Abstract
Background and Objectives
Spinal muscular atrophy (SMA) is a progressive neuromuscular disorder associated with continuous motor function loss and complications, such as scoliosis and contractures. Understanding the natural history of SMA is key to demonstrating the long-term outcomes of SMA treatments. This study reviews the natural history of motor function, scoliosis, and contractures in patients with SMA.
Methods
Electronic databases were searched from inception to June 27, 2022 (Embase, MEDLINE, and Evidence-Based Medicine Reviews). Observational studies, case-control studies, cross-sectional studies, and case series reporting on motor function (i.e., sitting, standing, and walking ability), scoliosis, and contracture outcomes in patients with types 1–3 SMA were included. Data on study design, baseline characteristics, and treatment outcomes were extracted. Data sets were generated from studies that reported Kaplan-Meier (KM) curves and pooled to generate overall KM curves.
Results
Ninety-three publications were included, of which 68 reported on motor function. Of these, 10 reported KM curves (3 on the probability of sitting in patients with types 2 and 3 SMA and 8 on the probability of walking/ambulation in patients with type 3 SMA). The median time to loss of sitting (95% CI) was 14.5 years (14.1–31.5) for the type 2 SMA sitter population (their maximum ability was independent sitting). The median time to loss of ambulation (95% CI) was 13.4 years (12.5–14.5) for type 3a SMA (disease onset at age younger than 3 years) and 44.2 years (43.0–49.4) for type 3b SMA (disease onset at age 3 years or older). Studies including scoliosis and contracture outcomes mostly reported non–time-to-event data.
Discussion
The results demonstrate that a high degree of motor function loss is inevitable, affecting patients of all ages. In addition, data suggest that untreated patients with types 2 and 3 SMA remain at risk of losing motor milestones during late adulthood, and patients with types 3a and 3b SMA are at risk of loss of ambulation over time. These findings support the importance of stabilization of motor function development even at older ages. Natural history data are key for the evaluation of SMA treatments as they contextualize the assessment of long-term outcomes.
Introduction
Spinal muscular atrophy (SMA) is an autosomal recessive, progressive neuromuscular disease and the leading genetic cause of infant mortality in the absence of treatment, with an estimated incidence of 1 in 10,000 live births.1 SMA is characterized by a deficiency in the survival of motor neuron (SMN) protein leading to progressive muscle denervation, skeletal muscular atrophy, overall weakness, and motor function loss.2 SMA severity varies among patients but is generally inversely correlated with the number of copies of the centromeric form of the SMN encoding gene, SMN2, although this is not absolute due to additional genetic and epigenetic disease modifiers.2,3
Patients with SMA are typically classified into types 0–4 based on the age at symptom onset, clinical severity, and maximum motor function achievement.4 Type 0 is the most severe form of SMA, resulting in fetal or neonatal death, whereas type 4 manifests during adulthood as mild muscle weakness.2,5 Type 1 is the most common SMA type, accounting for approximately 50%–80% of patients.2,6 Type 1 manifests at age 0–6 months2 and is characterized by symmetrical skeletal weakness, profound hypotonia, and respiratory deficiency; patients are generally unable to sit or walk, and death typically occurs by age 2 years if left untreated.2,5 Type 2 is generally diagnosed at age 7–18 months, characterized by muscle weakness in which patients can sit but typically not walk and associated with a reduced life expectancy when untreated.2,5 Type 3 manifests after age 18 months. Life expectancy is not affected, and patients are typically ambulatory, although the loss of ambulation is common if untreated.2,5 Complications, including scoliosis and joint contractures, are frequent in types 1–3 SMA; the occurrence of scoliosis has been associated with mobility difficulties, with patients who are unable to sit independently experiencing the highest rates.7 Frequency of contractures increases with SMA severity and in joints that are important for mobility, such as knees, hips, and elbows.7 Recent recommendations suggest that a reclassification of patients with SMA would be appropriate to acknowledge the disease spectrum and current clinical status, including mobility, particularly with the advent of disease-modifying treatments (DMTs).7
Since 2016, 3 DMTs with different treatment mechanisms to increase SMN protein levels have been introduced, leading to both short-term and long-term improvements in patients with SMA.8 Significantly improved lifespan and quality of life, greater achievement of motor milestones, reduced reliance on healthcare resources, and decreased symptom progression have been reported in patients treated with DMTs compared with untreated patients.9,10 The impact of DMTs on long-term significant life events, such as the occurrence of severe scoliosis or loss of ambulation, is still unknown. Because the adoption of DMTs becomes more widespread, SMA natural history data will become more difficult to obtain. However, these data remain critical to understand and effectively demonstrate long-term treatment outcomes and to evaluate the physical, social, and economic burden of the disease for patients and caregivers.11,12
The aim of this systematic literature review (SLR) was to identify studies reporting on motor function development, scoliosis, and contractures in the natural history of patients with types 1–3 SMA. In addition, pooled data sets were generated for key time-to-event data to inform the occurrence of disease-related events (e.g., motor milestone gains and losses, scoliosis, and contractures).
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
This SLR was reported according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses for Protocols 2020 guidelines.13 The predefined protocol was not registered. Ethical approval was deemed not necessary as data were analyzed from published studies in which informed consent was obtained by primary investigators.
Search Strategy and Selection Criteria
The search was performed on May 29, 2021, and updated on June 27, 2022. The following electronic databases were accessed using the Ovid platform: Embase, MEDLINE (including MEDLINE Epub ahead of print, MEDLINE in-process & other nonindexed citations, and MEDLINE daily), and Evidence-Based Medicine Reviews (incorporating: the Health Technology Assessment Database, National Health Service Economic Evaluation Database, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects, and the Cochrane Database of Systematic Reviews). Full search strategies are presented in eTables 1 and 2 (links.lww.com/WNL/D158). The results were screened based on their title and abstract, and the full texts of potentially relevant citations were reviewed. Additional hand searches of conference proceedings from 2018 to 2022, reference lists of included publications, and searches of health technology assessment body websites were conducted (eTable 3).
Eligibility criteria were based on the population, interventions, comparators, and outcomes framework14 to identify relevant data (eTable 4, links.lww.com/WNL/D158). Observational studies, case-control studies, cross-sectional studies, and case series reporting on motor function (i.e., sitting, standing, and walking ability), scoliosis, and contracture outcomes in patients with types 1–3 SMA were included.
Data Extraction
Data on study design, baseline characteristics, and outcomes were extracted into summary tables by a reviewer. Data inputs were independently checked against the source document by a second reviewer, and disputes were resolved by consensus.
Assessment of Bias and Quality of Evidence
To assess the degree of bias of eligible studies in the pooled analysis, the appropriate checklist from the Joanna Briggs Institute15 was used.
