Abstract
STUDY QUESTION
Are there associations between natural or surgical menopause and incident dementia by age at menopause?
SUMMARY ANSWER
Compared to age at menopause of 46–50 years, earlier natural menopause (≤40 and 41–45 years) was related to higher risk of all-cause dementia, while a U-shape relationship was observed between age at surgical menopause and risk of dementia.
WHAT IS KNOWN ALREADY
Menopause marks the end of female reproductive period. Age at menopause reflects the length of exposure to endogenous estrogen. Evidence on the association between age at natural, surgical menopause, and risk of dementia has been inconsistent.
STUDY DESIGN, SIZE, DURATION
A population-based cohort study involving 160 080 women who participated in the UK Biobank study.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Women with no dementia at baseline, and had no missing data on key exposure variables and covariates were included. Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% CIs on the association of categorical menopause age with incident all-cause dementia, Alzheimer’s disease (AD) and vascular dementia (VD). Restricted cubic splines were used to model the non-linear relationship between continuous age at natural, surgical menopause, and risk of dementia. In addition, we analyzed the interaction effect of ever-used menopausal hormone therapy (MHT) at baseline, income level, leisure activities, and age at menopause on risk of dementia.
MAIN RESULTS AND THE ROLE OF CHANCE
Compared to women with age at menopause of 46–50 years, women with earlier natural menopause younger than 40 years (1.36, 1.01–1.83) and 41–45 years (1.19, 1.03–1.39) had a higher risk of all-cause dementia, while late natural menopause >55 years was linked to lower risk of dementia (0.83, 0.71–0.98). Compared to natural menopause, surgical menopause was associated with 10% higher risk of dementia (1.10, 0.98–1.24). A U-shape relationship was observed between surgical menopause and risk of dementia. Women with surgical menopause before age 40 years (1.94, 1.38–2.73) and after age 55 years (1.65, 1.21–2.24) were both linked to increased risk of all-cause dementia. Women with early natural menopause without ever taking MHT at baseline had an increased risk of AD. Also, in each categorized age at the menopause level, higher income level or higher number of leisure activities was linked to a lowers risk of dementia.
LIMITATIONS, REASONS FOR CAUTION
Menopausal age was based on women’s self-report, which might cause recall bias.
WIDER IMPLICATION OF THE FINDINGS
Women who experienced natural menopause or had surgical menopause at an earlier age need close monitoring and engagement for preventive health measures to delay the development of dementia.
STUDY FUNDING/COMPETING INTERESTS
This work was supported by the Start-up Foundation for Scientific Research in Shandong University (202099000066), Science Fund Program for Excellent Young Scholars of Shandong Provence (Overseas) (2022HWYQ-030), and the National Natural Science Foundation of China (82273702). There are no competing interests.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: age, natural menopause, surgical menopause, dementia, Alzheimer’s disease
Introduction
With aging and the increase in life expectancy, dementia has become a great global public health challenge (Winblad et al., 2016). There were more than 50 million people living with dementia worldwide, and the number will reach 152 million by 2050 (Alzheimer’s Disease International, 2018). About two-thirds of Alzheimer’s disease (AD) patients are women (Prince et al., 2016; Sindi et al., 2021), and it is not just because women live longer and have more chances to develop AD. Common societal and lifestyle risk factors, such as education, exercise, smoking, and alcohol use, also cannot fully explain the sex difference (Podcasy and Epperson, 2016; Langa et al., 2017; Mielke, 2018). The reasons why women are more likely to develop AD remain less clear. Some female-specific risk factors, such as menopause and menopausal hormone therapy (MHT), might be related to the elevated risk of dementia in women.
Menopause marks the end of female reproductive period. Age at menopause reflects the length of exposure to endogenous estrogen. Compared to men of the same age, postmenopausal women had 1.5–3.0 times higher risk of AD (Morrison et al., 2006). The association between age at menopause and risk of dementia has been inconsistent. A recent systematic review that pooled findings from observational studies showed that later menopause was linked to a lower risk of all-cause dementia and AD (Fu et al., 2022). Another review found no association between menopausal age (later versus younger) and risk of dementia (Georgakis et al., 2016). Both of these two reviews did not separate natural and surgical menopause, and moderate heterogeneities among studies were observed when pooled estimates in these reviews. A specific review on surgical menopause and risk of dementia found surgical menopause at ≤45 years of age was associated with higher risk of dementia, compared to surgical menopause after age 45 years (Georgakis et al., 2019). Results also have been contradicted from single large-scale or long-term follow-up cohort studies (Najar et al., 2020; Yoo et al., 2020). Thus, additional large-scale cohort studies are necessary to replicate these findings.
Menopause concurs with the marked decline of endogenous estrogen. During menopause, women may take menopause hormone therapy (MHT) to reduce their vasomotor symptoms (Stuenkel, 2015). The association of menopausal age with dementia might be moderated by the use of MHT (Stute et al., 2021). In addition, Gong et al. (2022) found that lower SES level might strengthen the relationship of early menopause with dementia, and no clear interaction between smoking and menopause was observed. A study that involved 3568 people from Latin America did not observe an interaction between APOE genotype (none versus one or more APOE e4 alleles) and reproductive period (Prince et al., 2018). Thus, whether socioeconomic status (education and income level), behavior (smoking status and leisure activities), and genetic factors (apolipoprotein E (APOE) allele status) modify the association between age at menopause and dementia is worth a further analysis. Based on the above mentioned, we first aimed to examine the association between natural, surgical menopause and risk of all-cause dementia, AD, and vascular dementia (VD) by age at menopause. Then, we examined whether there was an interaction effect between MHT used or not at baseline, socioeconomic status, behavior, genetic factors, and age at menopause on risk of dementia.
