Pearls
There are 2 histologic subtypes of craniopharyngioma: adamantinomatous subtype and papillary subtype. The adamantinomatous subtype has a bimodal presentation, occurring between 5 and 15 years or between 45 and 60 years of age, whereas the papillary subtype typically occurs in adults aged 40–55 years.
Calcifications around the third ventricle, identified on CT brain, are one of the radiologic hallmarks of the adamantinomatous subtype of craniopharyngioma.
Posttreatment surveillance monitoring of craniopharyngiomas is essential given the risk of recurrence, which is estimated to be between 11% and 25% after gross total resection. The recurrence risk is higher for the adamantinomatous subtype.
Oy-sters
An MRI of the brain should be performed to rule out intracranial lesions in patients presenting with cerebral ventriculomegaly without identifiable cause on CT.
Although craniopharyngiomas commonly present with symptoms related to elevated intracranial pressure, altered mental status is also a common presentation, particularly in older patients.
Case Report
A 73-year-old generally healthy, highly functioning man previously able to live independently presented to the emergency department (ED) with 3 weeks of progressive behavioral and cognitive symptoms. He initially exhibited dyscalculia, which progressed to recurrent episodes of confusion, repetitive questioning, and memory loss. He also developed hyperphagia and urinary incontinence. On presentation to the ED, he was disoriented to time with a subtle wide-based gait. A CT of the brain demonstrated cerebral ventriculomegaly out of proportion to age-expected atrophy (Figure 1, A–D) and was read as concerning for normal pressure hydrocephalus (NPH). The patient was therefore discharged from the ED with a plan to pursue MRI brain outpatient and to follow-up in the NPH clinic for further management.
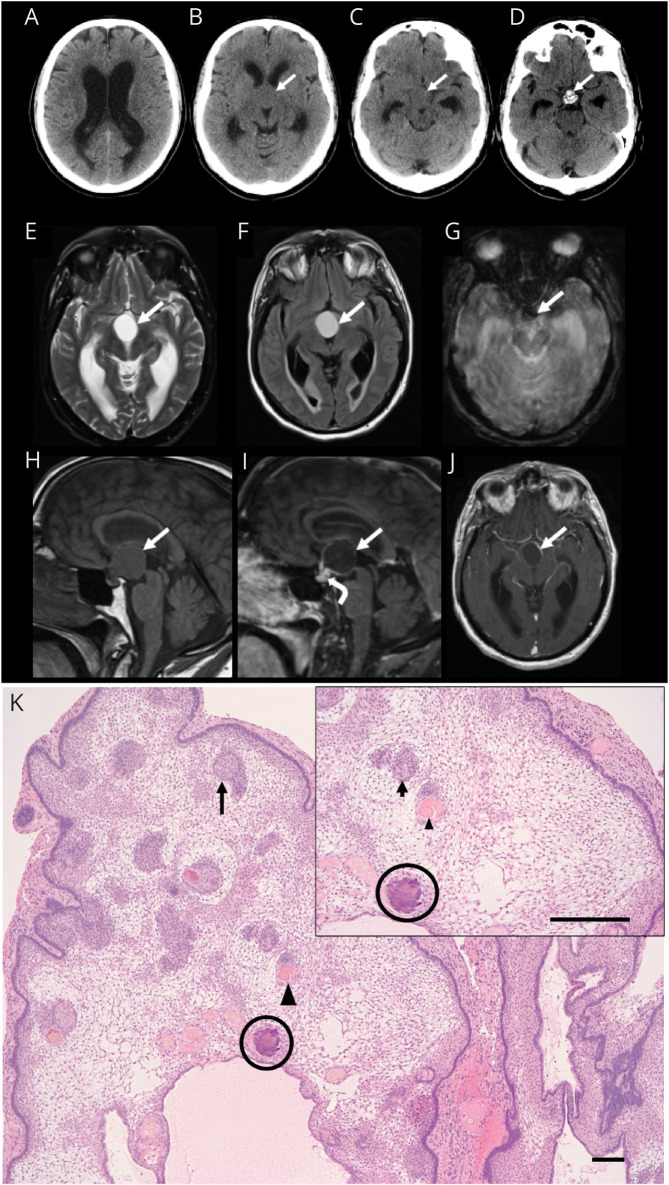
Figure 1. Neuroimaging and Histopathologic Findings of Adamantinomatous Craniopharyngioma.
