Abstract
The specific growth rate is a key control parameter in the industrial production of baker’s yeast. Nevertheless, quantitative data describing its effect on fermentative capacity are not available from the literature. In this study, the effect of the specific growth rate on the physiology and fermentative capacity of an industrial Saccharomyces cerevisiae strain in aerobic, glucose-limited chemostat cultures was investigated. At specific growth rates (dilution rates, D) below 0.28 h−1, glucose metabolism was fully respiratory. Above this dilution rate, respirofermentative metabolism set in, with ethanol production rates of up to 14 mmol of ethanol · g of biomass−1 · h−1 at D = 0.40 h−1. A substantial fermentative capacity (assayed offline as ethanol production rate under anaerobic conditions) was found in cultures in which no ethanol was detectable (D < 0.28 h−1). This fermentative capacity increased with increasing dilution rates, from 10.0 mmol of ethanol · g of dry yeast biomass−1 · h−1 at D = 0.025 h−1 to 20.5 mmol of ethanol · g of dry yeast biomass−1 · h−1 at D = 0.28 h−1. At even higher dilution rates, the fermentative capacity showed only a small further increase, up to 22.0 mmol of ethanol · g of dry yeast biomass−1 · h−1 at D = 0.40 h−1. The activities of all glycolytic enzymes, pyruvate decarboxylase, and alcohol dehydrogenase were determined in cell extracts. Only the in vitro activities of pyruvate decarboxylase and phosphofructokinase showed a clear positive correlation with fermentative capacity. These enzymes are interesting targets for overexpression in attempts to improve the fermentative capacity of aerobic cultures grown at low specific growth rates.
The quality of commercial baker’s yeast (Saccharomyces cerevisiae) is determined by many parameters, including storage stability, osmotolerance, freeze-thaw resistance, rehydration resistance of dried yeast, and color. In view of the primary role of baker’s yeast in dough, fermentative capacity (i.e., the specific rate of carbon dioxide production by yeast upon its introduction into dough) is a particularly important parameter (2).
In S. cerevisiae, high sugar concentrations and high specific growth rates trigger alcoholic fermentation, even under fully aerobic conditions (6, 18). Alcoholic fermentation during the industrial production of baker’s yeast is highly undesirable, as it reduces the biomass yield on the carbohydrate feedstock. Industrial baker’s yeast production is therefore performed in aerobic, sugar-limited fed-batch cultures. The conditions in such cultures differ drastically from those in the dough environment, which is anaerobic and with sugars at least initially present in excess (23).
Optimization of biomass productivity requires that the specific growth rate and biomass yield in the fed-batch process be as high as possible. In the early stage of the process, the maximum feasible growth rate is dictated by the threshold specific growth rate at which respirofermentative metabolism sets in. In later stages, the specific growth rate is decreased to avoid problems with the limited oxygen transfer and/or cooling capacity of industrial bioreactors (10, 27). The actual growth rate profile during fed-batch cultivation is controlled primarily by the feed rate profile of the carbohydrate feedstock (4, 22). Generally, an initial exponential feed phase is followed by phases with constant and declining feed rates, respectively (8).
From a theoretical point of view, the objective of suppressing alcoholic fermentation during the production phase may interfere with the aim of obtaining a high fermentative capacity in the final product. Process optimization has so far been based on strain selection and on empirical optimization of environmental conditions during fed-batch cultivation (e.g., pH, temperature, aeration rate, and feed profiles of sugar, nitrogen, and phosphorus [5, 10, 23]). For rational optimization of the specific growth rate profile, knowledge of the relation between specific growth rate and fermentative capacity is of primary importance. Nevertheless, quantitative data on this subject cannot be found in the literature.