Materials and methods
Study design and participants
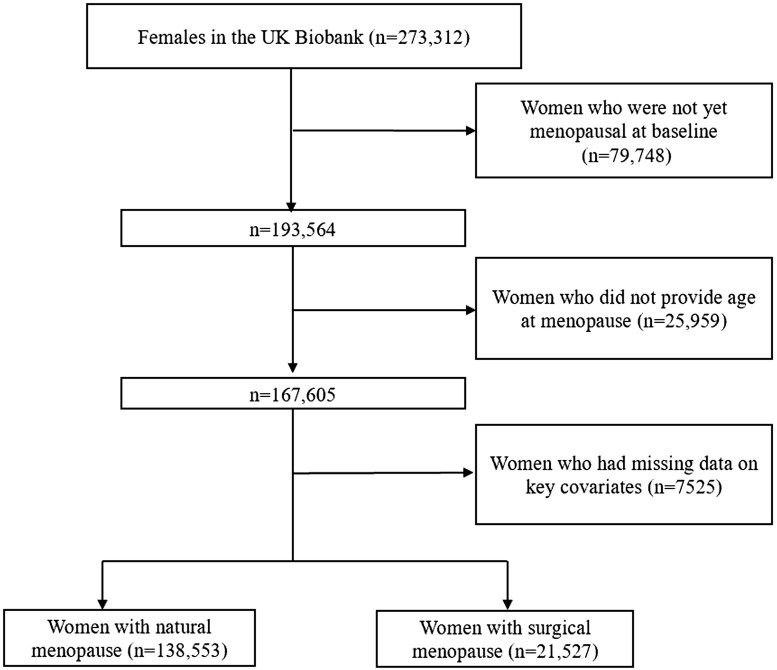
The study subjects came from the UK Biobank. The UK Biobank is a large population-based cohort study established in the UK from 2006 to 2010 (Sudlow et al., 2015). At recruitment, participants with an age range from 40 to 71 years old provided electronically signed consent forms, answered questions about sociodemographic, lifestyle, and health-related factors, and completed a series of physical measurements. All participants were linked to hospital data and national death registries from England, Scotland, and Wales (Gong et al., 2021) to determine the date of the first diagnosis of dementia after the baseline assessment. Totally, 1297 people were loss to follow-up because they had left the UK or had withdrawn consent for future linkage. UK Biobank received ethical approval from the UK National Health Service’s National Research Ethics Service (ref 11/NW/0382). This research was conducted under UK Biobank application number 68369. A prospective analysis was adopted based on postmenopausal women with no dementia at baseline, who had no missing data on key covariates, including ethnicity, education, income, cigarette smoking, alcohol drinking, leisure activities, BMI, cardiovascular disease (CVD) history, the E4 variant of apolipoprotein E (APOE e4), and MHT used status at baseline. A total of 160 080 women were included (Fig. 1).
Figure 1.
Flow diagram of study participant selection.
Exposure variables and outcome variables
In the present study, natural menopause was defined as an absence of menstruation over a period of 12 months and no experience of hysterectomy and/or oophorectomy before it. Surgical menopause was defined as the removal of both ovaries (bilateral oophorectomy) before natural menopause. Age at menopause was categorized as ≤40 (premature), 41–45 (early), 46–50, 51–55, and >55 years (late menopause). The primary outcome variable in this study was the first occurrence of all-cause dementia, including AD and VD. Physician-diagnosed dementia was ascertained from linkage data to primary care, hospital admission, and death register records. The International Classification of Diseases 10th Edition (ICD-10) codes F00, F01, F02, F03, G30, G31.0, G31.8, and ICD-9 code 290 were used to identify participants with all-cause dementia if one or more of these codes were recorded as a primary or secondary diagnosis. Incident AD was defined by ICD-10 codes F00, G30, and ICD-9 code 290. Incident VD was defined by ICD-10 code F01. Outcome adjudication for incident dementia was conducted by the UK Biobank Outcome Adjudication team.
Covariates
We included the following factors in the analyses as covariates because these have been shown to be associated with both age at menopause (Schoenaker et al., 2014; Zhu et al., 2019) and risk of dementia (Dong et al., 2022; Menesgere et al., 2023): age at baseline, ethnicity, education level, income, BMI, smoking status, physical activities, drinking status, leisure activities, CVD, APOE e4 carrier status, and ever-used MHT at baseline. Ethnicity was divided into white and non-white. Years of education were categorized as ≤10, 11–12, and >12 years. Total household income before tax was split into three groups: <£18 000, £18 000–30 999, and ≥£31 000. BMI was classified as <18.5, 18.5–24.9, 25–29.9, and ≥30 kg/m2. Cigarette smoking was classified as smoking almost all days, occasionally, ex-smoker, and never smoked. International Physical Activity Questionnaire (IPAQ) was used to calculate metabolic equivalent (MET) score, and physical activity level was categorized as low (<600 MET-min/week), medium (600–3000 MET-min/week), and high (≥3000 MET-min/week). Alcohol intake was categorized as daily, 3–4 times/week, 1–2 times/week, occasionally, and never. Leisure activities were assessed through participants’ choices of six activities and were categorized as none, one, and two or more. CVD history was classified into yes or no. APOE allele status was based on two single nucleotide polymorphisms: rs7412 and rs429358. Participants with APOE e4 allele (e3/e4, e4/e4, and occasionally e2/e4 genotypes) were compared with those with the e2/e2, e2/e3, or e3/e3 genotype. MHT used status was collected at baseline through a question ‘Have you ever used hormone replacement therapy (HRT)?’ and was classified as user or non-user. Detailed information on covariates collection and definitions is listed in Supplementary Table S1.
Statistical analysis
Baseline characteristics were presented as means and SD for continuous variables and as percentages (%) for categorical variables. Cox proportional hazard risk model was used to estimate hazard ratios (HR) and 95% CIs between age at menopause and risk of all-cause dementia, AD, and VD. When modeling the association with AD or VD, only those with AD or VD were included, i.e. women who had both AD and VD were excluded. Data were censored on 30 January 2021 or at the date of death. For women who experienced dementia, follow-up time was calculated as their age at diagnosis of dementia minus baseline age. For participants without experiencing dementia, follow-up time was defined as their age at the last follow-up (censored date) minus baseline age. When types of menopause were compared, women with natural menopause were used as the reference group, and when age at menopause was analyzed, women with age at menopause 46–50 years were used as the reference group. Covariates were adjusted sequentially, i.e. in Model 1, sociodemographic factors were adjusted (age at baseline, ethnicity, years of education, and income level). As dementia incidence increases exponentially with age and doubles about every 5 years beyond age 65, when age at baseline was adjusted, we used a quadratic form of age. In Model 2, lifestyle factors were further adjusted (smoking status, physical activity, alcohol consumption, leisure activities, and BMI level). In Model 3, CVD history and APOE e4 allele status were further adjusted. In Model 4, ever-used MHT at baseline was further adjusted. As CVD might be a mediator between age at menopause and dementia, we did analyses to see the changes in HR (95% CI) before and after CVD was adjusted. Also, we performed two sensitivity analyses, i.e. first only including dementia which occurred at least 5 years after menopause, and second by excluding women with ever-used MHT at baseline. Furthermore, we used a restricted cubic spline to model the non-linear relationships between continuous age at natural menopause, age at surgical menopause, and risk of dementia, with covariates being adjusted as in Model 4. In addition, we examined the interaction effect of MHT used status at baseline, education, income, smoking, leisure activities, and APOE e4 carrier status with categorized age at menopause by adding a product interaction term to Model 4. A two-sided P value of 0.05 or less indicated the significance of the interaction effect.
We used SAS (version 9.4, SAS Institute Inc, Cary, NC) in all statistical analyses. The PHREG procedure was used to fit the Cox proportional hazards regression models. All statistical tests were based on the two-sided 5% level of significance.