Axial CT images demonstrating hydrocephalus with lateral ventricle dilatation (A) secondary to a suprasellar mass (arrow, B and C) with intrinsic calcification at its base anterior to the dorsum sella (arrow, D). MRI brain axial T2-weighted (E), fluid-attenuated inversion recovery (F), gradient-echo (G), and sagittal T1-weighted (H) images demonstrate a proteinaceous cyst in the suprasellar cistern with rim enhancement on sagittal (I) and axial (J) postcontrast T1-weighted images (solid arrows) and solid enhancement at the base of the lesion (curved arrow, I). Susceptibility artifact on the axial gradient-echo image (arrow, G) corresponds to the calcification at the base of the lesion. Paraffin-embedded, hematoxylin and eosin–stained section (K) reveals a multilobulated mass that is composed of palisading epithelium surrounding a loose stellate reticulum. Whorls of squamous epithelium (arrow), nodules of anucleate squames (arrowhead), and calcifications (circle) are present within the reticulum (scale bar 10 μm).
Four days later, he re-presented to the ED with further progression of his cognitive symptoms, imbalance, and gait disturbance. He denied any other neurologic symptoms, including visual disturbance, headache, nausea, or vomiting. His neurologic examination was notable for intact orientation, normal comprehension to simple commands, and intact naming; however, impaired attention and recall were noted on the Montreal Cognitive Assessment (MoCA) tool (scoring 20 of 30 points). A subtle hypophonia and mild bilateral action tremor without evidence of rigidity or bradykinesia were noted. His gait was wide based and slow. An MRI examination of the brain showed mass effect at the level of the anterior third ventricle and foramen of monro and a partially solid and predominantly cystic mass in the suprasellar region with features consistent with a craniopharyngioma (Figure 1, E–J). A retrospective review of the CT brain obtained on initial presentation demonstrated calcifications around the third ventricle (Figure 1D, arrow). He subsequently underwent a right pterional craniotomy for craniopharyngioma resection and placement of a right frontal extraventricular drain (EVD). Postoperative CT and MRI brain demonstrated decreased ventriculomegaly with near-total resection of the suprasellar lesion except for a small focus of enhancement at the inferior aspect of the resection cavity, consistent with minimal residual tumor. The final histopathologic findings were consistent with adamantinomatous craniopharyngioma subtype (Figure 1K).
His postoperative course was complicated by diabetes insipidus, requiring desmopressin (DDAVP) as needed. After improved hydrocephalus on postoperative CT scans, his EVD was clamped and removed. Subsequently, he exhibited drastic improvement in his cognitive function, scoring 27/30 on repeat MoCA testing. While morning cortisol, thyroid function test (TSH), and pituitary hormones were normal before his surgery, he had low levels of adrenocorticotropic hormone, cortisol, TSH, T3, and T4 postoperatively. Levothyroxine and scheduled hydrocortisone were started given concern for central hypopituitarism. He was discharged on postoperative day 10.