Data Synthesis and Analysis
Although the search covered several functional outcomes, only studies reporting motor functions, scoliosis, and contractures were considered for data analysis (eTable 4, links.lww.com/WNL/D158). Numerical time-to-event data (i.e., mean or median time-to-event) were extracted and summarized. In addition, studies reporting non–time-to-event data (i.e., rates, mean baseline, follow-up, or change from baseline score) relating to the relevant outcomes were identified and described qualitatively (eTable 4).
Studies reporting time-to-event data as Kaplan-Meier (KM) curves for comparable outcomes were identified. A digitization process was conducted by scanning published KM curves from relevant reviewed studies. GetData Graph Digitizer version 2.26.0.20 was used to estimate a set of coordinates from each KM curve. After digitization, recreation of virtual individual patient data (IPD) was performed using the algorithm published by Guyot et al.16 in R statistical software.17 This was completed using the digitized coordinates and the number of patients at risk at the beginning of the follow-up period. A data set was created from each study arm comprising recreated time-to-event data, including a follow-up time variable and event status for all patients. Using the virtual IPD, KM curves were recreated for all study arms and compared with the figures in the publications.
To quality check the digitization process, summary statistics from the virtual IPD and original publication figures were compared, including estimates of median time to no longer sitting and time to loss of ambulation, and other KM percentages at specific time points.
Scenario analyses were conducted to explore the impact of differences in study populations for maximum function, age, and time axis definitions of the KM curves.
Data Availability
Data from this SLR are available within this article and in the Supplement. The search strategy is also available in the Supplement, and the studies excluded based on full-text analysis are available on request.
Results
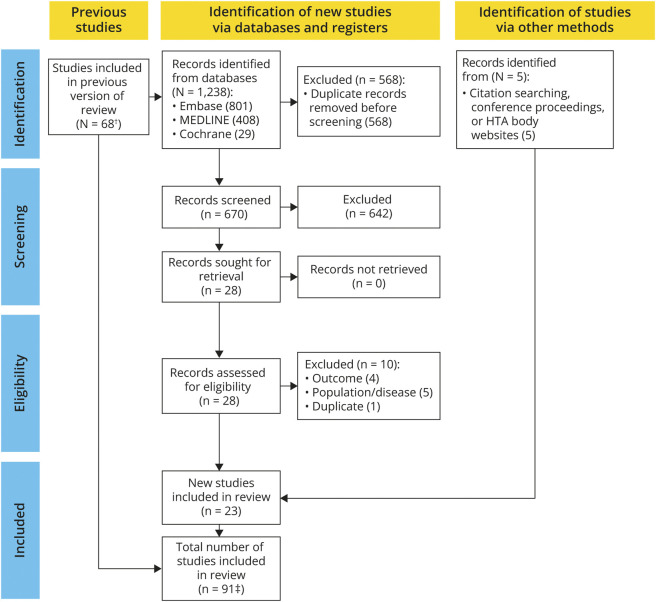
The original electronic database searches conducted in May 2021 identified 6,475 records (eFigure 1, links.lww.com/WNL/D158). After the removal of 1,448 duplicates, 5,027 titles and abstracts were screened and 91 articles were deemed potentially relevant before 26 were excluded based on the population, interventions, comparators, and outcomes framework criteria.14 Three additional records were identified through handsearching. The update search in June 2022 yielded an additional 23 records eligible for inclusion (Figure 1). A total of 91 publications were included in the SLR.
Figure 1. PRISMA Flow Diagram for the Systematic Literature Review (June 2021).
*A PRISMA flow diagram of the original search conducted in May 2021 is provided in eFigure 1 (links.lww.com/WNL/D158). †A total of 16 studies included in the review reported only respiratory and bulbar outcomes and are not considered further in this manuscript. HTA = health technology assessment; PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
The reviewed studies were typically based on national registries with global coverage across Europe, Asia, the United States, and Canada. Their publication date ranged from 1989 to 2022, with 33% of the studies published in 2001 or earlier. Studies included patients with a range of SMA types, including types 0/1–4, types 1–3, type 2 or 3, or type 1 SMA, with a single study reporting outcomes by SMN2 copy number rather than SMA type. Definitions of SMA types, SMA phenotype, patient characteristics, and study designs (e.g., timing of clinical assessments or confirmation of functional milestones) were inconsistent across the reviewed studies, and reporting of patient characteristics was limited. In addition, methods to assess motor milestones varied across the reviewed studies (e.g., patient questionnaires, review of medical records, or functional evaluations). Definitions of motor milestones also varied (e.g., inability to walk 100 m or the need for patients to use a wheelchair for outdoor activities). Loss of sitting and loss of walking/ambulation were not defined beyond the statement of loss of the corresponding ability. Studies reporting KM curves defined the time axis as age or duration of disease (in months or years), while Kaneko et al.18 defined it as the time from sitting independently.
There were 68 studies reporting on motor function gain or loss, of these 10 reported KM curve data, primarily for motor function loss; 11 reported numerical time-to-event data; and 65 reported non–time-to-event data on motor function. For scoliosis, 1 study reported KM curves, 9 reported numerical time-to-event data, and 24 reported non–time-to-event data. No KM curves or numerical time-to-event data were identified for contractures; however, 4 studies reported non–time-to-event data.
The studies reporting KM curves (n = 11) are indicated in eTable 5 (links.lww.com/WNL/D158); in total, these studies included 12 KM curves. The studies reporting numerical time-to-event data (n = 19) and non–time-to-event data (n = 76) are indicated in eTables 6 and 7, respectively. A total of 16 studies reported only respiratory and bulbar outcomes and were not considered further in this article.
Motor Function Outcomes
KM Curve Data
Two studies reported KM curve data for motor function gain. Carson et al.19 reported KM curves for the ability to roll back to front, sit independently, crawl on all fours, walk with assistance, walk independently, and ascend stairs in a specific population of Mennonite and Amish patients (SMA type not specified). Rudnik-Schöneborn et al.20 restricted inclusion of patients to those who had achieved motor function outcomes, with KM curves reaching 100% for the normal sitter and late sitter populations. Owing to differences in the enrolled populations and SMA type reporting, KM curve pooling of these 2 studies was not deemed appropriate.
Eight studies reported KM curve data for multiple motor function loss outcomes.18,21-27 These KM curves (eFigures 2 and 3, links.lww.com/WNL/D158) provided sufficient data to permit the generation of pooled data sets for the outcomes of loss of sitting and loss of ambulation. Owing to likely patient population overlap in 2 studies,26,27 only Zerres et al.27 was included in the pooled analysis. No publications were excluded from pooling based on high risk of bias.
Only 1 study by Wadman et al.21 reported a KM curve for the probability of losing the ability to stand in patients with types 2b, 3a, and 3b SMA; patients with types 2b and 3a showed a loss of standing largely in childhood, and patients with type 3b predominantly maintained the ability to stand during late adulthood.
