Adult oligodendrocyte precursor (or progenitor) cells (OPCs) are the most proliferative cells in the CNS and constitute approximately 10% of cells in the human brain.1,2 Whereas their primary function is to generate mature myelinating oligodendrocytes,2-4 there is increased evidence that OPCs have multiple myelination-independent functions in the nervous system both in health and disease.5 These cells constitute a heterogeneous population6 and have several unique properties (Figure).4,7-10 As proposed in a recent review,5 OPCs may function as sensors of multiple environmental signals, integrate these signals to subsequently influence surrounding non-neural cells, and bidirectionally interact with axons to regulate circuit maturation and remodeling.5One salient feature of OPCs is that they receive direct synaptic input11; express receptors for several neurotransmitters, including glutamate and γ-aminobutyric acid (GABA); and express a wide variety of ion channels.12-14 This allows OPCs to precisely detect neuronal activity that regulates their development and differentiation, which is critical for activity-dependent (adaptive) myelination.1,7-10 Reciprocally, OPCs may communicate with surrounding neurons and other cells by releasing several mediators through exocytosis15 or exosomes.16 OPCs have high metabolic demands,17,18 which make them vulnerable to oxidative damage during hypoxia-ischemia.19,20 They function as sensors of hypoxia and promote angiogenesis21 and respond to signals from astrocytes and microglia to contribute to neuroinflammation and glial scar formation.22-24 Oligodendrocyte precursor cells have the potential for remyelination after injury but may have a limited role in human demyelinating disease.25-27 Inability of proliferating OPCs to differentiate into mature oligodendrocytes leads to myelin loss in neurodegenerative disorders.28 Oligodendrocyte precursors are the primary cell type for development of gliomas.29 Thus, OPCs have a fundamental role both in the normal CNS and in a wide spectrum of pathologic conditions. There are recent reviews on the role of OPCs in health and disease,5,30 and only some salient concepts will be emphasized in this paper.
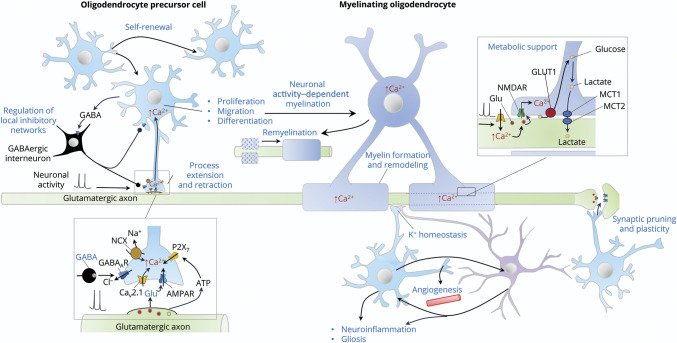
Figure. Functions of Adult Oligodendrocyte Precursor Cells.
Adult oligodendrocyte precursor cells (OPCs) are functionally heterogeneous. They extend motile processes, proliferate, and migrate, and some of them differentiate into myelinating oligodendrocytes. They receive direct synaptic inputs from unmyelinated glutamatergic (Glu) axons and fast-spiking GABAergic interneurons and have a critical role in neuronal activity (adaptive) myelination. Synaptic signals elicit calcium (Ca2+) transients in OPC processes and cell bodies to control OPC development. These transients can by triggered by activation of Ca2+-permeable alpha-amino-3-hydroxy-5-methyl isoxazole propionic acid receptors (AMPARs), voltage-gated channels (such as L-type, Cav2.1 channels), GABAA receptors (GABAARs), and purinergic P2X7 receptors activated by adenosine triphosphate (ATP). In OPCs, GABAAR activation results in depolarization that can promote Ca2+ influx indirectly through voltage-gated sodium (Na+) channels (Nav1.2, not shown) and the Na+/Ca2+ exchanger (NCX). Synaptic and other extracellular signals may promote OPC differentiation to oligodendrocytes for both activity-dependent myelination and remyelination after injury. OPCs may release GABA that inhibits fast-spiking inhibitory neurons; contact nodes of Ranvier, contributing to maintenance of extracellular of potassium (K+) homeostasis; participate in synaptic pruning and plasticity; and promote angiogenesis to support their high metabolic demands. OPCs cooperate and interact with astrocytes and microglia (not shown) in these processes and in mechanisms of neuroinflammation and gliosis. At the axon-myelin interface, N-methyl-D-aspartate receptors (NMDARs) mobilize glucose transporter 1 (GLUT-1), thereby promoting glucose intake by oligodendrocytes and production of lactate (and pyruvate), which is exported to axons through the monocarboxylic acid transporters (MCT)-1 and 2, thus providing an activity-dependent energy supply to axons.
Multiple Functions of Oligodendrocyte Precursor Cells
Development of Oligodendrocyte Precursor Cells
Oligodendrocyte precursor cells are progenitor cells that have the potential to differentiate into either myelinating oligodendrocytes or astrocytes according to environmental cues.31 They originate from the radial glia in the ventricular zone of the ventral forebrain brain and spinal cord and then expand and migrate to populate the gray and white matter of the entire CNS; they are subsequently replaced by OPCs born later in the dorsal cortex.4,32 Oligodendrocyte precursor cells are characterized by the expression of neuron-glia antigen 2 (NG2), a chondroitin sulfate proteoglycan encoded by the CSPG4 gene,33 and depend on platelet-derived growth factor (PDGF) secreted by neurons and astrocytes for their proliferation and migration.4,34-36 Fetal OPCs have a bipolar morphology and migrate along the vasculature37 and, when they encounter astrocytic end feet, detach from blood vessels,38 move into the brain parenchyma, acquire a multiprocessed morphology, and may then either continue to differentiate into mature myelinating oligodendrocytes or remain as adult OPCs.3 Ventrally born OPCs also regulate migration of cortical GABAergic interneurons away from microvessels.39 Adult OPCs self-renew and have a lifelong capacity to generate oligodendrocytes.4,40,41 This process is tightly controlled by intrinsic and extracellular signals, including epigenetic mechanisms and transcription factors that participate in positive feedback loops (reviewed in References 42-46).