Results
Characteristics of the participants
There were 160 080 women in this study, 138 553 with natural menopause and 21 527 with surgical menopause. The mean (SD) age at baseline was 59.2 ± 6.1 years, with a range from 40 to 71 years old. The mean (SD) age at the last follow-up was 72.1 ± 6.1 years. After a mean (SD) follow-up time of 12.1 ± 1.6 years from baseline, there were 1.8% (2427), 11.8% (16 279), and 9.9% (13 722) women with premature, early, and late natural menopause, respectively. The cumulative incidences of all-cause dementia, AD, and VD were 1.32% (1824, 27.3% cases from death certificates), 0.61% (841), and 0.25% (353), respectively. Compared with women without dementia, women with dementia were more likely to be less educated, obese and to be current smokers, with CVD (Table 1). Characteristics of women with surgical menopause were listed in Supplementary Table S2. Compared to women who were included, those excluded for missing age at menopause were relative older at baseline (mean age 61.7 versus 59.2 years) and had higher proportion of MHT used at baseline (65.3% versus 41.6%) (Supplementary Table S3). Women who were excluded for missing covariates had higher cumulative incidence of dementia (2.2% versus 1.3%) (Supplementary Table S4).
Table 1.
Characteristics by age at natural menopause and incident all-cause dementia events.
| Age at natural menopause |
Incident dementia |
||||||
|---|---|---|---|---|---|---|---|
| Characteristics | ≤40 years | 41–45 years | 46–50 years | 51–55 years | ≥55 years | No | Yes |
| (n = 2427) | (n = 16 279) | (n = 47 092) | (n = 59 033) | (n = 13 722) | (n = 136 729) | (n = 1824) | |
| Age at baseline | 57.0 ± 7.7 | 58.7 ± 7.0 | 58.6 ± 6.3 | 59.3 ± 5.6 | 61.6 ± 4.6 | 59.1 ± 6.0 | 64.5 ± 3.4 |
| Ethnicity | |||||||
| White | 2280 (93.9) | 15 342 (94.2) | 44 869 (95.3) | 57 083 (96.7) | 13 331 (97.2) | 131 139 (95.9) | 1766 (96.8) |
| Nonwhite | 147 (6.1) | 937 (5.8) | 2223 (4.7) | 1950 (3.3) | 391 (2.8) | 5590 (4.1) | 58 (3.2) |
| Education level | |||||||
| ≤10 | 1420 (58.5) | 8983 (55.3) | 24 090 (51.2) | 27 699 (46.9) | 6938 (50.6) | 67 989 (49.7) | 1141 (62.6) |
| 11–12 | 270 (11.1) | 1797 (11.0) | 5532 (11.7) | 7473 (12.7) | 1532 (11.1) | 16 422 (12.0) | 182 (10.0) |
| >12 | 737 (30.4) | 5499 (33.7) | 17 470 (37.1) | 23 861 (40.4) | 5252 (38.3) | 52 318 (38.3) | 501 (27.4) |
| Income (£) | |||||||
| <18 000 | 776 (32.0) | 4974 (30.6) | 12 690 (27.0) | 14 563 (24.7) | 3940 (28.7) | 40 326 (27.5) | 516 (46.1) |
| 18 000–30 999 | 592 (24.4) | 4322 (26.6) | 12 612 (26.8) | 15 975 (27.1) | 3858 (28.1) | 40 266 (27.4) | 304 (27.1) |
| ≥31 000 | 1059 (43.6) | 6983 (42.8) | 21 790 (46.2) | 28 495 (48.2) | 5924 (43.2) | 66 196 (45.1) | 300 (26.8) |
| BMI | |||||||
| <18.5 kg/m2 | 37 (1.5) | 158 (1.0) | 414 (0.9) | 406 (0.7) | 77 (0.6) | 1076 (0.8) | 16 (0.9) |
| 18.5–24.9 kg/m2 | 874 (36.0) | 6239 (38.3) | 19 282 (41.0) | 23 793 (40.3) | 4767 (34.7) | 54 293 (39.7) | 662 (36.3) |
| 25.0–29.9 kg/m2 | 848 (35.0) | 6070 (37.3) | 17 447 (37.1) | 22 316 (37.8) | 5379 (39.2) | 51 392 (37.6) | 668 (36.6) |
| ≥30kg/m2 | 668 (27.5) | 3812 (23.4) | 9949 (21.0) | 12 518 (21.2) | 3499 (25.5) | 29 968 (21.9) | 478 (26.2) |
| Cigarette smoking | |||||||
| Never smoker | 1225 (50.5) | 8602 (52.8) | 27 047 (57.4) | 36 118 (61.2) | 8280 (60.3) | 80 293 (58.7) | 603 (53.8) |
| Former smoker | 820 (33.8) | 5685 (34.9) | 15 719 (33.4) | 19 219 (32.6) | 4727 (34.5) | 45 493 (33.3) | 423 (37.8) |
| Current smoker | 382 (15.7) | 1992 (12.3) | 4326 (9.2) | 3696 (6.2) | 715 (5.2) | 10 943 (8.0) | 94 (8.4) |
| Alcohol drinking | |||||||
| Never drinker | 198 (8.2) | 1136 (7.0) | 2682 (5.7) | 2856 (4.8) | 687 (5.0) | 8166 (5.6) | 125 (11.2) |
| Former drinker | 132 (5.4) | 653 (4.0) | 1810 (3.8) | 1795 (3.0) | 450 (3.3) | 5233 (3.6) | 74 (6.6) |
| Current drinker | 2097 (86.4) | 14 490 (89.0) | 42 600 (90.5) | 54 382 (92.2) | 12 585 (91.7) | 133 389 (90.8) | 921 (82.2) |
| No. of leisure activities | |||||||
| 0 | 806 (33.2) | 4795 (29.5) | 12 971 (27.5) | 14 510 (24.6) | 3249 (23.6) | 35 757 (26.2) | 574 (31.5) |
| 1 | 985 (40.6) | 7027 (43.1) | 19 823 (42.1) | 24 402 (41.3) | 5660 (41.3) | 57 092 (41.8) | 805 (44.1) |
| ≥2 | 636 (26.2) | 4457 (27.4) | 14 298 (30.4) | 20 121 (34.1) | 4813 (35.1) | 43 880 (32.0) | 445 (24.4) |
| CVD | |||||||
| No | 2221 (91.5) | 15 130 (92.9) | 44 595 (94.7) | 56 240 (95.3) | 12 900 (94.0) | 129 604 (94.8) | 1482 (81.3) |
| Yes | 206 (8.5) | 1149 (7.1) | 2497 (5.3) | 2793 (4.7) | 822 (6.0) | 7125 (5.2) | 342 (18.7) |
| APOE e4 | |||||||
| No APOE e4 | 1807 (74.5) | 12 374 (76.0) | 35 849 (76.1) | 44 744 (75.8) | 10 353 (75.5) | 104 188 (76.2) | 939 (51.5) |
| One APOE e4 | 565 (23.3) | 3588 (22.0) | 10 303 (21.9) | 13 113 (22.2) | 3092 (22.5) | 29 956 (21.9) | 705 (38.7) |
| Two APOE e4 | 55 (2.2) | 317 (2.0) | 940 (2.00) | 1176 (2.0) | 277 (2.0) | 2585 (1.9) | 180 (9.8) |
| Ever-used MHT at baseline | |||||||
| No | 802 (33.0) | 7899 (48.5) | 28 276 (60.0) | 36 454 (61.8) | 7413 (54.0) | 79 952 (58.5) | 892 (48.9) |
| Yes | 1625 (67.0) | 8380 (51.5) | 18 816 (40.0) | 22 579 (38.2) | 6309 (46.0) | 56 777 (41.5) | 932 (51.1) |
CVD, cardiovascular disease; APOE, apolipoprotein E; MHT, menopausal hormone therapy.
