Abstract
Background and Objectives
Guillain-Barré syndrome (GBS) has been inconsistently associated with some coronavirus disease 2019 (COVID-19) vaccines. We aimed to quantify the risk of GBS according to the type of COVID-19 vaccine in a large population.
Methods
Using the French National Health Data System linked to the COVID-19 vaccine database, we analyzed all individuals aged 12 years or older admitted for GBS from December 27, 2020, to May 20, 2022. We estimated the relative incidence (RI) of GBS within 1–42 days after vaccination up to the first booster dose compared with baseline periods using a self-controlled case series design. We then derived the number of cases attributable to the vaccination. Analyses were adjusted for the period and stratified by age group, sex, and for the presence of severe acute respiratory syndrome coronavirus 2 or common acute infections.
Results
Of 58,530,770 people aged 12 years or older, 88.8% received at least 1 COVID-19 vaccine dose and 2,229 were hospitalized for GBS during the study period. Patients had a median age of 57 years, and 60% were male patients. The RI of GBS between 1–42 days was 2.5 (95% CI 1.8–3.6) for the first dose of ChAdOx1-S and 2.4 (95% CI 1.2–5.0) for the unique dose of Ad26.COV2.S vaccine. We estimated 6.5 attributable GBS cases per million persons having received a first dose of ChAdOx1-S and 5.7 cases per million for the Ad26.COV2.S vaccine. Except for the age group of 12–49 years after the second dose of the messenger RNA (mRNA)-1273 vaccine (RI 2.6, 95% CI 1.2–5.5), none of the RI estimates were found significantly increased for the mRNA vaccines.
Discussion
In summary, we found increased risks of GBS after the first administration of ChAdOx1-S and Ad26.COV2.S vaccines. In this comprehensive assessment at the French population level, there was no statistically significant increase in the risk of GBS after the administration of mRNA vaccines. This is reassuring in the context of the ongoing and future use of mRNA-based booster vaccination.
Introduction
Guillain-Barré syndrome (GBS) is a rare autoimmune disorder causing muscle weakness and paralysis that can potentially result in severe neurologic disabilities, respiratory failure, and death.1,2 Symptom onset is frequently preceded by an infectious illness in prior weeks. Known triggers include common respiratory and gastrointestinal infections, Zika virus infection,3 and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.4-6
An antigenic challenge caused by vaccination can also increase the risk of GBS, as revealed with the 1976 H1N1 influenza vaccine.7 After the emergence of reports of GBS in association with COVID-19 vaccination, the European Medicines Agency recommended adding a warning to the labeling of the ChAdOx1-S (Oxford-AstraZeneca)8 and Ad.26.COV2.S (Janssen/Johnson & Johnson) vaccines.9
Several reports from postmarketing surveillance and observational studies seem to confirm a slightly increased risk of GBS after the adenovirus-based vaccines, ChAdOx1-S and Ad.26.COV2.S.10-14 Conversely, those reports indicate no association with messenger RNA-based vaccines, BNT162b2 (Pfizer-BioNTech) and messenger RNA (mRNA)-1273 (Moderna). However, a few studies did not reach the same conclusions. In a study population of 8.3 million vaccinated people from the United Kingdom and Spain, no association was found with any of the 4 vaccines.15 Another study involving 16 million vaccinated people in Italy found an increased risk after the mRNA-1273 and ChAdOx1-S vaccines, but no association with the Ad.26.COV2.S vaccine.16
Although the reported absolute risks seem to be small, they could lead in the context of mass vaccination to substantial numbers of excess cases and in turn of potential severe complications. The COVID-19 vaccination program in France began on December 27, 2020, and as of May 20, 2022, 139 million doses of 4 vaccines (BNT162b2: 107 million, mRNA-1273: 23, ChAdOx1-S: 7.7, and Ad26.COV2.S: 1.0) were administered. In this study, we used the French National Health Data System (SNDS) which gathers details of hospital admissions, drug dispensing, COVID-19 vaccination, and SARS-CoV-2 testing at the individual level, with nationwide coverage. We estimated the relative incidence (RI) of GBS in the risk period after vaccination compared with baseline periods using a self-controlled case series design. We then derived the number of excess cases attributable to each vaccine.
Methods
Data Sources
Data were retrieved from the National Health Data System (SNDS), which provides comprehensive health care claims and hospitalization data for 99% of the French population (67 million inhabitants).17,18 Administrative and medical data on hospital stay were obtained from the French Hospital Discharge Database19 and linked at the individual level with the nationwide databases for COVID-19 vaccination (VAC-SI), SARS-CoV-2 diagnostic testing (SI-DEP), and drug prescription (DCIR).
Standard Protocol Approvals, Registrations, and Patient Consents
Our research group (EPI-PHARE) has permanent regulatory access to the SNDS, in application of the French Decree No. 2016-1871 of December 26, 2016, the French law articles Art. R. 1461-13 and R. 1461-14 from the French Public Health Code, and the French Data Protection Authority (Commission Nationale de l'Informatique et des Libertés [CNIL]) decision CNIL-2016-316. All data of the SNDS are pseudonymised. Written informed consent from the patients was not required under French regulations. This study was declared before initiation on the EPI-PHARE registry of studies under reference EP-0312.