Features of Adult Oligodendrocyte Precursor Cells
Adult OPCs extend multiple processes that are highly dynamic, constantly survey their local environment, and locally interact with one another to maintain unique territories though self-repulsion.47 The individual domains of adult OPCs overlap with those of astrocytes,48,49 and within these domains, the 2 cell types form multiple contacts with each other, with the same neuron, and with pericytes, thus forming distinct functional units.49 Oligodendrocyte precursors sense hypoxia through OPC-encoded hypoxia-inducible factor, which both stimulates their proliferation while arresting their maturation and stimulates angiogenesis through paracrine mechanisms.21
Neuron-Glia Synapses and Activity-Dependent Myelination
Neuronal activity regulates many aspects of OPC development and myelination.7,50,51 Adult OPCs are the only glial cells that receive direct synaptic input,11,12,52 express several types of voltage-gated and neurotransmitter-gated channels, sense level of extracellular potassium, and can generate local spikes and calcium (Ca2+) transients that affect their proliferation, migration, and differentiation.53-60 Thus, OPCs have a critical role in activity-dependent (adaptive) myelination, which is critical for circuit development and refinement. Human studies show dynamic, circuit-specific increase in white matter fractional anisotropy (reflecting partly increase in myelination) in response training in a variety of tasks.61,62 With aging, adult OPCs become both regionally and functionally heterogeneous and change their electrophysiologic activity and thus their differentiation potential.63Glutamatergic and GABAergic axons make synapses at discrete sites along processes of OPCs in both the gray and white matter.11,12,52 The transient activation of ionotropic glutamatergic and GABAergic receptors at these axoglial synapses elicits both global and localized Ca2+ transients that allow OPCs to precisely monitor ongoing neuronal activity and integrate synaptic inputs.57,64 The synchronization of Ca2+ transients in neighboring OPCs allows the formation of local networks in which different OPC subsets may respond to neuronal activity with either proliferation or differentiation.65 Unmyelinated glutamatergic axons innervate both their target neurons and neighboring OPCs11,12,58,66-68 and make synapses on OPCs within white matter tracts.69-71 The pattern of glutamatergic activity differentially affects OPC proliferation and differentiation.7,72 Vesicular release of glutamate from unmyelinated axon segments contacting OPC processes promotes myelin sheath formation.73 Studies in vitro indicate that this effect is mediated primarily through Ca2+-permeable alpha-amino-3-hydroxy-5-methyl isoxazole propionic acid receptors (AMPARs).12,64,74-77 These receptors also mediate use-dependent long-term potentiation at excitatory neuron-glia synapses78,79 and may be important for remyelination in response to neuronal activity after injury.80 By contrast, N-methyl-D-aspartate receptors (NMDARs) are present primarily in OPC processes, do not receive glutamatergic synapses, and have a role in myelin maintenance in oligodendrocytes.81,82 Synaptic GABAergic inputs to OPCs primarily originate from fast-spiking interneurons that also target the proximal domains of neighboring neurons83 Local GABAergic circuits affect OPCs through synaptic and extrasynaptic GABAA and GABAB receptors.12,84,85 Activation of synaptic GABAA receptors triggers small depolarization and increase in intracellular Ca2+ of OPCs66,86-88 and may finely tune the capacity of self-maintenance of the OPCs.89 GABAB receptors may promote OPC differentiation and myelination.90 Other signals, such as adenosine triphosphate (ATP), may also trigger intracellular Ca2+ transients in OPCs, either through influx through ion channels or release from intracellular stores.57,91 For example, ATP may be released from vesicles in unmyelinated or premyelinated segments of axons in an activity-dependent manner or in response to injury92 and, both directly or through its byproduct adenosine, may act through different subtypes of purinergic receptors to affect different aspects of OPC development.93 The level of intracellular Ca2+ affects proliferation of subsets of OPCs65 and activates transcription factors necessary for oligodendrocyte differentiation.94 Studies in vitro show that as oligodendrocytes develop and express maturation markers, they exhibit a progressive decrease in voltage-gated sodium and potassium channels and a loss of sodium-dependent spiking activity, concomitant with an increase in inwardly rectifying potassium channel activity and a switch in AMPAR composition.95
Role of Oligodendrocyte Precursor Cells in Synaptic Plasticity
Adult OPCs also contribute to the organization and plasticity of neuronal circuits by mechanisms independent of myelinogenesis. In response to activity-dependent neural signals at axo-glia synapses, OPCs may release several mediators through exocytosis15 or exosomes,16 and these mediators may reciprocally affect surrounding neurons and synapses. For example, OPCs may release GABA,13 which elicits GABAAR-mediated inhibition of local GABAergic interneurons, thus participating in organization of inhibitory microcircuits.83,96 OPCs may also regulate long-term potentiation at glutamatergic synapses through activity-dependent cleavage of the NG2 proteoglycan97 and secretion of several neuromodulatory factors.97,98 Adult OPCs, like microglia and astrocytes, may also shape neuronal networks through pruning synapses and axonal branches and contribute to the refinement of neuronal circuits during cortical development.99,100 These cells may also release several guidance molecules that can regulate axonal growth and synaptogenesis.101,102 For example, studies in the visual system show that OPCs fine-tune neuronal circuits through axonal remodeling.103 Similar to astrocytes, OPCs contact the nodes of Ranvier, where they may help maintaining extracellular K+ homeostasis.83
Energy Demands of Adult Oligodendrocyte Precursor Cells
Adult OPCs are major consumers of glucose in the CNS18 and are characterized by a highly active pentose phosphate pathway.17,18 They also produce acetyl coenzyme A that is metabolized in the tricarboxylic acid cycle.18 Compared with mature oligodendrocytes, adult OPCs consume approximately two-fold more oxygen104 and contain more mitochondria to sustain oxidative phosphorylation and ATP production required for myelin formation.105 This dependence on mitochondrial respiration decreases after myelination is complete.105 To satisfy their high energy demand, adult OPCs directly promote angiogenesis in the setting of hypoxia by producing several proangiogenic factors to secure delivery of energy substrates.21,106
Reciprocal Oligodendrocyte-Axon Interactions
Whereas the expression of synaptic receptors and ion channels becomes downregulated in mature myelinating oligodendrocytes,54 axon-oligodendrocyte communication and localized Ca2+ activity continue to regulate different aspects of adaptive myelination.8,107-109 Oligodendrocytes primarily use lactate both as a metabolic fuel and as a precursor for lipid synthesis and myelin formation.17 In mature oligodendrocytes, both NMDARs and GABABRs may be relevant in activity-dependent myelin growth and maintenance.82,90 Mitochondrial release of Ca2+ at axonal and paranodal regions may also promote myelin remodeling independently of neuronal activity.110 In oligodendrocytes, stimulation of NMDARs mobilizes glucose transporter 1, leading to its incorporation into the myelin compartment; this allows activity-dependent glucose uptake and support of fast-spiking axons by releasing lactate and pyruvate, which are shuttled to the axon through monocarboxylic transporters.111
Involvement of Adult Oligodendrocyte Precursor Cells in Neurologic Diseases
Adult OPCs may contribute to the pathogenesis of neurologic disorders by several mechanisms including cell degeneration and death, loss of function, and acquisition of an aberrant phenotype.30
Hypoxia-Ischemia
The high oxygen demands and expression of Ca2+-permeable channels in OPCs predisposes them to cytoplasmic Ca2+ overload, oxidative stress, and excitotoxicity in the setting of hypoxia-ischemia.19,20 Furthermore, Ca2+ overload at the axon-myelin interface leads to myelin sheath retraction and breakdown, for example, through activation of proteases such as calpain.19,81,112,113 Periventricular leukomalacia in premature neonates114,115 and hypoxic-ischemic injury116 are typical examples of white matter injury due to excitotoxic OPC damage and death. Periventricular leukomalacia reflects selective vulnerability of perinatal late OPCs to oxidative stress.117-119 In response to degeneration of OPCs, there is early and robust proliferation of neighboring OPCs, which fail to differentiate and initiate myelination despite the presence of intact-appearing axons.119 The persistence of these arrested populations of susceptible OPCs renders chronic white matter lesions vulnerable to recurrent hypoxia-ischemia.120 The deep white matter is susceptible to injury in ischemic stroke.116 In addition to excitotoxic damage by glutamate and ATP,112 hypoxia reduces GABAergic signaling through synaptic GABAAR in OPCs, leading to their excessive proliferation and delayed differentiation.87 One potential neuroprotective mechanism of hypothermia in the setting of ischemia is to prevents apoptosis of adult premyelinating OPCs.121