Age at natural menopause and dementia
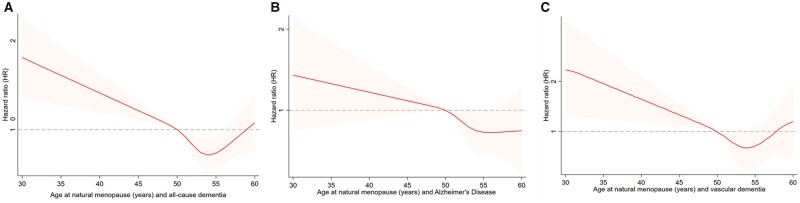
Compared to women with age at natural menopause 46–50 years, women with premature menopause (≤40 years) and early menopause (41–45 years) had a higher risk of all-cause dementia, with HR (95% CI) of 1.36 (1.01, 1.83), and 1.19 (1.03, 1.37), respectively, while women with menopausal age 51–55 years and late menopause (>55 years) were linked to lower risk of dementia, with HR (95% CI) of 0.83 (0.74, 0.92) and 0.83 (0.71, 0.98), respectively. When the associations with AD and VD were analyzed separately, we found the HRs in earlier and later menopause groups had the same direction as the HRs with all-cause dementia, although the 95% CIs spanned one (Table 2; Supplementary Table S5). When CVD was adjusted, the HR values were slightly weakened (Supplementary Table S6). Sensitivity analyses that only included dementia which occurred 5 years after menopause or only included women without ever-used MHT at baseline showed similar findings to Table 2 (Supplementary Tables S7 and S8). When restricted cubic splines were used to model the non-linear relationships between continuous age at natural menopause and dementia, we found that the dose–response relationship was consistent with the trend of association when categorical age of menopause was used (Fig. 2).
Table 2.
Associations between age at natural menopause and all-cause dementia, Alzheimer's disease (AD) and vascular dementia (VD).
| Dementia | Years | Women | Dementia | Incidence rate (per 10 000 person-years) | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||||
| All-cause dementia | ≤40 | 2427 | 49 | 16.9 | 1.60 (1.20, 2.15) | 1.51 (1.13, 2.03) | 1.37 (1.02, 1.84) | 1.36 (1.01, 1.83) |
| 41–45 | 16 279 | 289 | 14.6 | 1.25 (1.09, 1.44) | 1.23 (1.07, 1.42) | 1.20 (1.04, 1.38) | 1.19 (1.03, 1.37) | |
| 46–50 | 47 092 | 634 | 11.1 | 1 | 1 | 1 | 1 | |
| 51–55 | 59 033 | 653 | 9.1 | 0.80 (0.71, 0.89) | 0.82 (0.73, 0.91) | 0.83 (0.74, 0.92) | 0.83 (0.74, 0.92) | |
| >55 | 13 722 | 199 | 12.0 | 0.81 (0.69, 0.95) | 0.83 (0.71, 0.98) | 0.84 (0.71, 0.98) | 0.83 (0.71, 0.98) | |
| Alzheimer’s disease (AD) | ≤40 | 2427 | 24 | 8.3 | 1.71 (1.13, 2.59) | 1.63 (1.07, 2.47) | 1.49 (0.98, 2.27) | 1.48 (0.98, 2.25) |
| 41–45 | 16 279 | 118 | 6.0 | 1.08 (0.87, 1.34) | 1.06 (0.86, 1.32) | 1.04 (0.84, 1.29) | 1.04 (0.84, 1.29) | |
| 46–50 | 47 092 | 300 | 5.3 | 1 | 1 | 1 | 1 | |
| 51–55 | 59 033 | 314 | 4.4 | 0.81 (0.69, 0.95) | 0.83 (0.71, 0.97) | 0.84 (0.71, 0.98) | 0.84 (0.72, 0.98) | |
| >55 | 13 722 | 85 | 5.1 | 0.73 (0.57, 0.93) | 0.74 (0.58, 0.95) | 0.75 (0.58, 0.95) | 0.74 (0.58, 0.95) | |
| Vascular dementia (VD) | ≤40 | 2427 | 12 | 4.1 | 1.99 (1.10, 3.60) | 1.84 (1.01, 3.33) | 1.61 (0.89, 2.92) | 1.59 (0.88, 2.88) |
| 41–45 | 16 279 | 62 | 3.2 | 1.35 (0.99, 1.83) | 1.31 (0.96, 1.79) | 1.26 (0.93, 1.72) | 1.25 (0.92, 1.70) | |
| 46–50 | 47 092 | 122 | 2.1 | 1 | 1 | 1 | 1 | |
| 51–55 | 59 033 | 120 | 1.7 | 0.76 (0.59, 0.98) | 0.79 (0.61, 1.02) | 0.80 (0.62, 1.03) | 0.80 (0.62, 1.03) | |
| >55 | 13 722 | 37 | 2.2 | 0.75 (0.52, 1.08) | 0.77 (0.53, 1.12) | 0.78 (0.54, 1.13) | 0.78 (0.54, 1.12) |
Model 1: age at baseline, ethnicity, BMI, education level, and income level were adjusted; Model 2: leisure activities, cigarette smoking, and alcohol drinking were further adjusted based on Model 1; Model 3: cardiovascular disease (CVD) and APOE (apolipoprotein E) were further adjusted based on Model 2; Model 4: ever-used menopausal hormone therapy (MHT) at baseline was further adjusted based on Model 3. HR, hazard ratio.
Figure 2.
Non-linear relationships between age at natural menopause and dementia. (A) all-cause dementia, (B) Alzheimer’s disease, and (C) vascular dementia.
Surgical menopause and dementia
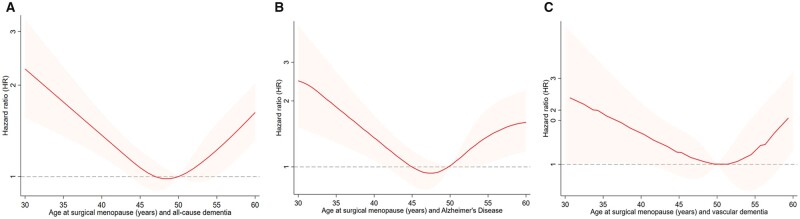
Compared to natural menopause, surgical menopause was associated with 10% higher risk of dementia (HR 1.10, 95% CI 0.98–1.24) (Table 3). Women with surgical menopause before age 40 years and after age 55 years were linked to an increased risk of all-cause dementia, with HR (95% CI) of 1.94 (1.38, 2.73) and 1.65 (1.21, 2.24), respectively. When the associations with AD and VD were analyzed, surgical menopause before age 40 years was linked to higher risk of AD (2.16, 1.32–3.54) and surgical menopause after age 55 was linked to higher risk of VD (1.95, 1.04–4.02) (Table 4). Restricted cubic spline models showed a U-shape relationship between continuous surgical menopause and risk of dementia (Fig. 3).