Study Population
The study population included all individuals 12 years or older with a GBS recorded as main or related diagnosis at hospital discharge between December 27, 2020, and May 20, 2022. We identified the syndrome based on the International Classification of Diseases, 10th Revision, code G61.0. The index date was defined as the admission date of the inpatient care where GBS was first diagnosed within the study period.
Exposure and Covariates
The risk period was taken to be 1–42 days after the receipt of a vaccine dose. This 6-week interval is consensually used as the period when the risk of GBS is present after vaccine receipt.7 For a finer characterization of the risk, we also considered the period subdivided into three 14-day intervals (i.e., 1–14, 15–28, and 29–42 days). Information on vaccinations was reported by all COVID-19 vaccine providers and included details on vaccine brand, the number of doses administered, and the date of each injection for all people vaccinated in France.
We characterized SARS-CoV-2 infection from a hospital admission for COVID-19 or a positive SARS-CoV-2 test, itself defined by a positive PCR or antigenic testing. Common respiratory and gastrointestinal infection episodes were identified by the prescription of specific drugs: respiratory tract antibacterials, drugs for obstructive airway diseases, cough and cold preparations, antidiarrheals, and intestinal anti-infective agents, as in a previous study.20
Statistical Analyses
We undertook a self-controlled case series study (SCCS) to assess among the cases of GBS if the diagnosis occurred preferentially in the defined risk period after a vaccine injection.21 The SCCS design offers the benefit of excluding potential bias owing to the fact that vaccinated individuals may differ from unvaccinated and of controlling for time-invariant confounders at the individual level, such as comorbidities or genetic susceptibility.
Given that the outcome of interest (GBS) could affect a subsequent exposure (COVID-19 vaccination), the standard SCCS method could not be used and an extension for event-dependent exposure was used.22,23
For each combination of vaccine and dose, we estimated the incidence of GBS during the 6-week risk period relative to the unexposed period. We accounted for the second booster dose in the model to properly define the unexposed period, but owing to its limited use over the study period, estimates of RI were obtained up to the first booster (third dose). In addition, we evaluated the RI for three 14-day intervals that subdivide the initial risk period. The model included a time-varying variable controlling for the background rate of GBS, regardless of vaccination.
In separate models, we also evaluated the risk of GBS in the 42 days after SARS-CoV-2 infection or common acute infections. We performed stratified analyses by age group (12–49 years vs 50 years or older) and sex. We corrected the estimates for multiple testing using the method of Benjamini and Hochberg,24 setting the false discovery rate (FDR) at 5%. Among the set of estimates of RI for each subgroup analysis, we thus considered a change in incidence as statistically significant where the null hypothesis (i.e., no change) remained rejected after applying the FDR controlling procedure.
To contextualize the risk, we calculated the attributable risk (AR) as the number of events attributable to the vaccine per million people exposed, under the assumption that significantly positive associations denote a causal relation.21 With RI the relative incidence, nr the number of events in the risk period, and ne the number of exposed subjects within the population (in millions), the AR was obtained by:
 |
Note that the first fraction of the right term in Equation (1) corresponds to the attributable fraction, that is, the proportion of events arising within the risk period that may be attributed to the vaccine exposure.
We assessed the robustness of the results by replicating the analyses on several subsets of the population. First, to distinguish genuine incident events from potentially recurrent events triggered by a new exposure, we restricted the study sample to case patients without a history of GBS in the preceding 2 years. Second, to exclude any known risk factor other than vaccine exposure, we analyzed a subset of patients without respiratory or gastrointestinal infection and a subset of patients without SARS-CoV-2 infection within 42 days before the GBS onset. Third, to make sure that a potential remnant effect of a particular vaccine exposure did not affect the measure of association with another subsequent vaccine, we selected patients having received only 1 type of vaccine as part of the primary vaccination (homologous schemes).
Data collection used SAS Enterprise Guide version 4.3 software (SAS Institute, Cary, NC). All analyses were performed using R software version 4.2.1 and SCCS package version 1.6.25,26
Data Availability
According to data protection and the French regulation, the authors cannot publicly release the data from the SNDS. However, any person or structure, public or private and for-profit or nonprofit, is able to access SNDS data on authorization from the French Data Protection Office (CNIL) to perform a study, a research, or an evaluation of public interest (snds.gouv.fr/SNDS/Processus-d-acces-auxdonnees and indsante.fr/).