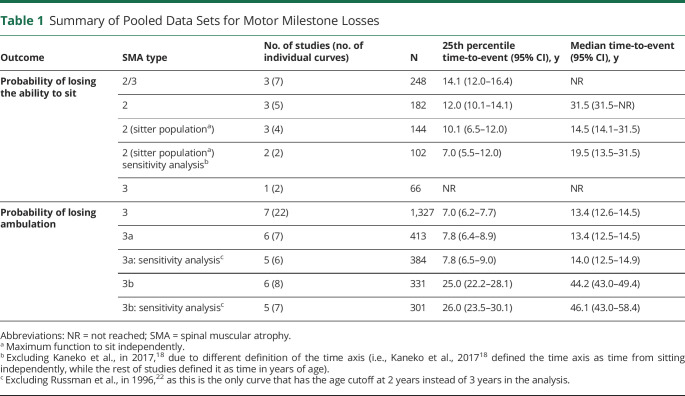
Pooled Data for Median Time to Loss of Sitting: Base-Case Analysis
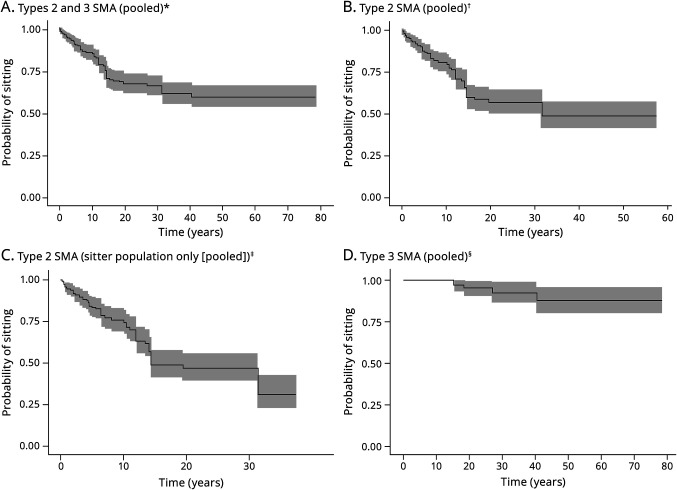
The median time to loss of sitting was evaluable for patients with types 2 and 3 SMA using pooled data from KM curves reported over 7 arms across 3 studies (Table 1 and eFigure 2, A–D, links.lww.com/WNL/D158).18,21,22 The median time to loss of sitting was not reached in the overall population (types 2 and 3 SMA; Figure 2A). In type 2 SMA (n = 182), the median time to loss of sitting was estimated to be 31.5 years (95% CI 31.5—not reached; Figure 2B). In the type 2 SMA sitter population (whose maximum ability was sitting independently, i.e., they were never able to stand or walk; n = 144), the median time to loss of sitting was estimated to be 14.5 years (95% CI 14.1–31.5; Figure 2C). In type 3 SMA (n = 66), the median time to loss of sitting was not reached using pooled data from 2 arms in 1 study21 (Figure 2D). The 25th percentiles for the time to loss of sitting ranged from 10.1 years to not reached across pooling scenarios (Table 1).
Table 1.
Summary of Pooled Data Sets for Motor Milestone Losses
| Outcome | SMA type | No. of studies (no. of individual curves) | N | 25th percentile time-to-event (95% CI), y | Median time-to-event (95% CI), y |
| Probability of losing the ability to sit | 2/3 | 3 (7) | 248 | 14.1 (12.0–16.4) | NR |
| 2 | 3 (5) | 182 | 12.0 (10.1–14.1) | 31.5 (31.5–NR) | |
| 2 (sitter populationa) | 3 (4) | 144 | 10.1 (6.5–12.0) | 14.5 (14.1–31.5) | |
| 2 (sitter populationa) sensitivity analysisb | 2 (2) | 102 | 7.0 (5.5–12.0) | 19.5 (13.5–31.5) | |
| 3 | 1 (2) | 66 | NR | NR | |
| Probability of losing ambulation | 3 | 7 (22) | 1,327 | 7.0 (6.2–7.7) | 13.4 (12.6–14.5) |
| 3a | 6 (7) | 413 | 7.8 (6.4–8.9) | 13.4 (12.5–14.5) | |
| 3a: sensitivity analysisc | 5 (6) | 384 | 7.8 (6.5–9.0) | 14.0 (12.5–14.9) | |
| 3b | 6 (8) | 331 | 25.0 (22.2–28.1) | 44.2 (43.0–49.4) | |
| 3b: sensitivity analysisc | 5 (7) | 301 | 26.0 (23.5–30.1) | 46.1 (43.0–58.4) |
Abbreviations: NR = not reached; SMA = spinal muscular atrophy.
Maximum function to sit independently.
Excluding Kaneko et al., in 2017,18 due to different definition of the time axis (i.e., Kaneko et al., 201718 defined the time axis as time from sitting independently, while the rest of studies defined it as time in years of age).
Excluding Russman et al., in 1996,22 as this is the only curve that has the age cutoff at 2 years instead of 3 years in the analysis.
Figure 2. Recreated Kaplan-Meier Curves for the Probability of Losing the Ability to Sit.
*The pooled curve across all 7 study arms (i.e., Kaneko et al., 2017 [types 2a and 2b], Russman et al., 1996 type 2, and Wadman et al., 2017 [types 2a, 2b, 3a, and 3b]), which is based on a total of 248 patients, is presented (along with the corresponding 95% CI). The follow-up length was variable across study arms, ranging from 1 year (Kaneko et al., 2017 [type 2a SMA]) to 79 years (Wadman et al., 2017 [type 3b]). The median time to no longer sitting in all pooled study arms was not reached. †The pooled curve across all 5 study arms (i.e., Kaneko et al., 2017 [types 2a and 2b], Russman et al., 1996 [type 2], and Wadman et al., 2017 [types 2a and 2b]), which is based on a total of 182 patients, is presented (along with the corresponding 95% CI). The median time to no longer sitting based on pooled study arms was estimated to be 31.5 years (95% CI 31.5–NR). ‡The pooled curve across all 4 study arms (i.e., Kaneko et al., 2017 type 2a, Kaneko et al., 2017 type 2b, Russman et al., 1996 type 2, and Wadman et al., 2017 type 2a), which is based on a total of 144 patients, is presented (along with the corresponding 95% CI). The median time for the sitter population based on the pooled curve across all 4 study arms was estimated to be 14.5 years (95% CI 14.1–31.5). In the sensitivity analysis (which excluded Kaneko et al., 2017 [types 2a and 2b SMA]), the median time to no longer sitting based on the pooled curve across the 2 study arms is estimated to be 19.5 years (95% CI 13.5–31.5). Compared with the base-case analysis, in the sensitivity analysis, the median increased by 5 years; however, the pooled curves seemed very similar both with and without the data from Kaneko et al., 2017 (types 2a and 2b SMA). §The pooled curve across both study arms, which is based on a total of 66 patients, is presented (along with the corresponding 95% CI). The data are immature for both type 3a and type 3b SMA, with no events observed in patients with type 3b SMA, and the median time to no longer sitting in type 3 SMA based on pooled study arms was not reached. NR = not reached; SMA = spinal muscular atrophy.