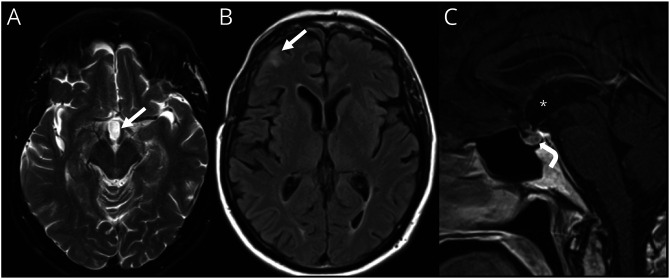
At 1-month follow-up, the patient exhibited further improvement in his cognition and resolution of the gait disturbance. However, he continued to have hyperphagia and complained of new insomnia. In addition, he was noted to have mild, patchy superior nasal visual field deficits. These deficits were attributed to bilateral optic neuropathies secondary to increased intracranial pressure (ICP) from obstructive hydrocephalus rather than optic chiasmal compression from the craniopharyngioma. He ultimately underwent gamma-knife stereotactic radiosurgery (dose 14 Gy) for the residual lesion. A repeat MRI of the brain 4 months after radiosurgery showed resolution of the previously noted postcontrast enhancement within the inferior resection cavity (Figure 2, A–C). He was continued on regular levothyroxine, hydrocortisone, transdermal testosterone, and as-needed desmopressin for management of hypopituitarism.
Figure 2. MRI Brain Postresection and Radiosurgery of the Adamantinomatous Craniopharyngioma.
Axial T2-weighted (A) and fluid-attenuated inversion recovery (B) MR brain images and sagittal postcontrast T1-weighted image (C) demonstrating postoperative changes after tumor resection and radiosurgery with resection cavity in suprasellar region (arrow, A; asterisk, C), gliosis in right frontal lobe beneath a craniotomy defect (arrow, B), resolution of hydrocephalus, and normal appearance to the pituitary gland (curved arrow, C).
Discussion
Craniopharyngiomas are low-grade, World Health Organization grade I, brain tumors arising from epithelial remnants of Rathke's pouch and can be either intrasellar or suprasellar in location.1 Two histologic subtypes of craniopharyngiomas predominate: the adamantinomatous and papillary subtypes.1 Each subtype is caused by different driver mutations. Alterations in the CTNNB1 gene, which encodes β-catenin, drive adamantinomatous craniopharyngioma tumorigenesis.1 Alterations in the CTNNB1 gene result in ineffective degradation of β-catenin, leading to its accumulation and overactivation of the WNT-β-catenin pathway.1 Alternatively, BRAFV600E alterations are the typical molecular feature of papillary craniopharyngiomas.1 The typical age of presentation varies between the 2 subtypes. The papillary subtype occurs primarily in adults aged 40–55 years. Meanwhile, the adamantinomatous subtype has a bimodal incidence: commonly presenting between 5 and 15 years or between 45 and 60 years of age.1
The most common presenting symptoms for craniopharyngioma across all age groups are headaches and visual disturbances, which are because of increased ICP from obstructive hydrocephalus and involvement of optic pathways, respectively.2,3 Other common presenting symptoms include ataxia/unsteadiness and symptoms related to hypothalamic-pituitary dysfunction, including sleep disturbance, weight dysregulation, and sexual dysfunction (such as decreased libido and amenorrhea).1,3 However, in older individuals, derangements in mental status are commonly observed.3,4
While the patient in this case report had evidence of obstructive hydrocephalus on neuroimaging, he did not present with typical symptoms of elevated ICP. Instead, he presented with progressive urinary incontinence, cognitive impairment, and gait disturbance (“wet, wacky, and wobbly”), which are pathognomonic for NPH. An MRI examination of the brain ultimately led to the identification of a midline craniopharyngioma as the cause of his obstructive hydrocephalus. In similar situations in which CT imaging findings are subtle or not easily identifiable, MRI can be useful in characterizing a possible underlying intracranial lesion. Retrospectively, the initial CT brain did demonstrate a suprasellar mass exerting mass effect on the foramen of monro with features characteristic of the adamantinomatous subtype of craniopharyngioma, specifically calcifications around the third ventricle (Figure 1D).1 In addition to coarse calcifications, the nonenhancing fluid-filled cyst with a thin rim of peripheral enhancement noted on this patient's MRI (Figure 1, E–J) is characteristic of adamantinomatous craniopharyngiomas.5 By contrast, the cystic component is typically hypointense on T-weighted imaging in the papillary subtype.3,5