Results
Characteristics of the Study Population
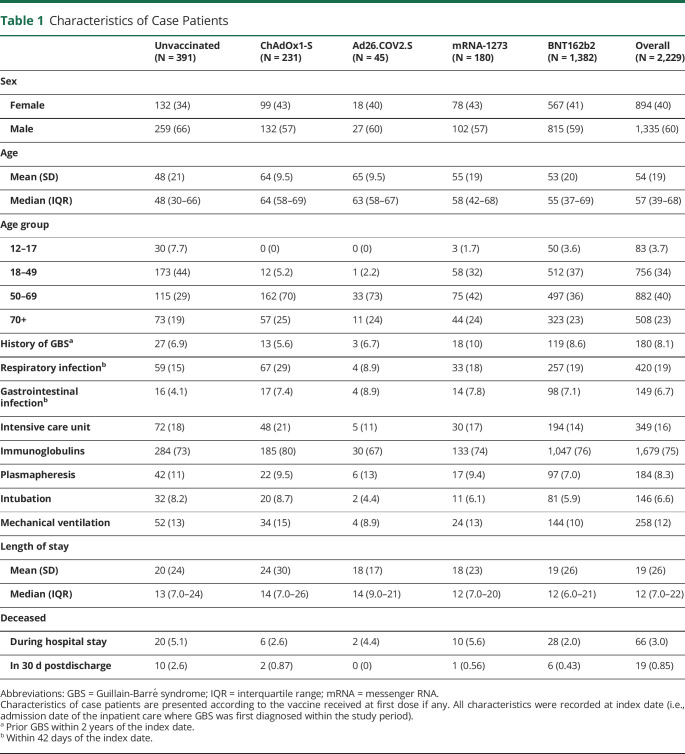
A total of 2,229 people were hospitalized for a GBS during the study period. Of these, 1,838 (82%) received at least 1 vaccine dose. The characteristics of case patients are presented in Table 1 according to the vaccine they received as a first dose, if any. The overall study population had a median age of 57 years and comprised 60% male patients. The median length of stay at hospital for these patients was 12 days, 16% were admitted to an intensive care unit, 6.6% required intubation, and 12% required mechanical ventilation. Immunoglobulins were given to 75%, and 8.3% underwent plasma exchange. A total of 85 patients died of any cause during their hospital stay (3%) or within 30 days after discharge (0.9%).
Table 1.
Characteristics of Case Patients
| Unvaccinated (N = 391) | ChAdOx1-S (N = 231) | Ad26.COV2.S (N = 45) | mRNA-1273 (N = 180) | BNT162b2 (N = 1,382) | Overall (N = 2,229) | |
| Sex | ||||||
| Female | 132 (34) | 99 (43) | 18 (40) | 78 (43) | 567 (41) | 894 (40) |
| Male | 259 (66) | 132 (57) | 27 (60) | 102 (57) | 815 (59) | 1,335 (60) |
| Age | ||||||
| Mean (SD) | 48 (21) | 64 (9.5) | 65 (9.5) | 55 (19) | 53 (20) | 54 (19) |
| Median (IQR) | 48 (30–66) | 64 (58–69) | 63 (58–67) | 58 (42–68) | 55 (37–69) | 57 (39–68) |
| Age group | ||||||
| 12–17 | 30 (7.7) | 0 (0) | 0 (0) | 3 (1.7) | 50 (3.6) | 83 (3.7) |
| 18–49 | 173 (44) | 12 (5.2) | 1 (2.2) | 58 (32) | 512 (37) | 756 (34) |
| 50–69 | 115 (29) | 162 (70) | 33 (73) | 75 (42) | 497 (36) | 882 (40) |
| 70+ | 73 (19) | 57 (25) | 11 (24) | 44 (24) | 323 (23) | 508 (23) |
| History of GBSa | 27 (6.9) | 13 (5.6) | 3 (6.7) | 18 (10) | 119 (8.6) | 180 (8.1) |
| Respiratory infectionb | 59 (15) | 67 (29) | 4 (8.9) | 33 (18) | 257 (19) | 420 (19) |
| Gastrointestinal infectionb | 16 (4.1) | 17 (7.4) | 4 (8.9) | 14 (7.8) | 98 (7.1) | 149 (6.7) |
| Intensive care unit | 72 (18) | 48 (21) | 5 (11) | 30 (17) | 194 (14) | 349 (16) |
| Immunoglobulins | 284 (73) | 185 (80) | 30 (67) | 133 (74) | 1,047 (76) | 1,679 (75) |
| Plasmapheresis | 42 (11) | 22 (9.5) | 6 (13) | 17 (9.4) | 97 (7.0) | 184 (8.3) |
| Intubation | 32 (8.2) | 20 (8.7) | 2 (4.4) | 11 (6.1) | 81 (5.9) | 146 (6.6) |
| Mechanical ventilation | 52 (13) | 34 (15) | 4 (8.9) | 24 (13) | 144 (10) | 258 (12) |
| Length of stay | ||||||
| Mean (SD) | 20 (24) | 24 (30) | 18 (17) | 18 (23) | 19 (26) | 19 (26) |
| Median (IQR) | 13 (7.0–24) | 14 (7.0–26) | 14 (9.0–21) | 12 (7.0–20) | 12 (6.0–21) | 12 (7.0–22) |
| Deceased | ||||||
| During hospital stay | 20 (5.1) | 6 (2.6) | 2 (4.4) | 10 (5.6) | 28 (2.0) | 66 (3.0) |
| In 30 d postdischarge | 10 (2.6) | 2 (0.87) | 0 (0) | 1 (0.56) | 6 (0.43) | 19 (0.85) |
Abbreviations: GBS = Guillain-Barré syndrome; IQR = interquartile range; mRNA = messenger RNA.