Pooled Data for Median Time to Loss of Ambulation: Base-Case Analysis
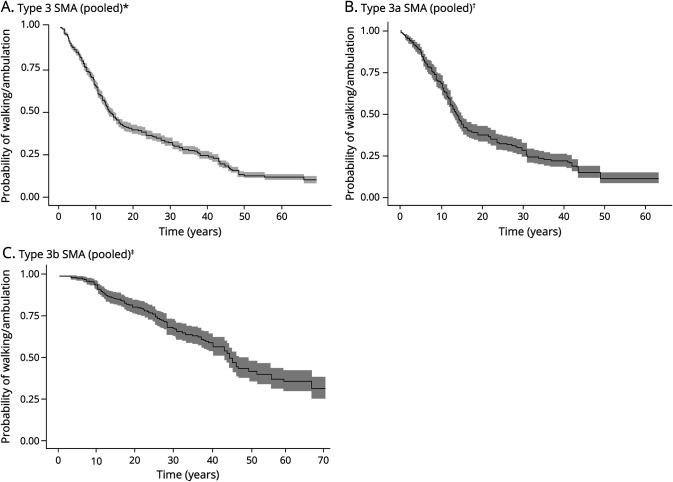
The median time to loss of ambulation was evaluable for patients with type 3 SMA using pooled data from KM curves reported over 22 arms across 7 studies (Table 1 and eFigure 3, A–C, links.lww.com/WNL/D158); in this overall population (n = 1,327), the median time to loss of ambulation was 13.4 years (95% CI 12.6–14.5; Figure 3A). For type 3a SMA (n = 413), the median time to loss of ambulation was 13.4 years (95% CI 12.5–14.5), with 7 arms across 6 unique studies (Figure 3B).18,21,22,24,25,27 For type 3b SMA (n = 331), the median time to loss of ambulation was 44.2 years (95% CI 43.0–49.4), with 8 arms across 6 unique studies (Figure 3C).18,21,22,24,25,27 The 25th percentiles for the probability of loss of ambulation ranged from 7.0–25.0 years across pooling scenarios (Table 1).
Figure 3. Recreated Kaplan-Meier Curves for the Probability of Losing Ambulation.
*The pooled curve for the 7 individual studies (i.e., Bladen et al., 2014, Chung et al., 2004, Kaneko et al., 2017, Lusakowska et al., 2021, Russman et al., 1996, Wadman et al., 2017, and Zerres et al., 1997) is presented (along with the corresponding 95% CI). The pooled curve across all 22 study arms (i.e., Bladen et al., 2014 [Argentina, Germany and Austria, Hungary, Serbia, Switzerland, the United Kingdom, and Ukraine], Chung et al., 2004 [type 3a and type 3b], Kaneko et al., 2017 [type 3a onset younger than 3 years and type 3b onset 3 years or older], Lusakowska et al., 2021 [type 3a 3 copies, type 3a 4 copies, type 3b 3 copies, and type 3b 4 copies], Russman et al., 1996 [type 3b younger than 2 years, type 3b 2 years or older], Wadman et al., 2017 [type 3a, type 3b onset younger than 12 years, and type 3b onset younger than 12 years], and Zerres et al., 1997 [type 3a and type 3b]), which is based on a total of 1,327 patients, is presented. †The pooled curve across all 7 study arms (i.e., Chung et al., 2004 [type 3a] and Kaneko et al., 2017 [type 3a onset younger than 3 years], Lusakowska et al., 2021 [type 3a 3 copies and type 3a 4 copies], Russman et al., 1996 [type 3b onset younger than 2 years], Wadman et al., 2017 [type 3a], and Zerres et al., 1997 [type 3a]), which is based on a total of 413 patients, is presented (along with the corresponding 95% CI). The median time to loss of walking/ambulation based on pooled study arms was estimated to be 13.4 years (95% CI 12.5–14.5). In the sensitivity analysis (which excluded Russman et al., 1996 [type 3b onset younger than 2 years]), the median time to loss of walking/ambulation across pooled study arms is estimated to be 14.0 years (95% CI 12.5–14.9). Compared with the base-case analysis, in the sensitivity analysis, the median increased by 7 months. ‡The pooled curve across all 8 study arms (i.e., Chung et al., 2004 [type 3b], Kaneko et al., 2017 [type 3b onset 3 years or older], Lusakowska et al., 2021 [type 3b 3 copies and type 3b 4 copies], Russman et al., 1996 [type 3b onset 2 years or older], Wadman et al., 2017 [type 3b onset younger than 12 years and type 3b onset older than 12 years], and Zerres et al., 1997 [type 3b]), which is based on a total of 331 patients, is presented (along with the corresponding 95% CI). The median to loss of walking/ambulation based on pooled study arms was estimated to be 44.2 years (95% CI 43.0–49.4). In the sensitivity analysis (which excluded Russman et al., 1996 type 3b 2 years or older), the median time to loss of walking/ambulation across pooled study arms is estimated to be 46.1 years (95% CI 43.0–58.4). Compared with the base-case analysis, the median increased by 1.9 years. SMA = spinal muscular atrophy.
Pooled Data for Motor Function Loss: Sensitivity Analyses
The study by Kaneko et al.18 defined the time axis as time from sitting independently, as opposed to age or duration of disease, as reported across the rest of studies. The sensitivity analysis of the sitter population, which excluded Kaneko et al.,18 increased the median time to loss of sitting by 5 years–19.5 years (95% CI 13.5–31.5) compared with the base-case analysis. However, the pooled curves indicated a similar trend with both the presence and absence of these data (Table 1 and eFigure 2C, links.lww.com/WNL/D158).
For the analysis of ambulation, to separate type 3a SMA from type 3b SMA, sensitivity analyses excluded the study by Russman et al.,22 as this study included a cut-off age at SMA onset of 2 years compared with 3 years in the other studies. This exclusion resulted in a small increase in the median time to loss of ambulation from 13.4 (95% CI 12.5–14.5) to 14.0 years (95% CI 12.5–14.9) in type 3a SMA and from 44.2 (95% CI 43.0–49.4) to 46.1 years (95% CI 43.0–58.4) in type 3b SMA (Table 1).