Table 3.
Associations between menopause type (natural menopause versus surgical menopause) and all-cause dementia, Alzheimer's disease (AD), and vascular dementia (VD).
| Dementia | Type | Women | Dementia | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|---|---|---|
| All cause dementia | Natural | 138 553 | 1824 | 1 | 1 | 1 | 1 |
| Surgical | 21 527 | 360 | 1.17 (1.04, 1.31) | 1.15 (1.03, 1.29) | 1.11 (0.99, 1.25) | 1.10 (0.98, 1.24) | |
| Alzheimer's disease (AD) | Natural | 138 553 | 841 | 1 | 1 | 1 | 1 |
| Surgical | 21 527 | 163 | 1.16 (0.98, 1.38) | 1.15 (0.97, 1.36) | 1.11 (0.93, 1.31) | 1.11 (0.93, 1.33) | |
| Vascular dementia (VD) | Natural | 138 553 | 353 | 1 | 1 | 1 | 1 |
| Surgical | 21 527 | 64 | 1.03 (0.78, 1.35) | 1.01 (0.77, 1.33) | 0.96 (0.73, 1.26) | 0.93 (0.71, 1.23) |
Model 1: age at baseline, ethnicity, BMI, education level, income level and age at menopause were adjusted; Model 2: leisure activities, cigarette smoking, and alcohol drinking were further adjusted based on Model 1; Model 3: cardiovascular disease (CVD) and APOE (apolipoprotein E) were further adjusted based on Model 2; Model 4: ever-used menopausal hormone therapy (MHT) at baseline was further adjusted based on Model 3. HR, hazard ratio.
Table 4.
Associations between age at surgical menopause and all-cause dementia, Alzheimer's disease (AD), and vascular dementia (VD).
| Dementia | Years | Women | Dementia | Incidence rate (per 10 000 person-years) | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||||
| All-cause dementia | ≤40 | 2862 | 64 | 18.5 | 2.23 (1.59, 3.13) | 2.08 (1.48, 2.92) | 1.98 (1.41, 2.79) | 1.94 (1.38, 2.73) |
| 41–45 | 4945 | 53 | 8.9 | 1.06 (0.74, 1.50) | 1.02 (0.72, 1.46) | 1.00 (0.70, 1.42) | 1.00 (0.70, 1.42) | |
| 46–50 | 6238 | 79 | 10.5 | 1 | 1 | 1 | 1 | |
| 51–55 | 3754 | 58 | 12.9 | 1.19 (0.85, 1.68) | 1.20 (0.85, 1.70) | 1.20 (0.85, 1.68) | 1.16 (0.82, 1.63) | |
| >55 | 3728 | 106 | 24.1 | 1.74 (1.29, 2.35) | 1.74 (1.29, 2.34) | 1.80 (1.33, 2.42) | 1.65 (1.21, 2.24) | |
| Alzheimer's disease (AD) | ≤40 | 2862 | 30 | 8.7 | 2.34 (1.44, 3.82) | 2.25 (1.38, 3.67) | 2.24 (1.37, 3.66) | 2.16 (1.32, 3.54) |
| 41–45 | 4945 | 19 | 3.2 | 0.81 (0.47, 1.42) | 0.80 (0.46, 1.39) | 0.78 (0.45, 1.37) | 0.78 (0.45, 1.37) | |
| 46–50 | 6238 | 37 | 4.9 | 1 | 1 | 1 | 1 | |
| 51–55 | 3754 | 34 | 7.6 | 1.44 (0.90, 2.30) | 1.46 (0.91, 2.33) | 1.44 (0.90, 2.31) | 1.38 (0.86, 2.21) | |
| >55 | 3728 | 43 | 9.8 | 1.45 (0.93, 2.27) | 1.46 (0.93, 2.28) | 1.54 (0.99, 2.42) | 1.37 (0.87, 2.17) | |
| Vascular dementia (VD) | ≤40 | 2862 | 11 | 3.2 | 2.42 (1.06, 5.50) | 2.24 (0.98, 5.14) | 1.84 (0.80, 4.22) | 1.79 (0.78, 4.11) |
| 41–45 | 4945 | 8 | 1.3 | 1.02 (0.42, 2.49) | 0.97 (0.40, 2.39) | 0.91 (0.37, 2.23) | 0.91 (0.37, 2.22) | |
| 46–50 | 6238 | 12 | 1.6 | 1 | 1 | 1 | 1 | |
| 51–55 | 3754 | 11 | 2.4 | 1.43 (0.63, 3.25) | 1.47 (0.65, 3.33) | 1.45 (0.64, 3.28) | 1.40 (0.62, 3.20) | |
| >55 | 3728 | 22 | 5.0 | 2.12 (1.04, 4.31) | 2.11 (1.04, 4.31) | 2.11 (1.03, 4.31) | 1.95 (1.04, 4.02) |
Model 1: age at baseline, ethnicity, BMI, education level, and income level were adjusted; Model 2: leisure activities, cigarette smoking, and alcohol drinking were further adjusted based on Model 1; Model 3: cardiovascular disease (CVD) and APOE (apolipoprotein E) were further adjusted based on Model 2; Model 4: ever-used menopausal hormone therapy (MHT) at baseline was further adjusted based on Model 3. HR, hazard ratio.
Figure 3.
Non-linear relationships between age at surgical menopause and dementia. (A) all-cause dementia, (B) Alzheimer’s disease, and (C) vascular dementia.
The roles of ever-used MHT at baseline, income, and leisure activities
After a formal test, there was a significant interaction between age at natural menopause and MHT used or not at baseline, income, and leisure activities. Women with early natural menopause without taking MHT at baseline had an increased risk of AD (1.36, 1.03–1.81), while no such relationship was observed in those taking MHT at baseline (1.03, 0.78–1.37) (Supplementary Fig. S1). In each age at the menopause categorized level, higher income level or higher number of leisure activities was linked to lower risk of dementia (Supplementary Figs S2 and S3).
Discussion
Our findings showed that compared to age at menopause of 46–50 years, earlier natural menopause (≤40 and 41–45 years) was related to higher risk of all-cause dementia, while later menopause (51–55 and >55 years) was linked to lower risk of dementia. There was a U-shape relationship between age at surgical menopause and risk of all-cause dementia. Income level, number of leisure activities, and whether used MHT at baseline modified the relationship between menopausal age and risk of dementia.
