 (filed: 2008): Therapeutic Compound, has a patent for SCN1A testing held by Bionomics Inc and licensed to various diagnostic companies, and has a patent molecular diagnostic/theranostic target for benign familial infantile epilepsy (BFIE) [PRRT2] 2011904493 & 2012900190 and PCT/AU2012/001321 (TECH ID: 2012-009). The remaining authors report no relevant discloses. Go to Neurology.org/N for full disclosures.
References
- 1.Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512-521. doi: 10.1111/epi.13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliver K, Scheffer I, Bennett M, Grinton B, Bahlo M, Berkovic S. Genes4Epilepsy: an epilepsy gene resource. Epilepsia. 2023;64(5):1368-1375. doi: 10.1111/epi.17547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheffer IE, Bennett CA, Gill D, et al. Exome sequencing for patients with developmental and epileptic encephalopathies in clinical practice. Dev Med Child Neurol. 2023;65(1):50-57. doi: 10.1111/dmcn.15308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer EE, Schofield D, Shrestha R, et al. Integrating exome sequencing into a diagnostic pathway for epileptic encephalopathy: evidence of clinical utility and cost effectiveness. Mol Genet Genomic Med. 2018;6(2):186-199. doi: 10.1002/MGG3.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Specchio N, Curatolo P. Developmental and epileptic encephalopathies: what we do and do not know. Brain. 2021;144(1):32-43. doi: 10.1093/brain/awaa371 [DOI] [PubMed] [Google Scholar]
- 6.McTague A, Howell KB, Cross JH, Kurian MA, Scheffer IE. The genetic landscape of the epileptic encephalopathies of infancy and childhood. Lancet Neurol. 2016;15(3):304-316. doi: 10.1016/S1474-4422(15)00250-1 [DOI] [PubMed] [Google Scholar]
- 7.Lee HY, Huang Y, Bruneau N, et al. Mutations in the gene PRRT2 cause paroxysmal kinesigenic dyskinesia with infantile convulsions. Cell Rep. 2012;1(1):2-12. doi: 10.1016/j.celrep.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heron SE, Grinton BE, Kivity S, et al. PRRT2 mutations cause benign familial infantile epilepsy and infantile convulsions with choreoathetosis syndrome. Am J Hum Genet. 2012;90(1):152-160. doi: 10.1016/j.ajhg.2011.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morales-Briceño H, Chang FCF, Wong C, et al. Paroxysmal dyskinesias with drowsiness and thalamic lesions in GABA transaminase deficiency. Neurology. 2019;92(2):94-97. doi: 10.1212/WNL.0000000000006744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly M, Park M, Mihalek I, et al. Spectrum of neurodevelopmental disease associated with the GNAO1 guanosine triphosphate-binding region. Epilepsia. 2019;60(3):406-418. doi: 10.1111/epi.14653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanger TD, Chen D, Fehlings DL, et al. Definition and classification of hyperkinetic movements in childhood. Mov Disord. 2010;25(11):1538-1549. doi: 10.1002/mds.23088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013. [Google Scholar]
- 13.Zuberi SM, Wirrell E, Yozawitz E, et al. ILAE classification and definition of epilepsy syndromes with onset in neonates and infants: position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022;63(6):1349-1397. doi: 10.1111/EPI.17239 [DOI] [PubMed] [Google Scholar]
- 14.Graziola F, Garone G, Grasso M, Capuano A. Cognitive assessment in GNAO1 neurodevelopmental disorder using an eye tracking system. J Clin Med. 2021;10(16):3541. doi: 10.3390/JCM10163541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YO, Korff CM, Villaluz MMG, et al. Head stereotypies in STXBP1 encephalopathy. Dev Med Child Neurol. 2013;55(8):769-772. doi: 10.1111/dmcn.12197 [DOI] [PubMed] [Google Scholar]
- 16.Deprez L, Weckhuysen S, Holmgren P, et al. Clinical spectrum of early-onset epileptic encephalopathies associated with STXBP1 mutations. Neurology. 2010;75(13):1159-1165. doi: 10.1212/WNL.0b013e3181f4d7bf [DOI] [PubMed] [Google Scholar]
- 17.Carvill GL, Heavin SB, Yendle SC, et al. Targeted resequencing in epileptic encephalopathies identifies de novo mutations in CHD2 and SYNGAP1. Nat Genet. 2013;45(7):825-830. doi: 10.1038/NG.2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graziola F, Garone G, Stregapede F, et al. Diagnostic yield of a targeted next-generation sequencing gene panel for pediatric-onset movement disorders: a 3-year cohort study. Front Genet. 2019;10:1026. doi: 10.3389/fgene.2019.01026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadleir LG, Mountier EI, Gill D, et al. Not all SCN1A epileptic encephalopathies are Dravet syndrome. Neurology. 2017;89(10):1035-1042. doi: 10.1212/WNL.0000000000004331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Specchio N, Pietrafusa N, Ferretti A, Trivisano M, Vigevano F. Successful use of fenfluramine in nonconvulsive status epilepticus of Dravet syndrome. Epilepsia. 2020;61(4):831-833. doi: 10.1111/EPI.16474 [DOI] [PubMed] [Google Scholar]