Multiple Sclerosis and Neuroinflammation
Oligodendrocyte precursor cells have a complex role in multiple sclerosis (MS) (reviewed in Reference 25). Adult OPCs seem to have only a limited role in myelin regeneration after demyelinating lesions in humans,27,122 whereas surviving oligodendrocytes upregulate their expression of myelination-related genes and are able to regenerate new myelin sheaths.27,123 The progressive oligodendrocyte depletion over the course of MS may reflect partly their defective proliferation and recruitment to the lesion.25,124-127 Studies in pluripotential stem cell–derived oligodendroglia from patients with MS show that OPCs conserve their ability to interact with axons and generate myelin, indicating that failure of remyelination does not reflect an intrinsic defect of OPCs.128 Some observations indicate that OPCs make new myelin more efficiently than preexisting oligodendrocytes.129 In the setting of demyelination, OPCs respond to cytokines and chemokines released by microglia and other immune cells, which may promote OPC proliferation and differentiation into oligodendrocytes.130,131 Oligodendrocyte precursors also have the potential to transdifferentiate into Schwann cells to assist remyelination.132 However, reactive OPCs can also actively participate in neuroinflammation both directly and through transformation into astrocytes, thereby contributing to impaired remyelination, reactive gliosis, and formation of the glial scar.22,133-136 Reactive astrocytes secrete different combinations of cytokines, extracellular matrix proteins, and growth factors that influence survival, proliferation, and differentiation of adult OPCs.24 Astrocytes derived from human-induced pluripotent stem cells of patients with MS can inhibit OPC differentiation and render OPCs able to adopt an immune profile.23 Demyelination primes OPCs to express genes encoding molecules involved in immune responses,137 including the receptor for interleukin (IL)–17, which inhibits OPC maturation and promotes inflammation.138 Demyelination also induces OPCs to phagocytose and present myelin debris to cytotoxic T cells.139 In active MS lesions, there is aberrant perivascular clustering of OPCs, which interferes with astrocyte end feet and endothelial tight junction integrity increasing permeability and disrupting the blood-brain barrier.140 There is also a cross talk between adult OPCs and microglia. Whereas redistribution of iron from ferritin in the microglia to adult OPCs may contribute to myelin repair,141 accumulation of extracellular iron triggers release of proinflammatory cytokines from microglia, which is harmful for adult OPCs.142
In response to injury, OPCs together with astrocytes and microglia proliferate and migrate to the lesion site and participate in the formation of the glial scar, where OPCs upregulate expression of several molecules that inhibit axonal growth, including the NG2 proteoglycan (chondroitin sulfate proteoglycan 4, CSPG4).143 These cells may also entrap dystrophic axons within lesions through the formation of synaptic-like contacts, which may stabilize remodeling axons.144,145
Neurodegeneration
With aging, adult OPCs decrease their proliferative activity,47 become functionally heterogeneous both within and between brain regions,63 and undergo many changes in their proteome, resulting in their reduced ability to differentiate into myelinating cells.146-150 Similarly, disease-associated oligodendroglia show a unique transcriptome profile that is shared across neurodegenerative disorders.151 These include Alzheimer disease (AD),152-155 amyotrophic lateral sclerosis (ALS),156,157 and multiple system atrophy (MSA),158 which show white matter pathology with proliferation of OPCs that are unable to differentiate into mature oligodendrocytes. In these disorders, cellular stress can affect the ability of OPCs to translate mRNAs into proteins required for myelination.28 For example, in early AD, OPCs accumulate in the vicinity of β-amyloid plaques but are unable to differentiate, leading to myelin loss.152-155 Impaired myelin formation may not directly result from Aβ accumulation159 but is promoted by the expression of the apolipoprotein E4 allele resulting in disruption of cholesterol metabolism.160 In ALS, impaired differentiation of OPCs into oligodendrocytes may reduce local energy supply to axons of motor neurons.156,157 However, OPCs derived from induced pluripotent stem cells of patients with ALS carrying a pathogenic C9ORF72 variant did not exhibit impairment of maturation and maintained their viability despite the presence of RNA foci.95 Multiple system atrophy is characterized by the accumulation of α-synuclein in the form of glial cytoplasmic inclusions (GCIs) in mature oligodendrocytes.161 The role of OPCs in the formation of GCIs in adult oligodendrocytes remains to be fully understood.158 In normal conditions, α-synuclein is expressed in OPCs but not in mature oligodendrocytes.162 Studies in experimental models show that abnormal α-synuclein impairs OPC differentiation into myelinating oligodendrocytes.162 However, whereas OPCs accumulate in MSA lesions, they do not show GCIs as do mature oligodendrocytes.162 Some studies suggest that GCI accumulation in oligodendrocytes may be due to their pruning of diseased axonal segments containing aggregated α-synuclein.163 However, adult oligodendrocytes do not seem to incorporate fibrillary aggregates, whereas OPCs do, suggesting that they may promote formation of inclusions.164
Glioma
Application of single-cell RNA sequencing in murine models of glioblastoma showed that OPCs disproportionately contribute to glioma formation.165 Genes enriched in gliomas harboring isocitrate dehydrogenase (IDH) variants, including astrocytomas and oligodendrogliomas, are upregulated in OPCs and are key regulators of OPC specification and maintenance.29 By contrast, genes involved in myelination are downregulated or absent in these tumors, indicating that all gliomas carrying IDH variants resemble early stages of OPC development with stalled oligodendrocyte differentiation.29 The fact that OPCs respond to neural activity7 is relevant because there are glutamatergic synaptic inputs to glioma cells that drive both glioma progression166,167 and migration of glioblastoma cells.168
Perspective
Adult OPCs constitute a unique proliferating, functionally heterogeneous population of cells that have features critical for neuronal activity–dependent myelination and remyelination, synaptic plasticity, circuit remodeling, and neuroinflammation. They sense and integrate multiple signals from neurons, glia, and other surrounding cells and release mediators that reciprocally affect migrating neurons, axons, synpases, blood vessels, and immune responses. The involvement of adult OPCs in neurologic disorders reflects their susceptibility to oxidative stress and their ability to undergo reprograming during inflammation and neurodegeneration. Whereas enhancing OPC recruitment to demyelinating lesions may be a reasonable approach to accelerate remyelination,25 the interactions among OPCs, neuroinflammation, and demyelination/remyelination are complex and probably depend on the disease state and environmental cues. The different effects of neuronal and non-neuronal signals on OPC proliferation, migration, and differentiation according to their developmental stage must be taken into consideration when designing pharmacologic tools to promote myelin formation and repair. Targeting specific transcriptional mechanisms using genetic approaches may also provide a potential therapeutic target for gliomas.
Glossary
- AD
Alzheimer disease
- ALS
amyotrophic lateral sclerosis
- ATP
adenosine triphosphate
- GCIs
glial cytoplasmic inclusions
- IDH
isocitrate dehydrogenase
- MS
multiple sclerosis
- MSA
multiple system atrophy
- NG2
neuron-glia antigen 2
- NMDARs
N-methyl-D-aspartate receptors
- OPCs
oligodendrocyte precursor cells
- PDGF
platelet-derived growth factor
Appendix. Author

| Name | Location | Contribution |
| Eduardo Benarroch, MD | Mayo Clinic, Rochester, MN | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
Study Funding
The author reports no targeted funding.