Age at natural menopause and all-cause dementia
Evidence on the association between age at natural menopause and all-cause dementia has been inconsistent (Geerlings et al., 2001; Prince et al., 2018; Yoo et al., 2020). A recent systematic review that pooled findings from 10 observational studies (involving over 4.7 million) showed that early menopause (<45 years) was linked to higher risk of all-cause dementia and AD, and dose–response meta-analyses also showed a linear relationship that the younger menopause, the higher risk of dementia. Nevertheless, this review did not distinguish natural or surgical menopause, and moderate heterogeneity was observed among studies (Fu et al., 2022). Another review included 13 observational studies (19 449 women were involved) that found no association between menopausal age (later versus younger) and risk of dementia, also with great heterogeneity among studies (Georgakis et al., 2016). However, when pooling the estimates, this review did not use uniform classification for age at menopause, and the reference level differed across studies (Georgakis et al., 2016). Recent large-scale or long-term follow-up cohort studies also showed contradicted findings. In a nationwide cohort study which involved 4.7 million Korean postmenopausal women, Yoo et al. reported that women with late menopause (≥55 years) had 21% lower risk of dementia compared to those who had menopause at age <40 years. However, this study used data from national health insurance service, and some key covariates, e.g. ever-used MHT at baseline, APOE e4 carrier status, and education level, were not available, which might cause some bias (Yoo et al., 2020). In contrast, a 44-year longitudinal study of Swedish postmenopausal women found that each 1 year later of menopause age was associated with 7% higher risk of dementia (1.07, 1.04–1.10). However, this study included postmenopausal women before the year 2002. The proportion of MHT prescriptions prior to 2002 was much higher than its prescription after 2002 (Zbuk and Anand, 2012), which may affect the association between menopausal age and dementia in a real-world context. Consistent with previous studies (Gilsanz et al., 2019; Yoo et al., 2020) after a series of covariates were adjusted, we observed that later menopause was linked to lower risk of all-cause dementia.
Age at natural menopause and AD
The association between age at menopause and risk of AD is also unclear (Yamada et al., 2009; Najar et al., 2020; Yoo et al., 2020). In Korean women, Yoo et al. (2020) found that later menopause (≥55 versus <40 years) was related to 20% lower risk of AD, while Najar et al. (2020) have found an opposite finding, i.e. each year later of menopause was associated with 7% higher risk of AD. In addition, Yamada et al. (2009) did not observe an association between them. In UK females, compared to women who had menopause at 46–50 years, we observed late age at natural menopause (>55 years) was associated with lower risk of AD, consistent with a Korean study (Yoo et al., 2020).
Age at natural menopause and VD
Few studies have examined the association between age at menopause and VD (Yamada et al., 2009; Yoo et al., 2020). The adult health study in Japan found no association between menopause age and VD (Yamada et al., 2009). The study in Korea found that the risk of VD decreased with the rise of age at menopause (Yoo et al., 2020). VD is usually caused by diseases that disrupt the blood supply to the brain (Vijayan and Reddy, 2016), and stroke was acknowledged a major risk factor for VD (Desmond et al., 2000; Ivan et al., 2004; Gamaldo et al., 2006). There has been a link between menopausal age and stoke. Compared to women with normal menopause, early menopause (<45 years) was linked to 50% higher risk of having stroke (Zhu et al., 2019). In our findings, a trend that early menopause might be related to higher risk of VD was also observed.
Surgical menopause and dementia
Evidence on the associations between surgical menopause and dementia risk remains limited. A review that included four studies and 12 731 people reported that overall surgical menopause was not associated with the risk of dementia (HR: 1.16, 95% CI: 0.96–1.43), but early surgical menopause (≤45 years) was associated with higher risk of dementia (1.70, 95% CI: 1.07–2.69) (Georgakis et al., 2019). Nevertheless, this review did not consider the association of later surgical menopause with dementia. In a recent study using Danish Nurse Cohort which involved 24 851 women over 60 years old, Cecilie et al. found the association between surgery menopause and dementia was 1.18 (0.89–1.56), while this study did not categorize age at surgical menopause, and their findings were limited by the statistical power (Uldbjerg et al., 2022). Using a broad categorization of age at surgical menopause, we found that compared to natural menopause, overall surgical menopause was linked to 10% higher risk of dementia. Besides, there was a U-shape relationship between age at surgical menopause and all-cause dementia risk. Both surgical menopause before 40 years and after 55 years were related to an elevated risk of dementia. We observed that women with later surgical menopause had an increased risk of dementia. However, this relationship should be interpreted with caution. This might be because people with later age at surgical menopause (e.g. ≥55 years) had elder age at baseline than people with later age at natural menopause (63.4 versus 61.6). Also, misclassifications of types of menopause may have occurred. Women who had earlier natural menopause before surgical menopause might not report their age at natural menopause and were classified into the surgical menopause group. Thus, the general trend for late surgical menopause and dementia might be artefactual.
Mechanisms
Several mechanisms have been proposed to explain the association between early menopause and increased risk of dementia. The ‘estrogen hypothesis’ suggests that estrogen has a protective effect against AD dementia. During menopause, the decline in circulating estrogen coincides with a decrease in brain bioenergetics and a shift to a metabolically impaired phenotype in these brain regions (Rettberg et al., 2014). Inadequate or absent compensatory bioenergetic adaptation to inadequate estrogen activation triggers not only hallmark symptoms of menopause but also cognitive changes that increase the risk of late-onset AD in postmenopausal women. Second, estrogen can activate cellular antioxidants, e.g. glutathione, to reduce Aβ deposition. Lack of estrogen in a long term enhances oxidative stress, which may increase neuronal aging and contribute to cognitive impairment. Studies have found that postmenopausal women have higher levels of Aβ than perimenopausal and premenopausal women (Rahman et al., 2020). Besides, some common factors such as adverse CVD risk factors, which were associated with both early reproductive aging and dementia risk might drive the association between them (Zhu et al., 2019).
Ever-used MHT at baseline, socioeconomic status, behavior, and dementia
Evidence on the association of MHT with dementia remains mixed in previous studies. One systematic review and meta-analysis showed that post-MHT was not associated with the risk of all-cause dementia and AD (O'Brien et al., 2014), while another review reported that MHT was linked to 8% higher risk of AD and 16% increased risk of all-cause dementia (Wu et al., 2020). Previous studies did not consider age at menopause when analyzing the effect of MHT. After combining age at menopause and ever-used MHT at baseline, we did not find that taking MHT at baseline increased risk of dementia. A recent population-based cohort study also reported that MHT use in postmenopausal women was not associated with an increased risk of developing dementia (Vinogradova et al., 2021). Income is an indicator of socioeconomic status, contributing in shaping cognitive reserve. Our findings indicated higher income level or higher number of leisure activities were linked to lowers risk of dementia across different ages at menopause. Low income and greater financial strain predict incident dementia (Röhr et al., 2022). Socioeconomic conditions are also associated with cognitive development in early life as well as modifiable risk factors in adulthood that may trigger neuropathological processes (e.g. smoking, physical activity, alcohol consumption, obesity, hypertension, diabetes, loneliness) (Cha et al., 2021). Leisure activities have been associated with lower risk of all-cause dementia, AD, and VD (Su et al., 2022). We observed leisure activities had a synergistic effect with age at menopause on the risk of dementia. Participation in leisure activities means more social connection, social support, and receiving diverse cognitive stimuli, which are all conducive in increasing cognitive reserve.