Characteristics of case patients are presented according to the vaccine received at first dose if any. All characteristics were recorded at index date (i.e., admission date of the inpatient care where GBS was first diagnosed within the study period).
Prior GBS within 2 years of the index date.
Within 42 days of the index date.
Risk of GBS Associated With Exposure
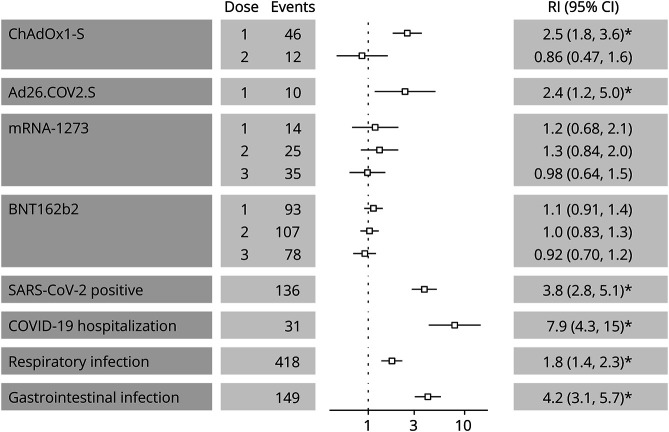
The risk of GBS within 42 days after either infection events or vaccine exposures is presented in Figure 1. We observed an increased risk of GBS within 42 days after either a positive SARS-CoV-2 test with RI of 3.8 (95% CI 2.8–5.1), a hospitalization for COVID-19 (RI 7.9, 95% CI 4.3–15), a respiratory infection (RI 1.8, 95% CI 1.4–2.3), or a gastrointestinal infection (RI 4.2, 95% CI 3.1–5.7). The RI for days 1–42 after vaccine exposure was 2.5 (95% CI 1.8–3.6) for the first dose of ChAdOx1-S and 2.4 (95% CI 1.2–5.0) for the unique dose of Ad26.COV2.S vaccine. There was no significant increase in GBS incidence after the second dose of the ChAdOx1-S vaccine nor after any dose of the BNT162b2 or mRNA-1273 vaccines.
Figure 1. Relative Incidence of Guillain-Barré Syndrome in 1–42 Days of Exposure.
The leftmost column presents separate model results for the 4 studied vaccine exposures and for exposures to SARS-CoV-2 infection or common acute infections. Number of events and estimates of relative incidence (95% CI) are presented for a risk period of 42 days following each exposure. The vertical dotted line represents a relative incidence of 1. The asterisk (*) indicates a statistically significant estimate of relative incidence after applying the FDR controlling procedure. COVID-19 = coronavirus disease 2019; mRNA = messenger RNA; RI = relative incidence; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
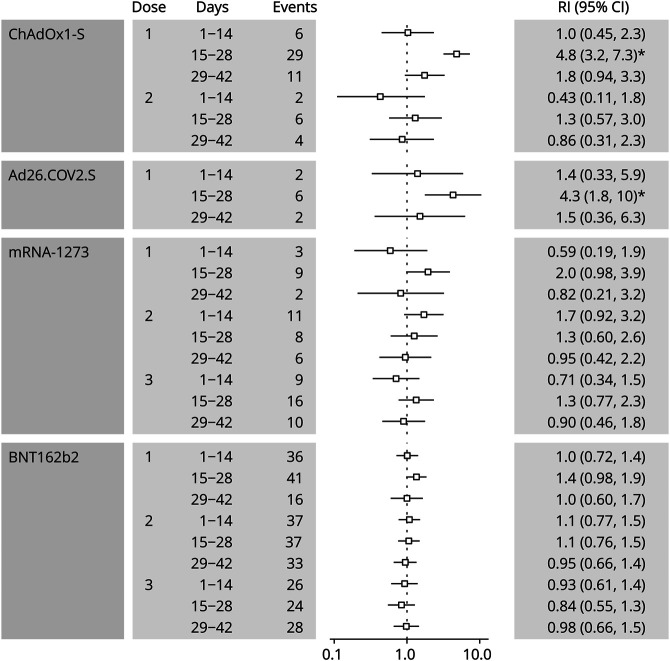
Analyses for the postvaccine risk period subdivided into 14-day intervals are presented in Figure 2. A significantly increased incidence was evident within 15–28 days after the first doses of the ChAdOx1-S (RI 4.8, 95% CI 3.2–7.3) and the Ad26.COV2.S vaccine (RI 4.3, 95% CI 1.8–10). We found RIs of 2.0 (95% CI 0.98–3.9) between days 15 and 28 after the first dose of mRNA-1273 and 1.4 (95% CI 0.98–1.9) between days 15 and 28 after the first dose of BNT162b2, although none of the estimates for mRNA vaccines reached statistical significance. Incidence of GBS also did not vary significantly across the 3 intervals after the second dose of the ChAdOx1-S vaccine.
Figure 2. Relative Incidence of Guillain-Barré Syndrome by 14-Day Interval After Vaccine Exposure.