Disclosure
The author reports no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Dimou L, Gotz M. Glial cells as progenitors and stem cells: new roles in the healthy and diseased brain. Physiol Rev. 2014;94(3):709-737. doi: 10.1152/physrev.00036.2013 [DOI] [PubMed] [Google Scholar]
- 2.Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24(2):476-488. doi: 10.1016/s1044-7431(03)00210-0 [DOI] [PubMed] [Google Scholar]
- 3.Jakovcevski I, Filipovic R, Mo Z, Rakic S, Zecevic N. Oligodendrocyte development and the onset of myelination in the human fetal brain. Front Neuroanat. 2009;3:5. doi: 10.3389/neuro.05.005.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergles DE, Richardson WD. Oligodendrocyte development and plasticity. Cold Spring Harb Perspect Biol. 2015;8(2):a020453. doi: 10.1101/cshperspect.a020453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao Y, Czopka T. Myelination-independent functions of oligodendrocyte precursor cells in health and disease. Nat Neurosci. 2023;26(10):1663-1669. doi: 10.1038/s41593-023-01423-3 [DOI] [PubMed] [Google Scholar]
- 6.Hayashi C, Suzuki N. Heterogeneity of oligodendrocytes and their precursor cells. Adv Exp Med Biol. 2019;1190:53-62. doi: 10.1007/978-981-32-9636-7_5 [DOI] [PubMed] [Google Scholar]
- 7.Gibson EM, Purger D, Mount CW, et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344(6183):1252304. doi: 10.1126/science.1252304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almeida RG, Lyons DA. On myelinated axon plasticity and neuronal circuit formation and function. J Neurosci. 2017;37(42):10023-10034. doi: 10.1523/JNEUROSCI.3185-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitew S, Gobius I, Fenlon LR, et al. Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat Commun. 2018;9(1):306. doi: 10.1038/s41467-017-02719-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fields RD. A new mechanism of nervous system plasticity: activity-dependent myelination. Nat Rev Neurosci. 2015;16(12):756-767. doi: 10.1038/nrn4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergles DE, Jabs R, Steinhauser C. Neuron-glia synapses in the brain. Brain Res Rev. 2010;63(1-2):130-137. doi: 10.1016/j.brainresrev.2009.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405(6783):187-191. doi: 10.1038/35012083 [DOI] [PubMed] [Google Scholar]
- 13.Serrano-Regal MP, Bayon-Cordero L, Ordaz RP, et al. Expression and function of GABA receptors in myelinating cells. Front Cell Neurosci. 2020;14:256. doi: 10.3389/fncel.2020.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habermacher C, Angulo MC, Benamer N. Glutamate versus GABA in neuron-oligodendroglia communication. Glia. 2019;67(11):2092-2106. doi: 10.1002/glia.23618 [DOI] [PubMed] [Google Scholar]
- 15.Pan L, Trimarco A, Zhang AJ, et al. Oligodendrocyte-lineage cell exocytosis and L-type prostaglandin D synthase promote oligodendrocyte development and myelination. Elife. 2023;12:e77441. doi: 10.7554/elife.77441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fruhbeis C, Frohlich D, Kuo WP, et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11(7):e1001604. doi: 10.1371/journal.pbio.1001604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez-Abarca LI, Tabernero A, Medina JM. Oligodendrocytes use lactate as a source of energy and as a precursor of lipids. Glia. 2001;36(3):321-329. doi: 10.1002/glia.1119 [DOI] [PubMed] [Google Scholar]
- 18.Amaral AI, Hadera MG, Tavares JM, Kotter MR, Sonnewald U. Characterization of glucose-related metabolic pathways in differentiated rat oligodendrocyte lineage cells. Glia. 2016;64(1):21-34. doi: 10.1002/glia.22900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matute C. Glutamate and ATP signalling in white matter pathology. J Anat. 2011;219(1):53-64. doi: 10.1111/j.1469-7580.2010.01339.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giacci M, Fitzgerald M. Oligodendroglia are particularly vulnerable to oxidative damage after neurotrauma in vivo. J Exp Neurosci. 2018;12:1179069518810004. doi: 10.1177/1179069518810004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuen TJ, Silbereis JC, Griveau A, et al. Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell. 2014;158(2):383-396. doi: 10.1016/j.cell.2014.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hampton DW, Rhodes KE, Zhao C, Franklin RJ, Fawcett JW. The responses of oligodendrocyte precursor cells, astrocytes and microglia to a cortical stab injury, in the brain. Neuroscience. 2004;127(4):813-820. doi: 10.1016/j.neuroscience.2004.05.028 [DOI] [PubMed] [Google Scholar]
- 23.Smith MD, Chamling X, Gill AJ, et al. Reactive astrocytes derived from human induced pluripotent stem cells suppress oligodendrocyte precursor cell differentiation. Front Mol Neurosci. 2022;15:874299. doi: 10.3389/fnmol.2022.874299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haindl MT, Kock U, Zeitelhofer-Adzemovic M, Fazekas F, Hochmeister S. The formation of a glial scar does not prohibit remyelination in an animal model of multiple sclerosis. Glia. 2019;67(3):467-481. doi: 10.1002/glia.23556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tepavčević V, Lubetzki C. Oligodendrocyte progenitor cell recruitment and remyelination in multiple sclerosis: the more, the merrier? Brain. 2022;145(12):4178-4192. doi: 10.1093/brain/awac307 [DOI] [PubMed] [Google Scholar]
- 26.Yeung MS, Zdunek S, Bergmann O, et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell. 2014;159(4):766-774. doi: 10.1016/j.cell.2014.10.011 [DOI] [PubMed] [Google Scholar]
- 27.Franklin RJM, Frisen J, Lyons DA. Revisiting remyelination: towards a consensus on the regeneration of CNS myelin. Semin Cell Dev Biol. 2021;116:3-9. doi: 10.1016/j.semcdb.2020.09.009 [DOI] [PubMed] [Google Scholar]
- 28.Hoch-Kraft P, Trotter J, Gonsior C. Missing in action: dysfunctional RNA metabolism in oligodendroglial cells as a contributor to neurodegenerative diseases? Neurochem Res. 2020;45(3):566-579. doi: 10.1007/s11064-019-02763-y [DOI] [PubMed] [Google Scholar]
- 29.Wei Y, Li G, Feng J, et al. Stalled oligodendrocyte differentiation in IDH-mutant gliomas. Genome Med. 2023;15(1):24. doi: 10.1186/s13073-023-01175-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yi C, Verkhratsky A, Niu J. Pathological potential of oligodendrocyte precursor cells: terra incognita. Trends Neurosci. 2023;46(7):581-596. doi: 10.1016/j.tins.2023.04.003 [DOI] [PubMed] [Google Scholar]
- 31.Raff MC, Abney ER, Miller RH. Two glial cell lineages diverge prenatally in rat optic nerve. Dev Biol. 1984;106(1):53-60. doi: 10.1016/0012-1606(84)90060-5 [DOI] [PubMed] [Google Scholar]
- 32.Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9(2):173-179. doi: 10.1038/nn1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishiyama A, Suzuki R, Zhu X. NG2 cells (polydendrocytes) in brain physiology and repair. Front Neurosci. 2014;8:133. doi: 10.3389/fnins.2014.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh J, Sharma K, Frost EE, Pillai PP. Role of PDGF-A-activated ERK signaling mediated FAK-paxillin interaction in oligodendrocyte progenitor cell migration. J Mol Neurosci. 2019;67(4):564-573. doi: 10.1007/s12031-019-1260-1 [DOI] [PubMed] [Google Scholar]
- 35.Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996;43(3):299-314. doi: [DOI] [PubMed] [Google Scholar]