Strengths and limitations
The strengths of this study include the large sample size which enabled us to examine the associations with dementia subtypes and to perform subgroup or combined analyses. Second, dementia outcomes were ascertained from primary care, hospital admissions, and mortality data, avoiding bias from self-reported data, and have been validated in a previous study. One study demonstrating positive predictive value for all-cause dementia was 80–87% (Wilkinson et al., 2019). Another study reported that the sensitivity and specificity of hospital dementia diagnoses were 78.0% and 92.0%, respectively (Sommerlad et al., 2018). In addition, the interaction effects of menopausal age with MHT used or not at baseline, socioeconomic, and behavioral factors on dementia were also considered.
Our study also has several limitations. First, menopausal age was based on women’s self-report, which might cause recall bias. However, previous studies have proved that the validity and reproducibility of self-reported age at menopause were good (den Tonkelaar, 1997). Second, we used lifestyle factors (e.g. smoking status and alcohol intake), BMI, and MHT reported at baseline (mid-age) as covariates rather than treating them as time-varying covariates, which may cause some bias. Third, detailed definitions of some covariates were lacking (e.g. number of cigarettes smoked per day and alcohol types), and leisure activities included multiple activities without being separated for analysis. Fourth, women who took MHT at baseline before the occurrence of the last menstrual period might cause menses to continue and would artificially raise the reported age at menopause. This could be a source of misclassification in MHT users. Fifth, we lacked information on age initiation, type, and duration of MHT use. This may affect the interpretation of our results to some extent. Sixth, participants of the UK Biobank are relatively healthier (e.g. lower rates of smoking), well educated, and less deprived than the general UK population. This may limit the generalizability and extrapolation of our findings to the broader population. Last, the present study included mainly white women living in the UK who reached menopause while remaining dementia free at baseline; this may limit the generalizability of the findings to other races.
In summary, compared to age at the menopause of 46–50 years, premature (≤40 years) and early (41–45 years) natural menopause were related to increased risk of all-cause dementia. There was a U-shape relationship between age at surgical menopause and risk of dementia, i.e. both surgical menopause before age 40 and after age 55 years were linked to higher risk of dementia. In clinical practice, late-life women who experienced natural menopause or had surgical menopause at an earlier age need close monitoring and engagement for preventive health measures and early diagnosis of dementia.
Supplementary Material
Acknowledgements
This research has been conducted using the UK Biobank Resource under Application Number 68369, and we are grateful to UK Biobank participants.
Contributor Information
Wenting Hao, Centre for Health Management and Policy Research, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, China; NHC Key Lab of Health Economics and Policy Research, Shandong University, Jinan, China.
Chunying Fu, Department of Epidemiology, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, China.
Caiyun Dong, Department of Epidemiology, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, China.
Chunmiao Zhou, Department of Epidemiology, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, China.
Huizi Sun, Department of Epidemiology, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, China.
Ziwei Xie, Department of Epidemiology, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, China.
Dongshan Zhu, Department of Epidemiology, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, China.
Data availability
The data described in the manuscript will be made available for researchers who apply to use the UK Biobank data set by registering and applying at https://www.ukbiobank.ac.uk/enable-your-research/register.
Authors’ roles
W.H. analyzed the data, prepared the figures and tables, and wrote the first draft of the manuscript. All authors participated in searching databases and prepared data. D.Z. contributed to the critical revision of the article.
Funding
National Natural Science Foundation of China (82273702), Science Fund Program for Excellent Young Scholars of Shandong Province (Overseas) (2022HWYQ-030), Taishan Scholars Project Special Fund (No. tsqnz20221103), and the Qilu Young Scholar (Tier-1) Program (202099000066).
Conflict of interest
None declared.
References
- Alzheimer's Disease International CP. World Alzheimer Report 2018. 2018. https://www.alzint.org/resource/world-alzheimer-report-2018/ (8 January 2022, date last accessed).
- Cha H, Farina MP, Hayward MD.. Socioeconomic status across the life course and dementia-status life expectancy among older Americans. SSM Popul Health 2021;15:100921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Tonkelaar I. Validity and reproducibility of self-reported age at menopause in women participating in the DOM-project. Maturitas 1997;27:117–123. [DOI] [PubMed] [Google Scholar]
- Desmond DW, Moroney JT, Paik MC, Sano M, Mohr JP, Aboumatar S, Tseng CL, Chan S, Williams JB, Remien RH. et al. Frequency and clinical determinants of dementia after ischemic stroke. Neurology 2000;54:1124–1131. [DOI] [PubMed] [Google Scholar]
- Dong C, Zhou C, Fu C, Hao W, Ozaki A, Shrestha N, Virani SS, Mishra SR, Zhu D.. Sex differences in the association between cardiovascular diseases and dementia subtypes: a prospective analysis of 464,616 UK Biobank participants. Biol Sex Differ 2022;13:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C, Hao W, Shrestha N, Virani SS, Mishra SR, Zhu D.. Association of reproductive factors with dementia: a systematic review and dose-response meta-analyses of observational studies. EClinicalMedicine 2022;43:101236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamaldo A, Moghekar A, Kilada S, Resnick SM, Zonderman AB, O'Brien R.. Effect of a clinical stroke on the risk of dementia in a prospective cohort. Neurology 2006;67:1363–1369. [DOI] [PubMed] [Google Scholar]
- Geerlings MI, Ruitenberg A, Witteman JC, van Swieten JC, Hofman A, van Duijn CM, Breteler MM, Launer LJ.. Reproductive period and risk of dementia in postmenopausal women. JAMA 2001;285:1475–1481. [DOI] [PubMed] [Google Scholar]
- Georgakis MK, Beskou-Kontou T, Theodoridis I, Skalkidou A, Petridou ET.. Surgical menopause in association with cognitive function and risk of dementia: a systematic review and meta-analysis. Psychoneuroendocrinology 2019;106:9–19. [DOI] [PubMed] [Google Scholar]
- Georgakis MK, Kalogirou EI, Diamantaras A-A, Daskalopoulou SS, Munro CA, Lyketsos CG, Skalkidou A, Petridou ET.. Age at menopause and duration of reproductive period in association with dementia and cognitive function: a systematic review and meta-analysis. Psychoneuroendocrinology 2016;73:224–243. [DOI] [PubMed] [Google Scholar]