Visual inspection of the recreated KM data sets identified outliers, most notably in the pooled analysis of the probability of losing ambulation in type 3 SMA. Three KM curves extracted from the study by Bladen et al.23 covering populations in Argentina, Hungary, and Serbia showed variations in the probability of losing ambulation, highlighting regional differences in patient outcomes. The KM curve for type 3b with a disease onset after 12 years of age from Wadman et al.21 was a visual outlier with no function loss observed before 60 years. However, this curve was based on a small population (n = ∼12), and removal of these data had minimal impact on the pooled curve and the median time-to-event (44.2 years [95% CI 43.0–49.4] with Wadman et al.21 included vs 44.2 years [95% CI 39.9–46.0] after exclusion).
Numerical Time-to-Event Data
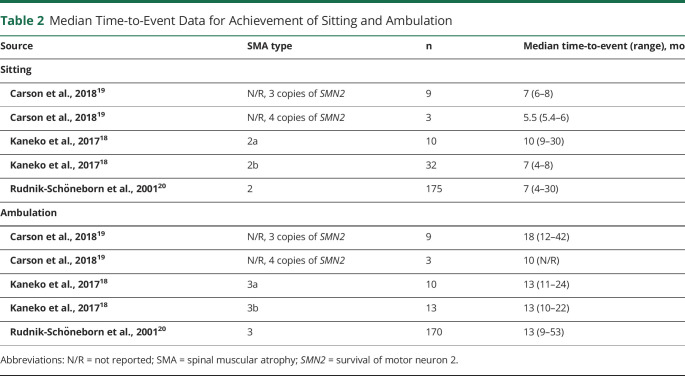
Numerical time-to-event data for motor function gain were reported across 4 studies.18-21 Of those, 3 studies reported median times to sitting in type 2 SMA (5.5–10 months) and median time to ambulation in type 3 SMA (10–18 months) (Table 2).18-20 All median values for time-to-event data on motor function gain were of similar magnitude, with overlapping ranges, suggesting that most patients fit within normal World Health Organization–defined motor milestone acquisitions.28 However, the associated upper ranges of median milestone acquisition indicated that there were a proportion of patients with delayed milestones.
Table 2.
Median Time-to-Event Data for Achievement of Sitting and Ambulation
| Source | SMA type | n | Median time-to-event (range), mo |
| Sitting | |||
| Carson et al., 201819 | N/R, 3 copies of SMN2 | 9 | 7 (6–8) |
| Carson et al., 201819 | N/R, 4 copies of SMN2 | 3 | 5.5 (5.4–6) |
| Kaneko et al., 201718 | 2a | 10 | 10 (9–30) |
| Kaneko et al., 201718 | 2b | 32 | 7 (4–8) |
| Rudnik-Schöneborn et al., 200120 | 2 | 175 | 7 (4–30) |
| Ambulation | |||
| Carson et al., 201819 | N/R, 3 copies of SMN2 | 9 | 18 (12–42) |
| Carson et al., 201819 | N/R, 4 copies of SMN2 | 3 | 10 (N/R) |
| Kaneko et al., 201718 | 3a | 10 | 13 (11–24) |
| Kaneko et al., 201718 | 3b | 13 | 13 (10–22) |
| Rudnik-Schöneborn et al., 200120 | 3 | 170 | 13 (9–53) |
Abbreviations: N/R = not reported; SMA = spinal muscular atrophy; SMN2 = survival of motor neuron 2.
Numerical time-to-event data for motor function loss were reported across 8 studies.18,22-27,29 The median time to loss of sitting in type 2 SMA was reported at age 11 years in type 2a SMA18 and 14 years in type 2b SMA.22 There was notable variability in the mean/median time to loss of ambulation across these studies (range 11.9–522 months) which is likely due to patient population heterogeneity, changes in standard of care over time, territories, and differing definitions of time to loss of ambulation (i.e., age at loss, time since gaining ambulation function, or interval between disease onset and loss of ambulation).18,22-27,29 The percentage of patients remaining ambulatory at 10 years ranged from 58%25 to 73%26 for type 3a and 89%25 to 97%26 for type 3b SMA. The probability of remaining ambulatory after 10 years generally increased with the age at onset.27
Non–Time-to-Event Data
Reporting of non–time-to-event data across 65 studies included motor function scores and/or the proportion of patients achieving, maintaining, or losing motor milestones, with ambulation and sitting most frequently reported. However, the parameters and populations assessed varied across studies.
The proportion of patients achieving the ability to sit independently and ambulation was reported in 10 studies. In patients with type 1 SMA, 1 study reported that 11.8% of patients achieved sitting and none achieved ambulation.30 For type 2 SMA, achievement of sitting and ambulation was reported for 47.0%–98.0% and 0%–2.5% of patients, respectively, across 7 studies.27,30-35 In patients with type 3 SMA, who typically learn to sit and walk in most cases, 42.4%–100% achieved sitting and 30.3%–100% achieved ambulation across 8 studies.25,30-33,35-37 These broad ranges reflected the heterogeneous nature of the available data sets, which included young patients with delayed motor function. The proportion of patients achieving standing was sparsely reported, with 24.4% of 168 patients with type 2 SMA achieving standing in a study by Zerres et al.27 and 50% of 8 patients with type 3 SMA in Kroksmark et al.36 A limited number of studies reported on the proportion of patients losing motor functions.38-40 Owing to the heterogeneity in follow-up duration and population demographics in reporting these values, no trends were identified. Although many of the studies reported motor function scores, these have been reviewed recently for patients with types 2 and 3 SMA by Coratti et al.,41 identifying a trend toward negative changes in motor function scores for untreated pediatric and adult patients.
Scoliosis Outcomes
Wijngaarde et al.42 reported KM-estimated median age at scoliosis surgery for patients with types 1c, 2a, and 2b SMA of 14.6, 9.6, and 13 years, respectively. Owing to the limited data on scoliosis outcomes, pooled analysis was not feasible.
Nine studies reported numerical time-to-event data on the mean or median age at scoliosis surgery in patients aged 7.9–25.1 years, with most patients aged younger than 15 years.21,34,38,40,42-46 No consistent trends were identified for studies reporting data by SMA type, highlighting heterogeneity across data sets.
Non–time-to-event data were reported in 24 studies. Generally, these studies indicated that a more severe SMA phenotype was associated with a higher probability of developing scoliosis and undergoing scoliosis surgery.21,23,34,38,42,43 Wijngaarde et al.42 reported that patients with more severe SMA (types 1c and 2) had an approximately 80% lifetime probability of scoliosis surgery. Ambulatory ability was associated with an older age at scoliosis surgery and reduced risk of surgery42; 71% of patients who lost the ability to walk younger than 10 years, and 22% of patients who remained ambulatory at 10 years were at risk of scoliosis surgery.42 Preservation of ambulation for 1 extra year in these patients reduced the risk of scoliosis surgery by 15%.42 Coratti et al.38 reported that the mean age at scoliosis surgery was higher in ambulatory vs nonambulatory patients with type 3 SMA (mean 16 vs 12 years).