- 36.Watzlawik JO, Warrington AE, Rodriguez M. PDGF is required for remyelination-promoting IgM stimulation of oligodendrocyte progenitor cell proliferation. PLoS One. 2013;8(2):e55149. doi: 10.1371/journal.pone.0055149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai HH, Niu J, Munji R, et al. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science. 2016;351(6271):379-384. doi: 10.1126/science.aad3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su Y, Wang X, Yang Y, et al. Astrocyte endfoot formation controls the termination of oligodendrocyte precursor cell perivascular migration during development. Neuron. 2023;111(2):190-201.e8. doi: 10.1016/j.neuron.2022.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lepiemme F, Stoufflet J, Javier-Torrent M, Mazzucchelli G, Silva CG, Nguyen L. Oligodendrocyte precursors guide interneuron migration by unidirectional contact repulsion. Science. 2022;376(6595):eabn6204. doi: 10.1126/science.abn6204 [DOI] [PubMed] [Google Scholar]
- 40.Nishiyama A, Boshans L, Goncalves CM, Wegrzyn J, Patel KD. Lineage, fate, and fate potential of NG2-glia. Brain Res. 2016;1638(Pt B):116-128. doi: 10.1016/j.brainres.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young KM, Psachoulia K, Tripathi RB, et al. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77(5):873-885. doi: 10.1016/j.neuron.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elbaz B, Popko B. Molecular control of oligodendrocyte development. Trends Neurosci. 2019;42(4):263-277. doi: 10.1016/j.tins.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cantone M, Kuspert M, Reiprich S, et al. A gene regulatory architecture that controls region-independent dynamics of oligodendrocyte differentiation. Glia. 2019;67(5):825-843. doi: 10.1002/glia.23569 [DOI] [PubMed] [Google Scholar]
- 44.Emery B, Lu QR. Transcriptional and epigenetic regulation of oligodendrocyte development and myelination in the central nervous system. Cold Spring Harb Perspect Biol. 2015;7(9):a020461. doi: 10.1101/cshperspect.a020461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Moyon S, Hernandez M, Casaccia P. Epigenetic control of oligodendrocyte development: adding new players to old keepers. Curr Opin Neurobiol. 2016;39:133-138. doi: 10.1016/j.conb.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koreman E, Sun X, Lu QR. Chromatin remodeling and epigenetic regulation of oligodendrocyte myelination and myelin repair. Mol Cell Neurosci. 2018;87:18-26. doi: 10.1016/j.mcn.2017.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16(6):668-676. doi: 10.1038/nn.3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu G, Wang W, Zhou M. Spatial organization of NG2 glial cells and astrocytes in rat hippocampal CA1 region. Hippocampus. 2014;24(4):383-395. doi: 10.1002/hipo.22232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wigley R, Butt AM. Integration of NG2-glia (synantocytes) into the neuroglial network. Neuron Glia Biol. 2009;5(1-2):21-28. doi: 10.1017/S1740925X09990329 [DOI] [PubMed] [Google Scholar]
- 50.Baraban M, Mensch S, Lyons DA. Adaptive myelination from fish to man. Brain Res. 2016;1641(Pt A):149-161. doi: 10.1016/j.brainres.2015.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mount CW, Monje M. Wrapped to adapt: experience-dependent myelination. Neuron. 2017;95(4):743-756. doi: 10.1016/j.neuron.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mount CW, Yalcin B, Cunliffe-Koehler K, Sundaresh S, Monje M. Monosynaptic tracing maps brain-wide afferent oligodendrocyte precursor cell connectivity. Elife. 2019;8:e49291. doi: 10.7554/eLife.49291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karadottir R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11(4):450-456. doi: 10.1038/nn2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Biase LM, Nishiyama A, Bergles DE. Excitability and synaptic communication within the oligodendrocyte lineage. J Neurosci. 2010;30(10):3600-3611. doi: 10.1523/JNEUROSCI.6000-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gould E, Kim JH. SCN2A contributes to oligodendroglia excitability and development in the mammalian brain. Cell Rep. 2021;36(10):109653. doi: 10.1016/j.celrep.2021.109653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheli VT, Santiago Gonzalez DA, Spreuer V, Paez PM. Voltage-gated Ca2+ entry promotes oligodendrocyte progenitor cell maturation and myelination in vitro. Exp Neurol. 2015;265:69-83. doi: 10.1016/j.expneurol.2014.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paez PM, Lyons DA. Calcium signaling in the oligodendrocyte lineage: regulators and consequences. Annu Rev Neurosci. 2020;43:163-186. doi: 10.1146/annurev-neuro-100719-093305 [DOI] [PubMed] [Google Scholar]
- 58.Sun W, Matthews EA, Nicolas V, Schoch S, Dietrich D. NG2 glial cells integrate synaptic input in global and dendritic calcium signals. Elife. 2016;5:e16262. doi: 10.7554/eLife.16262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu H, Yang X, Yang J, et al. IL-17 inhibits oligodendrocyte progenitor cell proliferation and differentiation by increasing K(+) channel Kv1.3. Front Cell Neurosci. 2021;15:679413. doi: 10.3389/fncel.2021.679413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maldonado PP, Angulo MC. Multiple modes of communication between neurons and oligodendrocyte precursor cells. Neuroscientist. 2015;21(3):266-276. doi: 10.1177/1073858414530784 [DOI] [PubMed] [Google Scholar]
- 61.Taubert M, Draganski B, Anwander A, et al. Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections. J Neurosci. 2010;30(35):11670-11677. doi: 10.1523/JNEUROSCI.2567-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hofstetter S, Tavor I, Tzur Moryosef S, Assaf Y. Short-term learning induces white matter plasticity in the fornix. J Neurosci. 2013;33(31):12844-12850. doi: 10.1523/JNEUROSCI.4520-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spitzer SO, Sitnikov S, Kamen Y, et al. Oligodendrocyte progenitor cells become regionally diverse and heterogeneous with age. Neuron. 2019;101(3):459-471.e5. doi: 10.1016/j.neuron.2018.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moura DMS, Brennan EJ, Brock R, Cocas LA. Neuron to oligodendrocyte precursor cell synapses: protagonists in oligodendrocyte development and myelination, and targets for therapeutics. Front Neurosci. 2021;15:779125. doi: 10.3389/fnins.2021.779125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marisca R, Hoche T, Agirre E, et al. Functionally distinct subgroups of oligodendrocyte precursor cells integrate neural activity and execute myelin formation. Nat Neurosci. 2020;23(3):363-374. doi: 10.1038/s41593-019-0581-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin SC, Bergles DE. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat Neurosci. 2004;7(1):24-32. doi: 10.1038/nn1162 [DOI] [PubMed] [Google Scholar]
- 67.Mangin JM, Kunze A, Chittajallu R, Gallo V. Satellite NG2 progenitor cells share common glutamatergic inputs with associated interneurons in the mouse dentate gyrus. J Neurosci. 2008;28(30):7610-7623. doi: 10.1523/JNEUROSCI.1355-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin SC, Huck JH, Roberts JD, Macklin WB, Somogyi P, Bergles DE. Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron. 2005;46(5):773-785. doi: 10.1016/j.neuron.2005.04.025 [DOI] [PubMed] [Google Scholar]
- 69.Osterstock G, Le Bras B, Arulkandarajah KH, et al. Axoglial synapses are formed onto pioneer oligodendrocyte precursor cells at the onset of spinal cord gliogenesis. Glia. 2018;66(8):1678-1694. doi: 10.1002/glia.23331 [DOI] [PubMed] [Google Scholar]
- 70.Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10(3):311-320. doi: 10.1038/nn1850 [DOI] [PubMed] [Google Scholar]