The management of craniopharyngioma consists of surgical resection, radiotherapy, or a combination thereof. Gross total resection (GTR) is desired, but can be limited to preserve the hypothalamus, particularly because hypothalamic injury can decrease survival and affect quality of life.1,6 Adjunctive radiotherapy is used in cases where complete resection is not achieved. There is growing interest in targeted therapies directed against the molecular signaling pathways characteristic to each subtype of craniopharyngioma to avoid further hypothalamic injury in patients with tumor recurrence.1 For example, there are several reports of patients with the papillary subtype treated with BRAF or MEK inhibitors that have demonstrated significant tumor regression.7
Tumor recurrence rates differ between the 2 histopathologic subtypes, with the adamantinomatous subtype showing significantly higher rates of recurrence (estimated to be approximately 26%), compared with the papillary subtype (estimated to be approximately 14%).8-10 The difference in rate of recurrence between the 2 subtypes is likely secondary to differences in their molecular and morphologic features.6,8,10,11 Adamantinomatous craniopharyngiomas are larger in size and more adherent to adjacent brain structures, which may be due to their molecular characteristics and calcifications.6,12 By contrast, papillary craniopharyngiomas are typically well demarcated, which often results in successful GTR.13 In addition to recurrence risk, metabolic syndrome resulting from hypothalamic obesity and alterations in pituitary hormones likely affect long-term survival.1,2,11 Currently, there are no evidence-based guidelines regarding surveillance and monitoring for tumor recurrence posttreatment. MRI is typically performed annually initially with frequency of surveillance imaging dependent on the degree of residual tumor. Other aspects of care that require regular monitoring include evaluation of endocrine function to monitor hypopituitarism and regular vision assessments. Several studies have demonstrated impairments of memory, learning, processing speed, and executive function secondary to hypothalamic involvement and complications of treatment. Thus, repeated neuropsychological testing should be considered as clinically indicated.14
In the case presented in this report, craniopharyngioma with ensuing obstructive hydrocephalus was not identified on initial CT imaging, which led to management delay. While most patients with craniopharyngioma present with symptoms of elevated ICP,2,15 the patient in this case presented primarily with progressive cognitive disturbance. The patient also described hyperphagia and later sleep dysregulation, clues indicating hypothalamic dysfunction. Initial CT imaging demonstrated significant ventriculomegaly and calcifications in the solid portion of the tumor, a subtle finding that was not initially visualized. This case highlights the relevance of calcifications around the third ventricle on CT, a radiologic hallmark of the adamantinomatous subtype of craniopharyngioma.1 It also illustrates how brain MRI can be used to characterize intracranial lesions with subtle and not easily recognizable features on CT imaging. Finally, both the adamantinomatous subtype itself and presence of calcifications portend an increased risk of recurrence and worse prognosis8,9,15; thus, close monitoring and follow-up are important, with frequency dependent on subtype.