- 21.Howell KB, McMahon JM, Carvill GL, et al. SCN2A encephalopathy: a major cause of epilepsy of infancy with migrating focal seizures. Neurology. 2015;85(11):958-966. doi: 10.1212/WNL.0000000000001926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myers CT, McMahon JM, Schneider AL, et al. De novo mutations in SLC1A2 and CACNA1A are important causes of epileptic encephalopathies. Am J Hum Genet. 2016;99(2):287-298. doi: 10.1016/j.ajhg.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dzinovic I, Škorvánek M, Necpál J, et al. Dystonia as a prominent presenting feature in developmental and epileptic encephalopathies: a case series. Parkinsonism Relat Disord. 2021;90:73-78. doi: 10.1016/j.parkreldis.2021.08.007 [DOI] [PubMed] [Google Scholar]
- 24.von Spiczak S, Helbig KL, Shinde DN, et al. DNM1 encephalopathy: a new disease of vesicle fission. Neurology. 2017;89(4):385-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katherine M. Stereotypic movement disorders. Semin Pediatr Neurol. 2018;25:19-24. doi: 10.1016/j.spen.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 26.Jinnah HA, Alterman R, Klein C, et al. Deep brain stimulation for dystonia: a novel perspective on the value of genetic testing. J Neural Transm. 2017;124(4):417-430. doi: 10.1007/s00702-016-1656-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HJ, Jeon B. Arching deep brain stimulation in dystonia types. J Neural Transm. 2021;128(4):539-547. doi: 10.1007/s00702-021-02304-4 [DOI] [PubMed] [Google Scholar]
- 28.Koy A, Cirak S, Gonzalez V, et al. Deep brain stimulation is effective in pediatric patients with GNAO1 associated severe hyperkinesia. J Neurol Sci. 2018;391:31-39. doi: 10.1016/j.jns.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 29.Tikkanen AU, Buxton K, Ullrich CK, Stone SSD, Nimec DL. The palliative use of intrathecal baclofen in Niemann-Pick disease type C. Pediatrics. 2019;144(5):e20191438. doi: 10.1542/peds.2019-1438 [DOI] [PubMed] [Google Scholar]
- 30.Lake W, Shah H. Intrathecal baclofen infusion for the treatment of movement disorders. Neurosurg Clin N Am. 2019;30(2):203-209. doi: 10.1016/j.nec.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 31.Brandsma R, van Egmond ME, Tijssen MAJ, et al. Diagnostic approach to paediatric movement disorders: a clinical practice guide. Dev Med Child Neurol. 2021;63(3):252-258. doi: 10.1111/dmcn.14721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spagnoli C, Fusco C, Percesepe A, Leuzzi V, Pisani F. Genetic neonatal-onset epilepsies and developmental/epileptic encephalopathies with movement disorders: a systematic review. Int J Mol Sci. 2021;22:4202. doi: 10.3390/ijms22084202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papandreou A, Danti FR, Spaull R, Leuzzi V, Mctague A, Kurian MA. The expanding spectrum of movement disorders in genetic epilepsies. Dev Med Child Neurol. 2020;62(2):178-191. doi: 10.1111/dmcn.14407 [DOI] [PubMed] [Google Scholar]
- 34.Erro R, Bhatia KP, Espay AJ, Striano P. The epileptic and nonepileptic spectrum of paroxysmal dyskinesias: channelopathies, synaptopathies, and transportopathies. Mov Disord. 2017;32(3):310-318. doi: 10.1002/mds.26901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schirinzi T, Garone G, Travaglini L, et al. Phenomenology and clinical course of movement disorder in GNAO1 variants: results from an analytical review. Parkinsonism Relat Disord. 2019;61:19-25. doi: 10.1016/j.parkreldis.2018.11.019 [DOI] [PubMed] [Google Scholar]
- 36.Trivisano M, Ferretti A, Bebin E, et al. Defining the phenotype of FHF1 developmental and epileptic encephalopathy. Epilepsia. 2020;61(7):e71-e78. doi: 10.1111/EPI.16582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myers KA, Marini C, Carvill GL, et al. Phenotypic spectrum of seizure disorders in MBD5-associated neurodevelopmental disorder. Neurol Genet. 2021;7(2):e579. doi: 10.1212/NXG.0000000000000579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bayat A, Kløvgaard M, Johannesen KM, et al. Deciphering the premature mortality in PIGA-CDG: an untold story. Epilepsy Res. 2021;170:106530. doi: 10.1016/J.EPLEPSYRES.2020.106530 [DOI] [PubMed] [Google Scholar]
- 39.Trivisano M, Pavia GC, Ferretti A, Fusco L, Vigevano F, Specchio N. Generalized tonic seizures with autonomic signs are the hallmark of SCN8A developmental and epileptic encephalopathy. Epilepsy Behav. 2019;96:219-223. doi: 10.1016/J.YEBEH.2019.03.043 [DOI] [PubMed] [Google Scholar]
- 40.Carvill GL, McMahon JM, Schneider A, et al. Mutations in the GABA transporter SLC6A1 cause epilepsy with myoclonic-atonic seizures. Am J Hum Genet. 2015;96(5):808-815. doi: 10.1016/j.ajhg.2015.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.