- 71.Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10(3):321-330. doi: 10.1038/nn1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nagy B, Hovhannisyan A, Barzan R, Chen TJ, Kukley M. Different patterns of neuronal activity trigger distinct responses of oligodendrocyte precursor cells in the corpus callosum. PLoS Biol. 2017;15(8):e2001993. doi: 10.1371/journal.pbio.2001993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hines JH, Ravanelli AM, Schwindt R, Scott EK, Appel B. Neuronal activity biases axon selection for myelination in vivo. Nat Neurosci. 2015;18(5):683-689. doi: 10.1038/nn.3992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kougioumtzidou E, Shimizu T, Hamilton NB, et al. Signalling through AMPA receptors on oligodendrocyte precursors promotes myelination by enhancing oligodendrocyte survival. Elife. 2017;6:e28080. doi: 10.7554/eLife.28080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen TJ, Kula B, Nagy B, et al. In vivo regulation of oligodendrocyte precursor cell proliferation and differentiation by the AMPA-receptor subunit GluA2. Cell Rep. 2018;25(4):852-861.e7. doi: 10.1016/j.celrep.2018.09.066 [DOI] [PubMed] [Google Scholar]
- 76.Barron T, Kim JH. Neuronal input triggers Ca(2+) influx through AMPA receptors and voltage-gated Ca(2+) channels in oligodendrocytes. Glia. 2019;67(10):1922-1932. doi: 10.1002/glia.23670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kukley M. Recent insights into the functional role of AMPA receptors in the oligodendrocyte lineage cells in vivo. Int J Mol Sci. 2023;24(4):4138. doi: 10.3390/ijms24044138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ge WP, Yang XJ, Zhang Z, et al. Long-term potentiation of neuron-glia synapses mediated by Ca2+-permeable AMPA receptors. Science. 2006;312(5779):1533-1537. doi: 10.1126/science.1124669 [DOI] [PubMed] [Google Scholar]
- 79.Birey F, Kokkosis AG, Aguirre A. Oligodendroglia-lineage cells in brain plasticity, homeostasis and psychiatric disorders. Curr Opin Neurobiol. 2017;47:93-103. doi: 10.1016/j.conb.2017.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gautier HO, Evans KA, Volbracht K, et al. Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Nat Commun. 2015;6:8518. doi: 10.1038/ncomms9518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438(7071):1162-1166. doi: 10.1038/nature04302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lundgaard I, Luzhynskaya A, Stockley JH, et al. Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol. 2013;11(12):e1001743. doi: 10.1371/journal.pbio.1001743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Orduz D, Maldonado PP, Balia M, et al. Interneurons and oligodendrocyte progenitors form a structured synaptic network in the developing neocortex. Elife. 2015;4:e06953. doi: 10.7554/eLife.06953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Velez-Fort M, Audinat E, Angulo MC. Central role of GABA in neuron-glia interactions. Neuroscientist. 2012;18(3):237-250. doi: 10.1177/1073858411403317 [DOI] [PubMed] [Google Scholar]
- 85.Balia M, Velez-Fort M, Passlick S, et al. Postnatal down-regulation of the GABAA receptor γ2 subunit in neocortical NG2 cells accompanies synaptic-to-extrasynaptic switch in the GABAergic transmission mode. Cereb Cortex. 2015;25(4):1114-1123. doi: 10.1093/cercor/bht309 [DOI] [PubMed] [Google Scholar]
- 86.Kirchhoff F, Kettenmann H. GABA triggers a [Ca2+]i increase in murine precursor cells of the oligodendrocyte lineage. Eur J Neurosci. 1992;4(11):1049-1058. doi: 10.1111/j.1460-9568.1992.tb00131.x [DOI] [PubMed] [Google Scholar]
- 87.Zonouzi M, Scafidi J, Li P, et al. GABAergic regulation of cerebellar NG2 cell development is altered in perinatal white matter injury. Nat Neurosci. 2015;18(5):674-682. doi: 10.1038/nn.3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tong XP, Li XY, Zhou B, et al. Ca(2+) signaling evoked by activation of Na(+) channels and Na(+)/Ca(2+) exchangers is required for GABA-induced NG2 cell migration. J Cell Biol. 2009;186(1):113-128. doi: 10.1083/jcb.200811071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Balia M, Benamer N, Angulo MC. A specific GABAergic synapse onto oligodendrocyte precursors does not regulate cortical oligodendrogenesis. Glia. 2017;65(11):1821-1832. doi: 10.1002/glia.23197 [DOI] [PubMed] [Google Scholar]
- 90.Serrano-Regal MP, Luengas-Escuza I, Bayon-Cordero L, et al. Oligodendrocyte differentiation and myelination is potentiated via GABA(B) receptor activation. Neuroscience. 2020;439:163-180. doi: 10.1016/j.neuroscience.2019.07.014 [DOI] [PubMed] [Google Scholar]
- 91.Li T, Wang L, Ma T, et al. Dynamic calcium release from endoplasmic reticulum mediated by ryanodine receptor 3 is crucial for oligodendroglial differentiation. Front Mol Neurosci. 2018;11:162. doi: 10.3389/fnmol.2018.00162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Agresti C, Meomartini ME, Amadio S, et al. ATP regulates oligodendrocyte progenitor migration, proliferation, and differentiation: involvement of metabotropic P2 receptors. Brain Res Brain Res Rev. 2005;48(2):157-165. doi: 10.1016/j.brainresrev.2004.12.005 [DOI] [PubMed] [Google Scholar]
- 93.Welsh TG, Kucenas S. Purinergic signaling in oligodendrocyte development and function. J Neurochem. 2018;145(1):6-18. doi: 10.1111/jnc.14315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weider M, Starost LJ, Groll K, et al. Nfat/calcineurin signaling promotes oligodendrocyte differentiation and myelination by transcription factor network tuning. Nat Commun. 2018;9(1):899. doi: 10.1038/s41467-018-03336-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Livesey MR, Magnani D, Cleary EM, et al. Maturation and electrophysiological properties of human pluripotent stem cell-derived oligodendrocytes. Stem Cells. 2016;34(4):1040-1053. doi: 10.1002/stem.2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang X, Liu Y, Hong X, et al. NG2 glia-derived GABA release tunes inhibitory synapses and contributes to stress-induced anxiety. Nat Commun. 2021;12(1):5740. doi: 10.1038/s41467-021-25956-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sakry D, Neitz A, Singh J, et al. Oligodendrocyte precursor cells modulate the neuronal network by activity-dependent ectodomain cleavage of glial NG2. PLoS Biol. 2014;12(11):e1001993. doi: 10.1371/journal.pbio.1001993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sakry D, Yigit H, Dimou L, Trotter J. Oligodendrocyte precursor cells synthesize neuromodulatory factors. PLoS One. 2015;10(5):e0127222. doi: 10.1371/journal.pone.0127222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Auguste YSS, Ferro A, Kahng JA, et al. Oligodendrocyte precursor cells engulf synapses during circuit remodeling in mice. Nat Neurosci. 2022;25(10):1273-1278. doi: 10.1038/s41593-022-01170-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Buchanan J, Elabbady L, Collman F, et al. Oligodendrocyte precursor cells ingest axons in the mouse neocortex. Proc Natl Acad Sci USA. 2022;119(48):e2202580119. doi: 10.1073/pnas.2202580119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goldberg JL, Vargas ME, Wang JT, et al. An oligodendrocyte lineage-specific semaphorin, Sema5A, inhibits axon growth by retinal ganglion cells. J Neurosci. 2004;24(21):4989-4999. doi: 10.1523/JNEUROSCI.4390-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Duan Y, Wang SH, Song J, et al. Semaphorin 5A inhibits synaptogenesis in early postnatal- and adult-born hippocampal dentate granule cells. Elife. 