Appendix. Authors
| Name | Location | Contribution |
| Laura E. Schroeder, MD, PhD | Brown University, Providence, RI | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Michael Kritselis, DO | Brown University, Providence, RI | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Nupur Lala, MD | Brown University, Providence, RI | Drafting/revision of the article for content, including medical writing for content |
| Jerrold Boxerman, MD, PhD | Brown University, Providence, RI | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Saud Alhusaini, MD, PhD | Brown University, Providence, RI | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
Study Funding
No targeted funding reported.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Müller HL, Merchant TE, Warmuth-Metz M, Martinez-Barbera JP, Puget S. Craniopharyngioma. Nat Rev Dis Primers. 2019;5(1):75. doi: 10.1038/s41572-019-0125-9 [DOI] [PubMed] [Google Scholar]
- 2.Karavitaki N, Brufani C, Warner JT, et al. Craniopharyngiomas in children and adults: systematic analysis of 121 cases with long-term follow-up. Clin Endocrinol (Oxf). 2005;62(4):397-409. doi: 10.1111/j.1365-2265.2005.02231.x [DOI] [PubMed] [Google Scholar]
- 3.Karavitaki N, Cudlip S, Adams CBT, Wass JAH. Craniopharyngiomas. Endocr Rev. 2006;27(4):371-397. doi: 10.1210/er.2006-0002 [DOI] [PubMed] [Google Scholar]
- 4.Pascual JM, Prieto R, Castro-Dufourny I, et al. Craniopharyngiomas primarily involving the hypothalamus: a model of neurosurgical lesions to elucidate the neurobiological basis of psychiatric disorders. World Neurosurg. 2018;120:e1245-e1278. doi: 10.1016/j.wneu.2018.09.053 [DOI] [PubMed] [Google Scholar]
- 5.Sartoretti-Schefer S, Wichmann W, Aguzzi A, Valavanis A. MR differentiation of adamantinous and squamous-papillary craniopharyngiomas. AJNR Am J Neuroradiol. 1997;18(1):77-87. [PMC free article] [PubMed] [Google Scholar]
- 6.Prieto R, Pascual JM, Hofecker V, et al. Craniopharyngioma adherence: a reappraisal of the evidence. Neurosurg Rev. 2020;43(2):453-472. doi: 10.1007/s10143-018-1010-9 [DOI] [PubMed] [Google Scholar]
- 7.Juratli TA, Jones PS, Wang N, et al. Targeted treatment of papillary craniopharyngiomas harboring BRAF V600E mutations. Cancer. 2019;125(17):2910-2914. doi: 10.1002/cncr.32197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tavangar SM, Larijani B, Mahta A, Hosseini SMA, Mehrazine M, Bandarian F. Craniopharyngioma: a clinicopathological study of 141 cases. Endocr Pathol. 2004;15(4):339-344. doi: 10.1385/ep:15:4:339 [DOI] [PubMed] [Google Scholar]
- 9.Adamson TE, Wiestler OD, Kleihues P, Yaşargil MG. Correlation of clinical and pathological features in surgically treated craniopharyngiomas. J Neurosurg. 1990;73(1):12-17. doi: 10.3171/jns.1990.73.1.0012 [DOI] [PubMed] [Google Scholar]
- 10.Wu J, Wu X, Yang YQ, et al. Association of histological subtype with risk of recurrence in craniopharyngioma patients: a systematic review and meta-analysis. Neurosurg Rev. 2022;45(1):139-150. doi: 10.1007/s10143-021-01563-9 [DOI] [PubMed] [Google Scholar]
- 11.Pereira AM, Schmid EM, Schutte PJ, et al. High prevalence of long-term cardiovascular, neurological and psychosocial morbidity after treatment for craniopharyngioma. Clin Endocrinol (Oxf). 2005;62(2):197-204. doi: 10.1111/j.1365-2265.2004.02196.x [DOI] [PubMed] [Google Scholar]
- 12.Cheng J, Shao Q, Pan Z, You J. Analysis and long-term follow-up of the surgical treatment of children with craniopharyngioma. J Craniofac Surg. 2016;27(8):e763-e766. doi: 10.1097/scs.0000000000003176 [DOI] [PubMed] [Google Scholar]
- 13.Stache C, Hölsken A, Fahlbusch R, et al. Tight junction protein claudin-1 is differentially expressed in craniopharyngioma subtypes and indicates invasive tumor growth. Neuro Oncol. 2014;16(2):256-264. doi: 10.1093/neuonc/not195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Özyurt J, Müller HL, Thiel CM. A systematic review of cognitive performance in patients with childhood craniopharyngioma. J Neurooncol. 2015;125(1):9-21. doi: 10.1007/s11060-015-1885-z [DOI] [PubMed] [Google Scholar]
- 15.Iglesias P, Nocete I, Moure Rodríguez MD, et al. Craniopharyngioma in the elderly: a multicenter and nationwide study in Spain. Neuroendocrinology. 2021;111(10):925-936. doi: 10.1159/000512161 [DOI] [PubMed] [Google Scholar]