Number of events and estimates of relative incidence (95% CI) are presented for three 14-day intervals dividing the risk period of 42 days following a vaccine dose. The vertical dotted line represents a RI of 1. The asterisk (*) indicates a statistically significant estimate of relative incidence after applying the FDR controlling procedure. RI = relative incidence.
Subgroup Estimates by Sex and Age Class
The results of subgroup analyses by age group and sex are presented in eTables 1 and 2 (links.lww.com/WNL/D139), respectively. An increased GBS incidence within 42 days after the first dose of the ChAdOx1-S vaccine is evident both in the 12–49 years and 50 years or older age groups. The increased risk after the Ad26.COV2.S vaccine is only observed in individuals aged 50 years or older (of note, this vaccine happened to be only recommended from age 55 years). In individuals younger than 50 years, the second dose of the mRNA-1273 vaccine was significantly associated with increased incidence of GBS (RI 2.6, 95% CI 1.2–5.5).
We observed an increased incidence after the first dose of the ChAdOx1-S vaccine in both male (RI 1.9, 95% CI 1.2–3.2) and female (RI 3.6, 95% CI 2.2–5.8) individuals. The risk after the Ad26.COV2.S vaccine was significantly increased in women (RI 4.0, 95% CI 1.4–11) but not among men (RI 1.6, 95% CI 0.53–4.6). There was no evidence of association between GBS and the mRNA-based vaccines in the analysis stratified by sex.
Attributable Risk
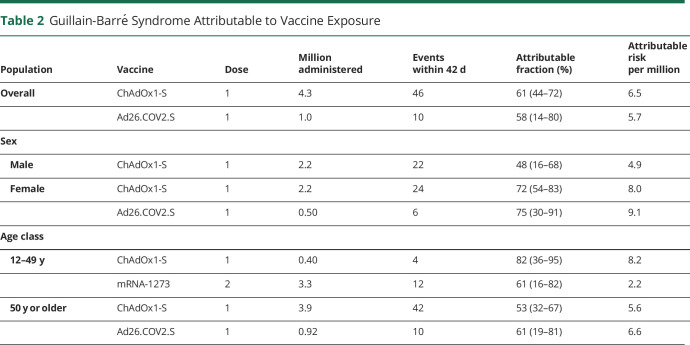
For statistically significant associations, we estimated the number of events attributable to vaccine exposure (Table 2). The fraction of GBS cases attributable to vaccination was estimated at 61% (95% CI 44–72) for the ChAdOx1-S vaccine and 58% (95% CI 14–80) for the Ad26.COV2.S vaccine in people aged 12 years or older and 61% (95% CI 16–82) for the mRNA-1273 vaccine in the age group of 12–49 years. This corresponds to an AR of 6.5 and 5.7 GBS cases per million persons having received a first dose of ChAdOx1-S or the Ad26.COV2.S vaccine, respectively. In people aged 12–49 years receiving a second dose of the mRNA-1273 vaccine, this translates to 2.2 events per million.
Table 2.
Guillain-Barré Syndrome Attributable to Vaccine Exposure
| Population | Vaccine | Dose | Million administered | Events within 42 d | Attributable fraction (%) | Attributable risk per million |
| Overall | ChAdOx1-S | 1 | 4.3 | 46 | 61 (44–72) | 6.5 |
| Ad26.COV2.S | 1 | 1.0 | 10 | 58 (14–80) | 5.7 | |
| Sex | ||||||
| Male | ChAdOx1-S | 1 | 2.2 | 22 | 48 (16–68) | 4.9 |
| Female | ChAdOx1-S | 1 | 2.2 | 24 | 72 (54–83) | 8.0 |
| Ad26.COV2.S | 1 | 0.50 | 6 | 75 (30–91) | 9.1 | |
| Age class | ||||||
| 12–49 y | ChAdOx1-S | 1 | 0.40 | 4 | 82 (36–95) | 8.2 |
| mRNA-1273 | 2 | 3.3 | 12 | 61 (16–82) | 2.2 | |
| 50 y or older | ChAdOx1-S | 1 | 3.9 | 42 | 53 (32–67) | 5.6 |
| Ad26.COV2.S | 1 | 0.92 | 10 | 61 (19–81) | 6.6 |
The results for the sensitivity analyses are presented in eTable 3 (links.lww.com/WNL/D139). Overall, findings of the main analysis were not altered when restricting the population to patients without a history of GBS in past 2 years nor to patients without SARS-CoV-2 infection in the 42 days before the GBS or to patients with a homologous scheme for the primary vaccination.
Discussion
In this nationwide study involving a 58.5 million population aged 12 years or older having received 139 million doses of COVID-19 vaccines, we provide estimates of the risk of GBS by vaccine type.
The risk of GBS within 42 days after the administration of the first dose of ChAdOx1-S vaccine or of the Ad26.COV2.S vaccine was significantly increased, with incidence spiking between 14 and 28 days after receipt. We estimate that each adenovirus-based vaccine caused approximately 6 excess cases of GBS per million persons. The elevated risk was consistently found in people free of SARS-CoV-2 or common acute infections, across sex and in different age categories. Besides, we find the highest relative risks of GBS after an infective illness and particularly among patient hospitalized for COVID-19.