2014;3:e04390. doi: 10.7554/eLife.04390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xiao Y, Petrucco L, Hoodless LJ, Portugues R, Czopka T. Oligodendrocyte precursor cells sculpt the visual system by regulating axonal remodeling. Nat Neurosci. 2022;25(3):280-284. doi: 10.1038/s41593-022-01023-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rone MB, Cui QL, Fang J, et al. Oligodendrogliopathy in multiple sclerosis: low glycolytic metabolic rate promotes oligodendrocyte survival. J Neurosci. 2016;36(17):4698-4707. doi: 10.1523/JNEUROSCI.4077-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meyer N, Rinholm JE. Mitochondria in myelinating oligodendrocytes: slow and out of breath? Metabolites. 2021;11(6):359. doi: 10.3390/metabo11060359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kishida N, Maki T, Takagi Y, et al. Role of perivascular oligodendrocyte precursor cells in angiogenesis after brain ischemia. J Am Heart Assoc. 2019;8(9):e011824. doi: 10.1161/JAHA.118.011824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Micu I, Plemel JR, Caprariello AV, Nave KA, Stys PK. Axo-myelinic neurotransmission: a novel mode of cell signalling in the central nervous system. Nat Rev Neurosci. 2018;19(1):49-58. doi: 10.1038/nrn.2017.128 [DOI] [PubMed] [Google Scholar]
- 108.Miller RH. Calcium control of myelin sheath growth. Nat Neurosci. 2018;21(1):2-3. doi: 10.1038/s41593-017-0043-7 [DOI] [PubMed] [Google Scholar]
- 109.Hughes AN, Appel B. Oligodendrocytes express synaptic proteins that modulate myelin sheath formation. Nat Commun. 2019;10(1):4125. doi: 10.1038/s41467-019-12059-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Battefeld A, Popovic MA, de Vries SI, Kole MHP. High-frequency microdomain Ca(2+) transients and waves during early myelin internode remodeling. Cell Rep. 2019;26(1):182-191.e5. doi: 10.1016/j.celrep.2018.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Funfschilling U, Supplie LM, Mahad D, et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485(7399):517-521. doi: 10.1038/nature11007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Matute C, Ransom BR. Roles of white matter in central nervous system pathophysiologies. ASN Neuro. 2012;4(2):e00079. doi: 10.1042/AN20110060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438(7071):1167-1171. doi: 10.1038/nature04301 [DOI] [PubMed] [Google Scholar]
- 114.Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93(2):F153-F161. doi: 10.1136/adc.2006.108837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(1):110-124. doi: 10.1016/S1474-4422(08)70294-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang Y, Liu G, Hong D, Chen F, Ji X, Cao G. White matter injury in ischemic stroke. Prog Neurobiol. 2016;141:45-60. doi: 10.1016/j.pneurobio.2016.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Back SA, Han BH, Luo NL, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22(2):455-463. doi: 10.1523/JNEUROSCI.22-02-00455.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rao VTS, Khan D, Cui QL, et al. Distinct age and differentiation-state dependent metabolic profiles of oligodendrocytes under optimal and stress conditions. PLoS One. 2017;12(8):e0182372. doi: 10.1371/journal.pone.0182372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Back SA. White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol. 2017;134(3):331-349. doi: 10.1007/s00401-017-1718-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Segovia KN, McClure M, Moravec M, et al. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol. 2008;63(4):520-530. doi: 10.1002/ana.21359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ichinose M, Kamei Y, Iriyama T, et al. Hypothermia attenuates apoptosis and protects contact between myelin basic protein-expressing oligodendroglial-lineage cells and neurons against hypoxia-ischemia. J Neurosci Res. 2014;92(10):1270-1285. doi: 10.1002/jnr.23418 [DOI] [PubMed] [Google Scholar]
- 122.Yeung MSY, Djelloul M, Steiner E, et al. Dynamics of oligodendrocyte generation in multiple sclerosis. Nature. 2019;566(7745):538-542. doi: 10.1038/s41586-018-0842-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jakel S, Agirre E, Mendanha Falcao A, et al. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature. 2019;566(7745):543-547. doi: 10.1038/s41586-019-0903-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wolswijk G. Oligodendrocyte survival, loss and birth in lesions of chronic-stage multiple sclerosis. Brain. 2000;123(Pt 1):105-115. doi: 10.1093/brain/123.1.105 [DOI] [PubMed] [Google Scholar]
- 125.Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346(3):165-173. doi: 10.1056/NEJMoa010994 [DOI] [PubMed] [Google Scholar]
- 126.Wolswijk G. Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain. 2002;125(Pt 2):338-349. doi: 10.1093/brain/awf031 [DOI] [PubMed] [Google Scholar]
- 127.Blakemore WF, Chari DM, Gilson JM, Crang AJ. Modelling large areas of demyelination in the rat reveals the potential and possible limitations of transplanted glial cells for remyelination in the CNS. Glia. 2002;38(2):155-168. doi: 10.1002/glia.10067 [DOI] [PubMed] [Google Scholar]
- 128.Mozafari S, Starost L, Manot-Saillet B, et al. Multiple sclerosis iPS-derived oligodendroglia conserve their properties to functionally interact with axons and glia in vivo. Sci Adv. 2020;6(49):eabc6983. doi: 10.1126/sciadv.abc6983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Luo JXX, Cui QL, Yaqubi M, et al. Human oligodendrocyte myelination potential; relation to age and differentiation. Ann Neurol. 2022;91(2):178-191. doi: 10.1002/ana.26288 [DOI] [PubMed] [Google Scholar]
- 130.Lloyd AF, Miron VE. The pro-remyelination properties of microglia in the central nervous system. Nat Rev Neurol. 2019;15(8):447-458. doi: 10.1038/s41582-019-0184-2 [DOI] [PubMed] [Google Scholar]
- 131.Foote AK, Blakemore WF. Inflammation stimulates remyelination in areas of chronic demyelination. Brain. 2005;128(Pt 3):528-539. doi: 10.1093/brain/awh417 [DOI] [PubMed] [Google Scholar]
- 132.Chen CZ, Neumann B, Forster S, Franklin RJM. Schwann cell remyelination of the central nervous system: why does it happen and what are the benefits? Open Biol. 2021;11(1):200352. doi: 10.1098/rsob.200352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wellman SM, Kozai TDY. In vivo spatiotemporal dynamics of NG2 glia activity caused by neural electrode implantation. Biomaterials. 2018;164:121-133. doi: 10.1016/j.biomaterials.2018.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Suzuki N, Sekimoto K, Hayashi C, Mabuchi Y, Nakamura T, Akazawa C. Differentiation of oligodendrocyte precursor cells from Sox10-Venus mice to oligodendrocytes and astrocytes. Sci Rep. 2017;7(1):14133. doi: 10.1038/s41598-017-14207-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hackett AR, Yahn SL, Lyapichev K, et al. Injury type-dependent differentiation of NG2 glia into heterogeneous astrocytes. Exp Neurol. 2018;308:72-79. doi: 10.1016/j.expneurol.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alonso G. NG2 proteoglycan-expressing cells of the adult rat brain: possible involvement in the formation of glial scar astrocytes following stab wound. Glia. 2005;49(3):318-338. doi: 10.1002/glia.20121 [DOI] [PubMed] [Google Scholar]