The chemostat cultivation system allows manipulation of the specific growth rate (which is equal to the dilution rate) while keeping other important growth conditions constant. Similar to industrial fed-batch cultivation, sugar-limited chemostat cultivation allows fully respiratory growth of S. cerevisiae on sugars (21, 37, 39). This is not possible in batch cultures, which by definition require high sugar concentrations, which lead to alcoholic fermentation, even during aerobic growth (6, 18, 37). Thus, as an experimental system, batch cultures bear little resemblance to the aerobic baker’s yeast production process. Indeed, we have recently shown that differences in fermentative capacity between a laboratory strain of S. cerevisiae and an industrial strain became apparent only in glucose-limited chemostat cultures but not in batch cultures (30).
The aim of the present study was to assess the effect of specific growth rate on fermentative capacity in an industrial baker’s yeast strain grown in aerobic, sugar-limited chemostat cultures. Furthermore, the effect of specific growth rate on in vitro activities of key glycolytic and fermentative enzymes was investigated in an attempt to identify correlations between fermentative capacity and enzyme levels.
MATERIALS AND METHODS
Strains and maintenance.
A pure culture of the prototrophic commercial baker’s yeast strain S. cerevisiae DS 28911 was obtained from Gist-Brocades BV, Delft, The Netherlands. Precultures were grown to stationary phase in shake flask cultures on mineral medium (35) adjusted to pH 6.0 and containing 2% (wt/vol) glucose. After addition of sterile glycerol (30%, vol/vol), 2-ml aliquots were stored in sterile vials at −70°C. These frozen stock cultures were used to inoculate precultures for chemostat cultivation.
Media.
The defined mineral medium contained the following (per liter): (NH4)2SO4, 5 g; KH2PO4, 3 g; MgSO4 · 7H2O, 0.5 g; EDTA, 15 mg; ZnSO4 · 7H2O, 4.5 mg; CoCl2 · 6H2O, 0.3 mg; MnCl2 · 4H2O, 1 mg; CuSO4 · 5H2O, 0.3 mg; CaCl2 · 2H2O, 4.5 mg; FeSO4 · 7H2O, 3 mg; NaMoO4 · 2H2O, 0.4 mg; H3BO3, 1 mg; KI, 0.1 mg; and silicone antifoam (BDH), 0.025 ml. The final vitamin concentrations per liter were as follows: biotin, 0.05 mg; calcium pantothenate, 1 mg; nicotinic acid, 1 mg; inositol, 25 mg; thiamine · HCl, 1 mg; pyridoxine · HCl, 1 mg; and para-aminobenzoic acid, 0.2 mg. The medium was prepared and sterilized as described previously (35). For chemostat cultivation, the glucose concentration in reservoir media was 7.5 g · liter−1 (0.25 mol of C · liter−1). Complex medium for YEPD-agar plates contained the following (per liter): yeast extract (Difco), 10 g; peptone from casein (Merck), 20 g; d-glucose, 20 g; and agar (Difco), 20 g.
Chemostat cultivation.
Aerobic chemostat cultivation was performed at 30°C in laboratory fermentors (Applikon, Schiedam, The Netherlands) at a stirrer speed of 800 rpm. The working volume of the cultures was kept at 1.0 liter by a peristaltic effluent pump coupled to an electrical level sensor. This setup ensured that under all growth conditions, biomass concentrations in samples taken directly from the culture differed by <1% from biomass concentrations in samples taken from the effluent line (15). The exact working volume was measured after each experiment. The pH was kept at 5.0 ± 0.1 by an ADI 1030 biocontroller, via the automatic addition of 2 mol of KOH · liter−1. The fermentor was flushed with air at a flow rate of 0.5 liter · min−1 with a Brooks 5876 mass-flow controller. The dissolved oxygen concentration was continuously monitored with an oxygen electrode (model 34 100 3002; Ingold) and remained above 60% air saturation. Steady-state data refer to cultures without detectable oscillations. Chemostat runs were started at a dilution rate (D) of 0.20 h−1. After steady states had been established at higher or lower dilution rates, the culture was brought back to D = 0.20 h−1 to check for hysteresis effects. These effects were not found (data not shown). A steady state was defined as the situation in which at least five volume changes had passed after the last change in growth conditions and in which the biomass concentration, the fermentative capacity, and the specific rates of carbon dioxide production and oxygen consumption had remained constant (<2% variation) over two volume exchanges. This typically required six to eight volume exchanges after each change in the dilution rate. In control experiments which, after the initial batch cultivation, were started at low dilution rates (<0.15 h−1) it was found that fermentative capacity required more volume changes to reach a constant value than did culture dry weight determination and gas analysis (data not shown). Chemostat cultures were routinely checked for purity by performing phase-contrast microscopy and by plating culture samples on YEPD-agar plates. The minor loss of ethanol in the off-gas due to evaporation, which occurs at high dilution rates (26, 36), was not accounted for in calculations of carbon recoveries of steady-state chemostat cultures. These calculations were based on a carbon content in dry yeast biomass of 48%.