These results add to the existing evidence suggestive of an association between the adenovirus-based COVID-19 vaccines and development of GBS. Although risk periods and metrics used may differ, our estimated population rates of vaccine-associated GBS are consistent with those reported in countries where these vaccines were largely rolled out. ARs for the ChAdOx1-S vaccine in the United Kingdom ranged from 3.8 to 11 cases per million people,12,13,27 and population rates (either reporting or incidence rates) with respect to the Ad26.COV2.S vaccine used in the United States ranged from 4.1 to 19 per million.10,11,14
Despite a much larger uptake in the population of mRNA-based vaccines (eTable 4, links.lww.com/WNL/D139), none of the RI estimates were found significantly increased for these vaccines, except for the 12–49 years age group after the second dose of mRNA-1273 and for a smaller AR than for adenovirus vaccines. Our overall findings considered along those of several other published reports do not support an increased risk of GBS after the primary vaccination with mRNA vaccines.10-13,15 In addition, we observed no increased risk after the booster vaccination with these vaccines.
Several characteristics of this study provide strength of evidence to our conclusions. First, to our knowledge, this study includes the largest number of individuals who developed a GBS while vaccinated for COVID-19. The large sample size comes with a long study period and the possibility of investigating both the primary and first booster vaccination with the mRNA-based vaccines. The reassuring findings regarding these vaccines used for booster have an important public health implication for the future vaccination campaigns.
Second, the assessment of cases and details of the 4 vaccine exposures were prospectively recorded, independently of the outcome and obtained from high-quality and comprehensive databases. As in previous studies using the same data sources, the observed characteristics of inpatient care in our sample, notably length of stay, intensive procedures, and therapies, were typical of GBS.20,28,29
Third, the self-controlled design allowed to control for time-invariant individual-level confounders, including unmeasured ones. We also adjusted for the period to account for changes in the baseline incidence of GBS. Last, the sensitivity analyses did not alter the main findings, lending support to the effect of adenovirus-based vaccines and at-most moderate association with the first administration of mRNA-based vaccines.
Our study has several limitations. First, cases included in this study were identified solely among hospitalized individuals. Although the severity of GBS most commonly requires intensive management, the number of cases after vaccination could be underestimated by discounting mild presentations that did not require hospitalization. However, this would bias the results only if the association between vaccination and GBS were substantially different according to the severity of symptoms, for example, if cases related to vaccination had a different probability of hospitalization. Furthermore, we observe an incidence of GBS (as shown monthly for the prepandemic period and our study period in the eFigure 1, links.lww.com/WNL/D139) in the upper range of age-standardized incidence for Western countries,30 making under diagnosis unlikely.
Second, we defined the history and recent episodes of gastrointestinal or respiratory infections from indirect treatment information. Although we found an expected level of association between those infections and the development of GBS,29 we might have underestimated or overestimated the presence of infection in assessing the association with vaccination. Similarly, we probably missed SARS-CoV-2 infections that were not diagnosed or only detected by at-home test without laboratory confirmation. However, the potential confounding effect of undocumented infections should not be important as the removal of observed infections, acute seasonal infections or SARS-CoV-2, did not change the findings.
In interpreting our conclusions, two important points should be noted. First, there was no evidence of an increased risk of GBS after the second or third dose of the vaccines. The RI of 1.7 in first 2 weeks after dose 2 of the mRNA-1273 vaccine might be due to a remnant effect of dose 1—the recommended 3 weeks delay between first 2 injections causing the greatest overlap of respective risk periods. We did not analyze the possible recurrence of GBS in the study period; therefore, our results indicate that the first event is only likely to be triggered by the first shot.
Second, we focused solely on the potential adverse event of developing a GBS after vaccines and did not address the benefit of vaccination at adverting SARS-CoV-2 infection and hospitalization. As already reported, we show a substantial risk of developing GBS after hospitalization for COVID-19 and after a mere positive test (with or without symptoms).6 Our risk estimates should be considered relative to the cases avoided by vaccination, which could outweigh the detrimental effect of the vaccines.
In summary, we found increased risks of GBS after the first administration of ChAdOx1-S and Ad26.COV2.S vaccines. The rate of excess cases attributable to these vaccines, assuming a causal effect, was in the order of 6 cases per million persons. In this comprehensive assessment at the French population level, there was no statistically significant increase in the risk of GBS after the administration of mRNA vaccines. This is reassuring in the context of the ongoing and future use of mRNA-based booster vaccination.