Contractures
Limited non–time-to-event data, predominantly flexion angles and the proportion of patients with contractures, were reported across 4 studies,31,36,37,47 which showed that the proportion of patients with upper limb contractures (fingers, wrist, elbow) and limitations in the range of movement across all contractures was larger in patients with type 2 vs type 3 SMA (eTable 7, links.lww.com/WNL/D158).
Discussion
Natural history data on SMA are essential to fully understand the impact of SMA treatment on long-term outcomes.11 This SLR aimed to identify data on motor function, scoliosis, and contractures associated with SMA and generate pooled data sets for key outcomes. As such, this article summarizes the breadth of natural history evidence related to motor function, scoliosis, and contractures in types 1–3 SMA over patients' lifetimes.
In this study, pooled data sets for time-to-event data showed that a high degree of motor function loss is inevitable over time in untreated patients, especially in the type 2 SMA sitter population, ambulatory patients with type 3 SMA, and across all ages. Analysis of the 25th percentiles from the pooled data sets also highlighted a motor function loss over the early years of patient follow-up. Identified trends were consistent with the known SMA phenotypes, with patients with type 2 SMA able to sit but unlikely to achieve ambulation and patients with type 3 SMA more likely to achieve ambulation. Our study showed that patients with types 2 and 3 SMA continued to be at risk of losing motor milestones during late adulthood and supported the importance of stabilization of motor milestones even at older ages. Despite a perception that patients with type 3 SMA are at lower risk of motor function loss, the identified literature showed that these patients were at risk of losing sitting, standing, and ambulation function over time.
This study highlighted the importance of acknowledging differences in SMA management across countries and the potential effect such differences could have on international studies. This is particularly relevant in the management of mild SMA symptoms where treatment guidelines are less stringent, and interventions depend on the evaluation of the treating physician.48 Before the introduction of DMTs, international guidelines were published in an attempt to help standardize the multidisciplinary care required for patients globally.48 However, access to relevant clinical specialists, scoliosis surgery, and other treatments is not conceivable for some regions.
This analysis was associated with several limitations. The clinical classification of SMA was established in the 1980s, and the gene locus for 5q SMA was identified in the 1990s. Although many of the studies did not explicitly confirm the 5q SMA genotype, only 2 were published before 1995, both reporting scoliosis outcomes.40,49 Therefore, the data included in the current review can be confidently related to 5q SMA in most cases. Some reviewed studies partly included data collected after the introduction of DMTs in December 2016 (eTables 5 and 6, links.lww.com/WNL/D158).25,34,38,45 These studies may partially represent the natural history of selected groups of patients who were not eligible to receive DMTs, which may have biased their results compared with earlier studies, and this may consequently be reflected in this SLR.
Several assumptions were required when creating the virtual IPD; studies used different time units (for age), and all IPD were recreated in years, the sample sizes were estimated for the relevant subgroups of patients in 2 studies, and only the total number of patients was reported rather than the number of patients at risk at specified time points. Owing to the nature of the reviewed studies, there was a risk of potential overlap in study populations, which may have led to some patients being included multiple times in the pooled analyses. Where potential overlap could be identified, only 1 study was included.
The outcomes of interest for the current work are nonfatal events, and the correct analysis of the data would incorporate the handling of the competing risk of death. However, the KM estimator used in the current analysis is known to inflate the outcome probability in the presence of competing events such as death; using the Aalen-Johansen estimator would have been more appropriate as it accounts for the competing risk of death.50
Limited data were available for the outcomes of interest, particularly for scoliosis and contractures. Notably, scoliosis data were most reported as age at scoliosis surgery. This is unlikely to accurately reflect the timeline of development of scoliosis in all patients, as those who did not undergo surgery due to expense, access, or disease severity were not considered in these data. However, given the paucity of reliable scoliosis data, it may be useful as a proxy for severe scoliosis in some countries. Regional differences in SMA management between countries (e.g., in rates of, and access to, scoliosis surgery) are likely to affect the outcomes of interest.
Despite limiting eligibility of publications to types 1–3 SMA, the included studies were heterogeneous in the definition of SMA phenotype and patient characteristics. Limited reporting of patient characteristics across the studies (e.g., baseline SMN2 copy number [eTable 5, links.lww.com/WNL/D158]), precluded a robust assessment of intertrial heterogeneity of the patient populations. Thus, cross-study differences in the KM estimates of the pooled data set could not be adjusted, and observations were treated as if they came from a single study, likely underestimating the variability of the estimates.
The methods of assessing motor milestones were generally extracted from medical records and questionnaires which may have been subject to observer and recall bias. Comparison across results was challenging due to the variability in reported data, and pooled data sets could only be generated from data on the loss of sitting and loss of ambulation. A coherent summary of non–time-to-event data, particularly for motor function, was not possible due to the inherent heterogeneity of these data.
Differences in study designs, patient populations, data collection methods, and outcome definitions were a key limitation. Finally, inconsistent definitions of SMA types were reported; thus, careful consideration of comparable SMA definitions was ensured before the selection of the KM curves included in the pooled analyses. Differences in severity and motor function between SMA types limited the relevance of pooled data. Further pooled analyses by SMA type were therefore conducted, which provided more meaningful results.
This study presented pooled data sets for time-to-event data on SMA natural history. A strength of this study was the accuracy of the estimation of time-to-outcome achievement for the generation of pooled data sets through virtual IPD, as demonstrated by the similar values of the median times obtained from time-to-event data. In addition, this study highlighted the substantial quantity of evidence related to motor function, especially loss of sitting and loss of ambulation. However, a paucity of natural history data on outcomes such as standing and heterogeneity in reporting limited further conclusions.
This study provided a comprehensive repository of the natural history data of patients with types 1–3 SMA. Pooled data from KM curves of published studies showed that patients with type 2 and type 3 SMA continued to be at risk of losing motor milestones during late adulthood.
Natural history data represent an important decision-making basis for the acceptance of new treatments and allow assessment of long-term treatment outcomes, which are currently relevant with the introduction of new DMTs to markets worldwide. Further research on SMA natural history would benefit from time-to-event data as opposed to non–time-to-event data, which have been most frequently reported in the literature.
Acknowledgment
Medical writing support was provided by Kirsty Alsop, PhD (Mtech Access, Bicester, United Kingdom), who helped to draft the manuscript, and Laura Pérez-Pachón, PhD (Nucleus Global, London, United Kingdom), who helped to draft the manuscript, respond to reviewer comments, and with copyediting and word processing, in accordance with Good Publication Practice (GPP) 2022 guidelines (ismpp.org/gpp-2022).