Gas analysis.
The exhaust gas was cooled in a condenser (2°C) and dried with a Perma Pure dryer type PD-625-12P. O2 and CO2 concentrations were determined with a Servomex type 1100A analyzer and a Beckman 864 infrared detector, respectively. Determination of the exhaust gas flow rate and calculation of specific rates of CO2 production and O2 consumption were performed as described previously (31, 38).
Determination of culture dry weight.
Culture samples (10 ml) were filtered over preweighed nitrocellulose filters (pore size, 0.45 μm; Gelman Sciences). After removal of medium, the filters were washed with demineralized water, dried in a Sharp type R-4700 microwave oven for 20 min at 360-W output, and weighed. Duplicate determinations gave results that varied by <1%.
Determination of fermentative capacity.
Samples containing exactly 100 mg (dry weight) of biomass from a steady-state chemostat culture were harvested by centrifugation at 5,000 × g for 5 min, washed once, and resuspended in 5 ml of 0.9% (wt/vol) NaCl solution. Subsequently, these cell suspensions were introduced into a thermostatted (30°C) vessel containing 10 ml of fivefold-concentrated mineral medium (pH 5.6). The volume was adjusted to 40 ml with demineralized water. After a 10-min incubation, 10 ml of a glucose solution (100 g · liter−1) was added and samples (1 ml) were taken at appropriate time intervals. The 10-ml headspace was continuously flushed with water-saturated carbon dioxide, at a flow rate of approximately 10 ml · min−1. The ethanol concentration in the supernatant was determined by a colorimetric assay (32) with partially purified alcohol oxidase from Hansenula polymorpha (a kind gift of Bird Engineering, Schiedam, The Netherlands). Fermentative capacity, calculated from the increase of the ethanol concentration during the first 30 min of the experiments, was expressed as millimoles of ethanol produced per gram of dry yeast biomass per hour. During this period, the increase in biomass concentration was negligible and the increase in ethanol concentration was linear with time and proportional to the amount of biomass present. When cells pregrown in a glucose-limited chemostat culture (D = 0.10 h−1) were used, the specific rate of ethanol production measured by this assay differed by less than 10% from the specific rate of carbon dioxide production in a 3% (wt/vol) sucrose-enriched dough (30).
Metabolite analysis.
Glucose in reservoir media and supernatants was determined enzymically with a glucose oxidase kit (Merck systems kit 14144; detection limit, ca. 5 μM). Ethanol, glycerol, and pyruvic acid were determined by high-pressure liquid chromatography analysis with an HPX-87H Aminex ion-exchange column (300 by 7.8 mm; Bio-Rad) at 60°C. The column was eluted with 5 mM sulfuric acid at a flow rate of 0.6 ml · min−1. Pyruvic acid was detected by a Waters 441 UV meter at 214 nm, coupled to a Waters 741 data module. Ethanol and glycerol were detected by an ERMA type ERC-7515A refractive index detector coupled to a Hewlett-Packard type 3390A integrator. Acetic acid was determined with Boehringer test kit 148261 (detection limit, ca. 0.2 mM).
Preparation of cell extracts.