Glossary
- AR
attributable risk
- CNIL
Commission Nationale de l'Informatique et des Libertés
- COVID-19
coronavirus disease 2019
- FDR
false discovery rate
- GBS
Guillain-Barré syndrome
- mRNA
messenger RNA
- RI
relative incidence
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SCCS
self-controlled case series study
- SNDS
French National Health Data System
Appendix. Authors
| Name | Location | Contribution |
| Stéphane Le Vu, PharmD, PhD | EPI-PHARE Scientific Interest Group in Epidemiology of Health Products (French National Agency for the Safety of Medicines and Health Products [ANSM], French National Health Insurance [CNAM]), Saint-Denis, France | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Marion Bertrand, MSc | EPI-PHARE Scientific Interest Group in Epidemiology of Health Products (French National Agency for the Safety of Medicines and Health Products [ANSM], French National Health Insurance [CNAM]), Saint-Denis, France | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Jérémie Botton, PharmD, PhD | EPI-PHARE Scientific Interest Group in Epidemiology of Health Products (French National Agency for the Safety of Medicines and Health Products [ANSM], French National Health Insurance [CNAM]), Saint-Denis; Université Paris-Saclay, Faculté de Pharmacie, Orsay, France | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Marie-Joelle Jabagi, PharmD, PhD | EPI-PHARE Scientific Interest Group in Epidemiology of Health Products (French National Agency for the Safety of Medicines and Health Products [ANSM], French National Health Insurance [CNAM]), Saint-Denis, France | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Jérôme Drouin, MSc | EPI-PHARE Scientific Interest Group in Epidemiology of Health Products (French National Agency for the Safety of Medicines and Health Products [ANSM], French National Health Insurance [CNAM]), Saint-Denis, France | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Laura Semenzato, MSc | EPI-PHARE Scientific Interest Group in Epidemiology of Health Products (French National Agency for the Safety of Medicines and Health Products [ANSM], French National Health Insurance [CNAM]), Saint-Denis, France | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Alain Weill, MD | EPI-PHARE Scientific Interest Group in Epidemiology of Health Products (French National Agency for the Safety of Medicines and Health Products [ANSM], French National Health Insurance [CNAM]), Saint-Denis, France | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Rosemary Dray-Spira, MD, PhD | EPI-PHARE Scientific Interest Group in Epidemiology of Health Products (French National Agency for the Safety of Medicines and Health Products [ANSM], French National Health Insurance [CNAM]), Saint-Denis, France | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Mahmoud Zureik, MD, PhD | EPI-PHARE Scientific Interest Group in Epidemiology of Health Products (French National Agency for the Safety of Medicines and Health Products [ANSM], French National Health Insurance [CNAM]), Saint-Denis; University Paris-Saclay, UVSQ, University Paris-Sud, Inserm, Anti-Infective Evasion and Pharmacoepidemiology, CESP, Montigny le Bretonneux, France | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
Footnotes
CME Course: NPub.org/cmelist
Study Funding
No targeted funding reported.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Shahrizaila N, Lehmann HC, Kuwabara S. Guillain-Barré syndrome. Lancet. 2021;397(10280):1214-1228. doi: 10.1016/S0140-6736(21)00517-1 [DOI] [PubMed] [Google Scholar]
- 2.Yuki N, Hartung HP. Guillain–Barré syndrome. N Engl J Med. 2012;366(24):2294-2304. doi: 10.1056/NEJMra1114525 [DOI] [PubMed] [Google Scholar]
- 3.Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387(10027):1531-1539. doi: 10.1016/S0140-6736(16)00562-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toscano G, Palmerini F, Ravaglia S, et al. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382(26):2574-2576. doi: 10.1056/NEJMc2009191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trujillo Gittermann LM, Valenzuela Feris SN, von Oetinger Giacoman A. Relation between COVID-19 and Guillain-Barré syndrome in adults: a systematic review. Neurol Engl Ed. 2020;35(9):646-654. doi: 10.1016/j.nrleng.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu E, Xie Y, Al-Aly Z. Long-term neurologic outcomes of COVID-19. Nat Med. 2022;28(11):2406-2415. doi: 10.1038/s41591-022-02001-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safranek TJ, Lawrence DN, Kurland LT, et al. Reassessment of the association between Guillain-Barré syndrome and receipt of swine influenza vaccine in 1976-1977: results of a two-state study. Expert Neurology Group. Am J Epidemiol. 1991;133(9):940-951. doi: 10.1093/oxfordjournals.aje.a115973 [DOI] [PubMed] [Google Scholar]
- 8.EMA. Meeting Highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 29 November–2 December 2021. European Medicines Agency. Published December 3, 2021. Accessed February 4, 2022. ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-29-november-2-december-2021. [Google Scholar]
- 9.EMA. COVID-19 Vaccine Janssen: Guillain-Barré Syndrome Listed as a Very Rare Side Effect. European Medicines Agency. Published July 22, 2021. Accessed January 26, 2023. ema.europa.eu/en/news/covid-19-vaccine-janssen-guillain-barre-syndrome-listed-very-rare-side-effect. [Google Scholar]