- Gilsanz P, Lee C, Corrada MM, Kawas CH, Quesenberry CP Jr, Whitmer RA.. Reproductive period and risk of dementia in a diverse cohort of health care members. Neurology 2019;92:e2005–e2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Harris K, Peters SAE, Woodward M.. Sex differences in the association between major cardiovascular risk factors in midlife and dementia: a cohort study using data from the UK Biobank. BMC Med 2021;19:110–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Harris K, Peters SAE, Woodward M.. Reproductive factors and the risk of incident dementia: a cohort study of UK Biobank participants. PLoS Med 2022;19:e1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivan CS, Seshadri S, Beiser A, Au R, Kase CS, Kelly-Hayes M, Wolf PA.. Dementia after stroke: the Framingham Study. Stroke 2004;35:1264–1268. [DOI] [PubMed] [Google Scholar]
- Langa KM, Larson EB, Crimmins EM, Faul JD, Levine DA, Kabeto MU, Weir DR.. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med 2017;177:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menesgere A, Sundarakumar JS, Shahul Hameed SK, Ravindranath V.. Comparison of risk factors for dementia among rural and urban elderly adults-data from two cohort studies in India. Alzheimers Dement 2023;19:2443–2449. [DOI] [PubMed] [Google Scholar]
- Mielke MM. Sex and gender differences in Alzheimer's disease dementia. Psychiatr Times 2018;35:14–17. [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Brinton RD, Schmidt PJ, Gore AC.. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci 2006;26:10332–10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najar J, Östling S, Waern M, Zettergren A, Kern S, Wetterberg H, Hällström T, Skoog I.. Reproductive period and dementia: a 44-year longitudinal population study of Swedish women. Alzheimers Dement 2020;16:1153–1163. [DOI] [PubMed] [Google Scholar]
- O'Brien J, Jackson JW, Grodstein F, Blacker D, Weuve J.. Postmenopausal hormone therapy is not associated with risk of all-cause dementia and Alzheimer's disease. Epidemiol Rev 2014;36:83–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podcasy JL, Epperson CN.. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin Neurosci 2016;18:437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M, Ali GC, Guerchet M, Prina AM, Albanese E, Wu YT.. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther 2016;8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince MJ, Acosta D, Guerra M, Huang Y, Jimenez-Velazquez IZ, Llibre Rodriguez JJ, Salas A, Sosa AL, Chua KC, Dewey ME. et al. Reproductive period, endogenous estrogen exposure and dementia incidence among women in Latin America and China; A 10/66 population-based cohort study. PLoS One 2018;13:e0192889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Schelbaum E, Hoffman K, Diaz I, Hristov H, Andrews R, Jett S, Jackson H, Lee A, Sarva H. et al. Sex-driven modifiers of Alzheimer risk: a multimodality brain imaging study. Neurology 2020;95:e166–e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettberg JR, Yao J, Brinton RD.. Estrogen: a master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol 2014;35:8–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhr S, Pabst A, Baber R, Engel C, Glaesmer H, Hinz A, Schroeter ML, Witte AV, Zeynalova S, Villringer A. et al. Social determinants and lifestyle factors for brain health: implications for risk reduction of cognitive decline and dementia. Sci Rep 2022;12:12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenaker DA, Jackson CA, Rowlands JV, Mishra GD.. Socioeconomic position, lifestyle factors and age at natural menopause: a systematic review and meta-analyses of studies across six continents. Int J Epidemiol 2014;43:1542–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindi S, Kåreholt I, Ngandu T, Rosenberg A, Kulmala J, Johansson L, Wetterberg H, Skoog J, Sjöberg L, Wang HX. et al. Sex differences in dementia and response to a lifestyle intervention: evidence from Nordic population-based studies and a prevention trial. Alzheimers Dement 2021;17:1166–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerlad A, Perera G, Singh-Manoux A, Lewis G, Stewart R, Livingston G.. Accuracy of general hospital dementia diagnoses in England: sensitivity, specificity, and predictors of diagnostic accuracy 2008-2016. Alzheimers Dement 2018;14:933–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuenkel CA. Menopausal hormone therapy: current considerations. Endocrinol Metab Clin North Am 2015;44:565–585. [DOI] [PubMed] [Google Scholar]
- Stute P, Wienges J, Koller AS, Giese C, Wesemüller W, Janka H, Baumgartner S.. Cognitive health after menopause: does menopausal hormone therapy affect it? Best Pract Res Clin Endocrinol Metab 2021;35:101565. [DOI] [PubMed] [Google Scholar]
- Su S, Shi L, Zheng Y, Sun Y, Huang X, Zhang A, Que J, Sun X, Shi J, Bao Y. et al. Leisure activities and the risk of dementia: a systematic review and meta-analysis. Neurology 2022;99:e1651–e1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M. et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uldbjerg CS, Wilson LF, Koch T, Christensen J, Dehlendorff C, Priskorn L, Abildgaard J, Simonsen MK, Lim YH, Jørgensen JT. et al. Oophorectomy and rate of dementia: a prospective cohort study. Menopause 2022;29:514–522. [DOI] [PubMed] [Google Scholar]
- Vijayan M, Reddy PH.. Stroke, vascular dementia, and Alzheimer's disease: molecular links. J Alzheimers Dis 2016;54:427–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova Y, Dening T, Hippisley-Cox J, Taylor L, Moore M, Coupland C.. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ 2021;374:n2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson T, Schnier C, Bush K, Rannikmäe K, Henshall DE, Lerpiniere C, Allen NE, Flaig R, Russ TC, Bathgate D. et al. ; Dementias Platform UK and UK Biobank. Identifying dementia outcomes in UK Biobank: a validation study of primary care, hospital admissions and mortality data. Eur J Epidemiol 2019;34:557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B, Amouyel P, Andrieu S, Ballard C, Brayne C, Brodaty H, Cedazo-Minguez A, Dubois B, Edvardsson D, Feldman H. et al. Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol 2016;15:455–532. [DOI] [PubMed] [Google Scholar]
- Wu M, Li M, Yuan J, Liang S, Chen Z, Ye M, Ryan PM, Clark C, Tan SC, Rahmani J. et al. Postmenopausal hormone therapy and Alzheimer's disease, dementia, and Parkinson's disease: a systematic review and time-response meta-analysis. Pharmacol Res 2020;155:104693. [DOI] [PubMed] [Google Scholar]
- Yamada M, Mimori Y, Kasagi F, Miyachi T, Ohshita T, Sasaki H.. Incidence and risks of dementia in Japanese women: radiation effects research foundation adult health study. J Neurol Sci 2009;283:57–61. [DOI] [PubMed] [Google Scholar]
- Yoo JE, Shin DW, Han K, Kim D, Won HS, Lee J, Kim SY, Nam GE, Park HS.. Female reproductive factors and the risk of dementia: a nationwide cohort study. Eur J Neurol 2020;27:1448–1458. [DOI] [PubMed] [Google Scholar]
- Zbuk K, Anand SS.. Declining incidence of breast cancer after decreased use of hormone-replacement therapy: magnitude and time lags in different countries. J Epidemiol Community Health 2012;66:1–7. [DOI] [PubMed] [Google Scholar]
- Zhu D, Chung H-F, Dobson AJ, Pandeya N, Giles GG, Bruinsma F, Brunner EJ, Kuh D, Hardy R, Avis NE. et al. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health 2019;4:e553–e564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Chung HF, Pandeya N, Dobson AJ, Hardy R, Kuh D, Brunner EJ, Bruinsma F, Giles GG, Demakakos P. et al. Premenopausal cardiovascular disease and age at natural menopause: a pooled analysis of over 170,000 women. Eur J Epidemiol 2019;34:235–246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data described in the manuscript will be made available for researchers who apply to use the UK Biobank data set by registering and applying at https://www.ukbiobank.ac.uk/enable-your-research/register.