Glossary
- DMT
disease-modifying treatment
- IPD
individual patient data
- KM
Kaplan-Meier
- SLR
systematic literature review
- SMA
spinal muscular atrophy
- SMN
survival of motor neuron
Appendix. Authors
| Name | Location | Contribution |
| Valerie Aponte Ribero, MSc | F. Hoffmann-La Roche Ltd., Basel, Switzerland | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Yasmina Martí, PhD | F. Hoffmann-La Roche Ltd., Basel, Switzerland | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Sarah Batson, PhD | Mtech Access Limited, Bicester, Oxfordshire, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Stephen Mitchell, PhD | Mtech Access Limited, Bicester, Oxfordshire, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Ksenija Gorni, MD, PhD | F. Hoffmann-La Roche Ltd., Basel, Switzerland | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Nicole Gusset, PhD | SMA Europe, Freiburg, Germany; SMA Schweiz, Heimberg, Switzerland | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Maryam Oskoui, MDCM | Departments of Pediatrics and Neurology Neurosurgery, McGill University, Montreal, Quebec, Canada | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Laurent Servais, MD, PhD | MDUK Oxford Neuromuscular Centre, Department of Paediatrics, University of Oxford, United Kingdom; Division of Child Neurology, Centre de Références des Maladies Neuromusculaires, Department of Pediatrics, University Hospital Liège & University of Liège, Liège, Belgium | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| C. Simone Sutherland, PhD | F. Hoffmann-La Roche Ltd., Basel, Switzerland | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
Study Funding
This study was funded by F. Hoffmann-La Roche Ltd., Basel, Switzerland.
Disclosure
V. Aponte Ribero was an employee of F. Hoffmann-La Roche Ltd. at the time of the study conduct. Y. Martí is employed by F. Hoffmann-La Roche Ltd. and owns stocks in Roche. S. Batson was employed as a consultant to F. Hoffmann-La Roche Ltd. S. Mitchell was employed as a consultant to F. Hoffmann-La Roche Ltd. K. Gorni is employed by F. Hoffmann-La Roche Ltd. and owns stocks in Roche. N. Gusset has conducted consultancy and lectures for Biogen, Novartis, and Roche. M. Oskoui's affiliation has received research support from Biogen, Roche-Genentech, Muscular Dystrophy Canada, and the Canadian Institutes of Health Research. L. Servais has conducted consultancy for Novartis, Roche, Biogen, Scholar Rock, and BioHaven, and he has received grants from Novartis, Roche, and Biogen. C.S. Sutherland was employed by F. Hoffmann-La Roche Ltd. and owns stocks in Roche. Go to Neurology.org/N for full disclosures.
References
- 1.Verhaart IEC, Robertson A, Wilson IJ, et al. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy: a literature review. Orphanet J Rare Dis. 2017;12(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Amico A, Mercuri E, Tiziano FD, Bertini E. Spinal muscular atrophy. Orphanet J Rare Dis. 2011;6:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maretina MA, Zheleznyakova GY, Lanko KM, Egorova AA, Baranov VS, Kiselev AV. Molecular factors involved in spinal muscular atrophy pathways as possible disease-modifying candidates. Curr Genomics. 2018;19(5):339-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wirth B. An update of the mutation spectrum of the survival motor neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA). Hum Mutat. 2000;15(3):228-237. [DOI] [PubMed] [Google Scholar]
- 5.Talbot K, Tizzano EF. The clinical landscape for SMA in a new therapeutic era. Gene Ther. 2017;24:529-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emmady PD, Bodle J. Werdnig Hoffmann disease. In: StatPearls. StatPearls Publishing LLC; 2023. [Google Scholar]
- 7.Gusset N, Stalens C, Stumpe E, et al. Understanding European patient expectations towards current therapeutic development in spinal muscular atrophy. Neuromuscul Disord. 2021;31(5):419-430. [DOI] [PubMed] [Google Scholar]
- 8.Erdos J, Wild C. Mid- and long-term (at least 12 months) follow-up of patients with spinal muscular atrophy (SMA) treated with nusinersen, onasemnogene abeparvovec, risdiplam or combination therapies: a systematic review of real-world study data. Eur J Paediatr Neurol. 2022;39:1-10. [DOI] [PubMed] [Google Scholar]
- 9.Ojala KS, Reedich EJ, DiDonato CJ, Meriney SD. In search of a cure: the development of therapeutics to alter the progression of spinal muscular atrophy. Brain Sci. 2021;11(2):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Zaidy S, Pickard AS, Kotha K, et al. Health outcomes in spinal muscular atrophy type 1 following AVXS-101 gene replacement therapy. Pediatr Pulmonol. 2019;54(2):179-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wijngaarde CA, Stam M, Otto LAM, et al. Population-based analysis of survival in spinal muscular atrophy. Neurology. 2020;94(15):e1634-e1644. [DOI] [PubMed] [Google Scholar]
- 12.Sutherland CS, Hudson P, Mitchell S, Paracha N. Systematic literature review to identify utility values in patients with spinal muscular atrophy (SMA) and their caregivers. Pharmacoeconomics. 2022;40(suppl 1):S39-S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amir-Behghadami M, Janati A. Population, intervention, comparison, outcomes and study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg Med J. 2020;37(6):387. [DOI] [PubMed] [Google Scholar]
- 15.Critical Appraisal Tools. Joanna Briggs Institute. Accessed July 11, 2023. https://jbi.global/critical-appraisal-tools [Google Scholar]
- 16.Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2021. [Google Scholar]
- 18.Kaneko K, Arakawa R, Urano M, Aoki R, Saiito K. Relationships between long-term observations of motor milestones and genotype analysis results in childhood-onset Japanese spinal muscular atrophy patients. Brain Dev. 2017;39(9):763-773. [DOI] [PubMed] [Google Scholar]
- 19.Carson VJ, Puffenberger EG, Bowser LE, et al. Spinal muscular atrophy within Amish and Mennonite populations: ancestral haplotypes and natural history. PLoS One. 2018;13(9):e0202104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudnik-Schöneborn S, Hausmanowa-Petrusewicz I, Borkowska J, Zerres K. The predictive value of achieved motor milestones assessed in 441 patients with infantile spinal muscular atrophy types II and III. Eur Neurol. 2001;45(3):174-181. [DOI] [PubMed] [Google Scholar]
- 21.Wadman RI, Stam M, Gijzen M, et al. Association of motor milestones, SMN2 copy and outcome in spinal muscular atrophy types 0-4. J Neurol Neurosurg Psychiatry. 2017;88(4):365-367. [DOI] [PubMed] [Google Scholar]