- 10.Abara WE, Gee J, Marquez P, et al. Reports of Guillain-Barré syndrome after COVID-19 vaccination in the United States. JAMA Netw Open. 2023;6(2):e2253845. doi: 10.1001/jamanetworkopen.2022.53845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanson KE, Goddard K, Lewis N, et al. Incidence of Guillain-Barré syndrome after COVID-19 vaccination in the Vaccine Safety Datalink. JAMA Netw Open. 2022;5(4):e228879. doi: 10.1001/jamanetworkopen.2022.8879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patone M, Handunnetthi L, Saatci D, et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med. 2021;27(12):2144-2153. doi: 10.1038/s41591-021-01556-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker JL, Schultze A, Tazare J, et al. Safety of COVID-19 vaccination and acute neurological events: a self-controlled case series in England using the OpenSAFELY platform. Vaccine. 2022;40(32):4479-4487. doi: 10.1016/j.vaccine.2022.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woo EJ, Mba-Jonas A, Dimova RB, Alimchandani M, Zinderman CE, Nair N. Association of receipt of the Ad26.COV2.S COVID-19 vaccine with presumptive Guillain-Barré syndrome, February-July 2021. JAMA. 2021;326(16):1606-1613. doi: 10.1001/jama.2021.16496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Raventós B, Roel E, et al. Association between COVID-19 vaccination, SARS-CoV-2 infection, and risk of immune mediated neurological events: population based cohort and self-controlled case series analysis. BMJ. 2022;376:e068373. doi: 10.1136/bmj-2021-068373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morciano C, Alegiani SS, Ippoliti FM, et al. Post-marketing active surveillance of Guillan Barré syndrome following vaccination with anti-COVID-19 vaccines in persons aged ≥12 years in Italy: a multi-database self-controlled case series study. medRxiv. Preprint posted online January 19, 2023. doi: 10.1101/2023.01.17.23284585 [DOI] [Google Scholar]
- 17.Semenzato L, Botton J, Drouin J, et al. Chronic diseases, health conditions and risk of COVID-19-related hospitalization and in-hospital mortality during the first wave of the epidemic in France: a cohort study of 66 million people. Lancet Reg Health Eur. 2021;8:100158. doi: 10.1016/j.lanepe.2021.100158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botton J, Jabagi MJ, Bertrand M, et al. Risk for myocardial infarction, stroke, and pulmonary embolism following COVID-19 vaccines in adults younger than 75 years in France. Ann Intern Med. 2022;175(9):1250-1257. doi: 10.7326/M22-0988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boudemaghe T, Belhadj I. Data resource profile: the French National Uniform Hospital Discharge Data Set Database (PMSI). Int J Epidemiol. 2017;46(2):392-392d. doi: 10.1093/ije/dyw359 [DOI] [PubMed] [Google Scholar]
- 20.Rudant J, Dupont A, Mikaeloff Y, Bolgert F, Coste J, Weill A. Surgery and risk of Guillain-Barré syndrome: a French nationwide epidemiologic study. Neurology. 2018;91(13):e1220-e1227. doi: 10.1212/WNL.0000000000006246 [DOI] [PubMed] [Google Scholar]
- 21.Farrington P, Whitaker H, Weldeselassie YG. Self-Controlled Case Series Studies: A Modelling Guide With R. 2nd ed. Chapman and Hall/CRC; 2018. doi: 10.1201/9780429491313 [DOI] [Google Scholar]
- 22.Farrington CP, Whitaker HJ, Hocine MN. Case series analysis for censored, perturbed, or curtailed post-event exposures. Biostatistics. 2009;10(1):3-16. doi: 10.1093/biostatistics/kxn013 [DOI] [PubMed] [Google Scholar]
- 23.Ghebremichael-Weldeselassie Y, Jabagi MJ, Botton J, et al. A modified self-controlled case series method for event-dependent exposures and high event-related mortality, with application to COVID-19 vaccine safety. Stat Med. 2022;41(10):1735-1750. doi: 10.1002/sim.9325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289-300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 25.R Core Team. R: A Language and Environment for Statistical Computing. Published online 2022. Accessed February 15, 2023. https://www.R-project.org/ [Google Scholar]
- 26.Weldeselassie YG, Whitaker H, Farrington P. SCCS: The Self-Controlled Case Series Method. Published online July 5, 2022. Accessed February 15, 2023. CRAN.R-project.org/package=SCCS. [Google Scholar]
- 27.Keh RYS, Scanlon S, Datta-Nemdharry P, et al. COVID-19 vaccination and Guillain-Barré syndrome: analyses using the National Immunoglobulin Database. Brain. 2023;146(2):739-748. doi: 10.1093/brain/awac067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delannoy A, Rudant J, Chaignot C, Bolgert F, Mikaeloff Y, Weill A. Guillain-Barré syndrome in France: a nationwide epidemiological analysis based on hospital discharge data (2008-2013). J Peripher Nerv Syst. 2017;22(1):51-58. doi: 10.1111/jns.12202 [DOI] [PubMed] [Google Scholar]
- 29.Grave C, Boucheron P, Rudant J, et al. Seasonal influenza vaccine and Guillain-Barré syndrome: a self-controlled case series study. Neurology. 2020;94(20):e2168-e2179. doi: 10.1212/WNL.0000000000009180 [DOI] [PubMed] [Google Scholar]
- 30.Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain-Barré syndrome: a systematic review and meta-analysis. Neuroepidemiology. 2011;36(2):123-133. doi: 10.1159/000324710 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
According to data protection and the French regulation, the authors cannot publicly release the data from the SNDS. However, any person or structure, public or private and for-profit or nonprofit, is able to access SNDS data on authorization from the French Data Protection Office (CNIL) to perform a study, a research, or an evaluation of public interest (snds.gouv.fr/SNDS/Processus-d-acces-auxdonnees and indsante.fr/).