- 137.Meijer M, Agirre E, Kabbe M, et al. Epigenomic priming of immune genes implicates oligodendroglia in multiple sclerosis susceptibility. Neuron. 2022;110(7):1193-1210.e13. doi: 10.1016/j.neuron.2021.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang C, Zhang CJ, Martin BN, et al. IL-17 induced NOTCH1 activation in oligodendrocyte progenitor cells enhances proliferation and inflammatory gene expression. Nat Commun. 2017;8:15508. doi: 10.1038/ncomms15508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kirby L, Jin J, Cardona JG, et al. Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat Commun. 2019;10(1):3887. doi: 10.1038/s41467-019-11638-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Niu J, Tsai HH, Hoi KK, et al. Aberrant oligodendroglial-vascular interactions disrupt the blood-brain barrier, triggering CNS inflammation. Nat Neurosci. 2019;22(5):709-718. doi: 10.1038/s41593-019-0369-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR. Oligodendrocytes and myelination: the role of iron. Glia. 2009;57(5):467-478. doi: 10.1002/glia.20784 [DOI] [PubMed] [Google Scholar]
- 142.Zhang X, Haaf M, Todorich B, et al. Cytokine toxicity to oligodendrocyte precursors is mediated by iron. Glia. 2005;52(3):199-208. doi: 10.1002/glia.20235 [DOI] [PubMed] [Google Scholar]
- 143.Tan AM, Zhang W, Levine JM. NG2: a component of the glial scar that inhibits axon growth. J Anat. 2005;207(6):717-725. doi: 10.1111/j.1469-7580.2005.00452.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Filous AR, Tran A, Howell CJ, et al. Entrapment via synaptic-like connections between NG2 proteoglycan+ cells and dystrophic axons in the lesion plays a role in regeneration failure after spinal cord injury. J Neurosci. 2014;34(49):16369-16384. doi: 10.1523/JNEUROSCI.1309-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Busch SA, Horn KP, Cuascut FX, et al. Adult NG2+ cells are permissive to neurite outgrowth and stabilize sensory axons during macrophage-induced axonal dieback after spinal cord injury. J Neurosci. 2010;30(1):255-265. doi: 10.1523/JNEUROSCI.3705-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Segel M, Neumann B, Hill MFE, et al. Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature. 2019;573(7772):130-134. doi: 10.1038/s41586-019-1484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rivera AD, Pieropan F, Chacon-De-La-Rocha I, et al. Functional genomic analyses highlight a shift in Gpr17-regulated cellular processes in oligodendrocyte progenitor cells and underlying myelin dysregulation in the aged mouse cerebrum. Aging Cell. 2021;20(4):e13335. doi: 10.1111/acel.13335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ito M, Muramatsu R, Kato Y, et al. Age-dependent decline in remyelination capacity is mediated by apelin-APJ signaling. Nat Aging. 2021;1(3):284-294. doi: 10.1038/s43587-021-00041-7 [DOI] [PubMed] [Google Scholar]
- 149.de la Fuente AG, Queiroz RML, Ghosh T, et al. Changes in the oligodendrocyte progenitor cell proteome with ageing. Mol Cell Proteomics. 2020;19(8):1281-1302. doi: 10.1074/mcp.RA120.002102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dimovasili C, Fair AE, Garza IR, et al. Aging compromises oligodendrocyte precursor cell maturation and efficient remyelination in the monkey brain. Geroscience. 2023;45(1):249-264. doi: 10.1007/s11357-022-00621-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kenigsbuch M, Bost P, Halevi S, et al. A shared disease-associated oligodendrocyte signature among multiple CNS pathologies. Nat Neurosci. 2022;25(7):876-886. doi: 10.1038/s41593-022-01104-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Behrendt G, Baer K, Buffo A, et al. Dynamic changes in myelin aberrations and oligodendrocyte generation in chronic amyloidosis in mice and men. Glia. 2013;61(2):273-286. doi: 10.1002/glia.22432 [DOI] [PubMed] [Google Scholar]
- 153.Dong YX, Zhang HY, Li HY, Liu PH, Sui Y, Sun XH. Association between Alzheimer's disease pathogenesis and early demyelination and oligodendrocyte dysfunction. Neural Regen Res. 2018;13(5):908-914. doi: 10.4103/1673-5374.232486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Butt AM, De La Rocha IC, Rivera A. Oligodendroglial cells in Alzheimer's disease. Adv Exp Med Biol. 2019;1175:325-333. doi: 10.1007/978-981-13-9913-8_12 [DOI] [PubMed] [Google Scholar]
- 155.Vanzulli I, Papanikolaou M, De-La-Rocha IC, et al. Disruption of oligodendrocyte progenitor cells is an early sign of pathology in the triple transgenic mouse model of Alzheimer's disease. Neurobiol Aging. 2020;94:130-139. doi: 10.1016/j.neurobiolaging.2020.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gong Z, Ba L, Zhang M. Dysfunction of the oligodendrocytes in amyotrophic lateral sclerosis. J Biomed Res. 2022;36(5):336-342. doi: 10.7555/JBR.36.20220009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Raffaele S, Boccazzi M, Fumagalli M. Oligodendrocyte dysfunction in amyotrophic lateral sclerosis: mechanisms and therapeutic perspectives. Cells. 2021;10(3):565. doi: 10.3390/cells10030565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hsiao JT, Tanglay O, Li AA, et al. Role of oligodendrocyte lineage cells in multiple system atrophy. Cells. 2023;12(5):739. doi: 10.3390/cells12050739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wu SL, Yu B, Cheng YJ, et al. Brain region-specific myelinogenesis is not directly linked to amyloid-beta in APP/PS1 transgenic mice. Exp Neurol. 2023;362:114344. doi: 10.1016/j.expneurol.2023.114344 [DOI] [PubMed] [Google Scholar]
- 160.Blanchard JW, Akay LA, Davila-Velderrain J, et al. APOE4 impairs myelination via cholesterol dysregulation in oligodendrocytes. Nature. 2022;611(7937):769-779. doi: 10.1038/s41586-022-05439-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Papp MI, Lantos PL. The distribution of oligodendroglial inclusions in multiple system atrophy and its relevance to clinical symptomatology. Brain. 1994;117(Pt 2):235-243. doi: 10.1093/brain/117.2.235 [DOI] [PubMed] [Google Scholar]
- 162.May VE, Ettle B, Poehler AM, et al. α-Synuclein impairs oligodendrocyte progenitor maturation in multiple system atrophy. Neurobiol Aging. 2014;35(10):2357-2368. doi: 10.1016/j.neurobiolaging.2014.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.De Nuccio F, Kashyrina M, Serinelli F, et al. Oligodendrocytes prune axons containing alpha-synuclein aggregates in vivo: lewy neurites as precursors of glial cytoplasmic inclusions in multiple system atrophy? Biomolecules. 2023;13(2):269. doi: 10.3390/biom13020269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kaji S, Maki T, Kinoshita H, et al. Pathological endogenous α-synuclein accumulation in oligodendrocyte precursor cells potentially induces inclusions in multiple system atrophy. Stem Cell Rep. 2018;10(2):356-365. doi: 10.1016/j.stemcr.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Weng Q, Wang J, Wang J, et al. Single-cell transcriptomics uncovers glial progenitor diversity and cell fate determinants during development and gliomagenesis. Cell Stem Cell. 2019;24(5):707-723.e8. doi: 10.1016/j.stem.2019.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Venkataramani V, Tanev DI, Strahle C, et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature. 2019;573(7775):532-538. doi: 10.1038/s41586-019-1564-x [DOI] [PubMed] [Google Scholar]
- 167.Venkatesh HS, Johung TB, Caretti V, et al. Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell. 2015;161(4):803-816. doi: 10.1016/j.cell.2015.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Venkataramani V, Yang Y, Schubert MC, et al. Glioblastoma hijacks neuronal mechanisms for brain invasion. Cell. 2022;185(16):2899-2917.e31. doi: 10.1016/j.cell.2022.06.054 [DOI] [PubMed] [Google Scholar]