- 22.Russman BS, Buncher CR, White M, Samaha FJ, Iannaccone ST. Function changes in spinal muscular atrophy II and III. The DCN/SMA Group. Neurology. 1996;47(4):973-976. [DOI] [PubMed] [Google Scholar]
- 23.Bladen CL, Thompson R, Jackson JM, et al. Mapping the differences in care for 5,000 spinal muscular atrophy patients, a survey of 24 national registries in North America, Australasia and Europe. J Neurol. 2014;261(1):152-163. [DOI] [PubMed] [Google Scholar]
- 24.Chung BH, Wong VC, Ip P. Spinal muscular atrophy: survival pattern and functional status. Pediatrics. 2004;114(5):e548-e553. [DOI] [PubMed] [Google Scholar]
- 25.Lusakowska A, Jedrzejowska M, Kaminska A, et al. Observation of the natural course of type 3 spinal muscular atrophy: data from the polish registry of spinal muscular atrophy. Orphanet J Rare Dis. 2021;16(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zerres K, Rudnik-Schöneborn S. Natural history in proximal spinal muscular atrophy. Clinical analysis of 445 patients and suggestions for a modification of existing classifications. Arch Neurol. 1995;52(5):518-523. [DOI] [PubMed] [Google Scholar]
- 27.Zerres K, Rudnik-Schoneborn S, Forrest E, Lusakowska A, Borkowska J, Hausmanowa-Petrusewicz I. A collaborative study on the natural history of childhood and juvenile onset proximal spinal muscular atrophy (type II and III SMA): 569 patients. J Neurol Sci. 1997;146(1):67-72. [DOI] [PubMed] [Google Scholar]
- 28.WHO Multicentre Growth Reference Study Goup. WHO Motor Development Study: windows of achievement for six gross motor development milestones. Acta Paediatr Suppl. 2006;450:86-95. [DOI] [PubMed] [Google Scholar]
- 29.Souchon F, Simard LR, Lebrun S, Rochette C, Lambert J, Vanasse M. Clinical and genetic study of chronic (types II and III) childhood onset spinal muscular atrophy. Neuromuscul Disord. 1996;6(6):419-424. [DOI] [PubMed] [Google Scholar]
- 30.Belter L, Jarecki J, Reyna SP, et al. The Cure SMA Membership Surveys: highlights of key demographic and clinical characteristics of individuals with spinal muscular atrophy. J Neuromuscul Dis. 2021;8(1):109-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chabanon A, Seferian AM, Daron A, et al. Prospective and longitudinal natural history study of patients with Type 2 and 3 spinal muscular atrophy: baseline data NatHis-SMA study. PLoS One. 2018;13(7):e0201004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coratti G, Carmela Pera M, Montes J, et al. Revised upper limb module in type II and III spinal muscular atrophy: 24-month changes. Neuromuscul Disord. 2022;32(1):36-42. [DOI] [PubMed] [Google Scholar]
- 33.Coratti G, Pera MC, Montes J, et al. Different trajectories in upper limb and gross motor function in spinal muscular atrophy. Muscle Nerve. 2021;64(5):552-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trucco F, Ridout D, Scoto M, et al. Respiratory trajectories in type 2 and 3 spinal muscular atrophy in the iSMAC cohort study. Neurology. 2021;96(4):e587-e599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaufmann P, McDermott MP, Darras BT, et al. Prospective cohort study of spinal muscular atrophy types 2 and 3. Neurology. 2012;79(18):1889-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kroksmark AK, Beckung E, Tulinius M. Muscle strength and motor function in children and adolescents with spinal muscular atrophy II and III. Eur J Paediatr Neurol. 2001;5(5):191-198. [DOI] [PubMed] [Google Scholar]
- 37.Salazar R, Montes J, Dunaway Young S, et al. Quantitative evaluation of lower extremity joint contractures in spinal muscular atrophy: implications for motor function. Pediatr Phys Ther. 2018;30(3):209-215. [DOI] [PubMed] [Google Scholar]
- 38.Coratti G, Messina S, Lucibello S, et al. Clinical variability in spinal muscular atrophy type III. Ann Neurol. 2020;88(6):1109-1117. [DOI] [PubMed] [Google Scholar]
- 39.Ge X, Bai J, Lu Y, Qu Y, Song F. The natural history of infant spinal muscular atrophy in China: a study of 237 patients. J Child Neurol. 2012;27(4):471-477. [DOI] [PubMed] [Google Scholar]
- 40.Granata C, Merlini L, Magni E, Marini ML, Stagni SB. Spinal muscular atrophy: natural history and orthopaedic treatment of scoliosis. Spine (Phila Pa 1976). 1989;14(7):760-762. [DOI] [PubMed] [Google Scholar]
- 41.Coratti G, Cutrona C, Pera MC, et al. Motor function in type 2 and 3 SMA patients treated with Nusinersen: a critical review and meta-analysis. Orphanet J Rare Dis. 2021;16(1):430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wijngaarde CA, Brink RC, de Kort FAS, et al. Natural course of scoliosis and lifetime risk of scoliosis surgery in spinal muscular atrophy. Neurology. 2019;93(2):e149-e158. [DOI] [PubMed] [Google Scholar]
- 43.Alvarez K, Suarez B, Palomino MA, et al. Observations from a nationwide vigilance program in medical care for spinal muscular atrophy patients in Chile. Arq Neuropsiquiatr. 2019;77(7):470-477. [DOI] [PubMed] [Google Scholar]
- 44.Mercuri E, Lucibello S, Pera MC, et al. Long-term progression in type II spinal muscular atrophy: a retrospective observational study. Neurology. 2019;93(13):e1241-e1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wadman RI, De Amicis R, Brusa C, et al. Feeding difficulties in children and adolescents with spinal muscular atrophy type 2. Neuromuscul Disord. 2021;31(2):101-112. [DOI] [PubMed] [Google Scholar]
- 46.Wadman RI, Wijngaarde CA, Stam M, et al. Muscle strength and motor function throughout life in a cross-sectional cohort of 180 patients with spinal muscular atrophy types 1c-4. Eur J Neurol. 2018;25(3):512-518. [DOI] [PubMed] [Google Scholar]
- 47.Johnson N, Paradis A, Dave V, et al. PRO6 Clinical and ambulatory characteristics in adult patients with spinal muscular atrophy: a natural history multi-country chart review study. Value Health. 2021;24:S198. [Google Scholar]
- 48.Wang CH, Finkel RS, Bertini ES, et al. Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol. 2007;22(8):1027-1049. [DOI] [PubMed] [Google Scholar]
- 49.Merlini L, Granata C, Bonfiglioli S, Marini ML, Cervellati S, Savini R. Scoliosis in spinal muscular atrophy: natural history and management. Dev Med Child Neurol. 1989;31(4):501-508. [DOI] [PubMed] [Google Scholar]
- 50.Stegherr R, Schmoor C, Lübbert M, Friede T, Beyersmann J. Estimating and comparing adverse event probabilities in the presence of varying follow-up times and competing events. Pharm Stat. 2021;20(6):1125-1146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from this SLR are available within this article and in the Supplement. The search strategy is also available in the Supplement, and the studies excluded based on full-text analysis are available on request.