For preparation of cell extracts, culture samples were harvested by centrifugation, washed twice with 10 mM potassium phosphate buffer (pH 7.5) containing 2 mM EDTA, concentrated fourfold, and stored at −20°C. Before being assayed, the samples were thawed, washed, and resuspended in 100 mM potassium phosphate buffer (pH 7.5) containing 2 mM MgCl2 and 1 mM dithiothreitol. Extracts were prepared by sonication with 0.7-mm-diameter glass beads at 0°C in an MSE sonicator (150-W output, 7-μm peak-to-peak amplitude) for 3 min at 0.5-min intervals. Unbroken cells were removed by centrifugation at 36,000 × g for 20 min at 4°C. In all cultures investigated, this method released 53% ± 4% of the total protein present in the cell samples. The supernatant was used as the cell extract.
Enzyme assays.
Enzyme assays were performed with a Hitachi 100-60 spectrophotometer at 30°C and 340 nm (E340 of reduced pyridine dinucleotide cofactors, 6.3 mM−1) with freshly prepared extracts. All enzyme activities are expressed as moles of substrate converted per minute per milligram of protein. When necessary, extracts were diluted in sonication buffer. All assays were performed with two concentrations of cell extract. The specific activities of these duplicate experiments differed by <10%.
Hexokinase (EC 2.7.1.1) was assayed by the method of Postma et al. (20). Phosphoglucose isomerase (EC 5.3.1.9) was assayed by the method of Bergmeyer (3) with minor modifications. The assay mixture contained 50 mM Tris-HCl buffer (pH 8.0), 5 mM MgCl2, 0.4 mM NADP+, 1.8 U of glucose-6-phosphate dehydrogenase (Boehringer) · ml−1, and cell extract. The reaction was started with 2 mM fructose-6-phosphate. Phosphofructokinase (EC 2.7.1.11) was assayed by the method of de Jong-Gubbels et al. (7) with minor modifications. The assay mixture contained 50 mM imidazole-HCl (pH 7.0), 5 mM MgCl2, 0.15 mM NADH, 0.10 mM fructose-2,6-diphosphate, 0.5 U of fructose-1,6-diphosphate aldolase (Boehringer) · ml−1, 0.6 U of glycerol-3-phosphate dehydrogenase (Boehringer) · ml−1, 1.8 U of triosephosphate isomerase (Boehringer) · ml−1, and cell extract. The endogenous activity was measured after addition of 0.25 mM fructose-6-phosphate. The reaction was started with 0.5 mM ATP. Fructose-1,6-diphosphate aldolase (EC 4.1.2.13) was assayed by the method of van Dijken et al. (28). Triosephosphate isomerase (EC 5.3.1.1) was assayed by the method of Bergmeyer (3) with minor modifications. The assay mixture contained 100 mM triethanolamine-HCl buffer (pH 7.6), 0.15 mM NADH, 8.5 U of glycerol-3-phosphate dehydrogenase (Boehringer) · ml−1, and cell extract. The reaction was started with 6 mM glyceraldehyde-3-phosphate. Glyceraldehyde-3-phosphate dehydrogenase (EC 1.2.1.12) was assayed by the method of Bergmeyer (3) with minor modifications. The assay mixture contained 100 mM triethanolamine-HCl buffer (pH 7.6), 1 mM ATP, 1 mM EDTA, 1.5 mM MgSO4, 0.15 mM NADH, 22.5 U of phosphoglycerate kinase (Boehringer) · ml−1, and cell extract. The reaction was started with 5 mM 3-phosphoglycerate. The assay of phosphoglycerate kinase (EC 2.7.2.3) was identical to that of glyceraldehyde-3-phosphate dehydrogenase, except that phosphoglycerate kinase was replaced by 8.0 U of glyceraldehyde-3-phosphate dehydrogenase (Boehringer) · ml−1.
Phosphoglycerate mutase (EC 2.7.5.3) was assayed by the method of Bergmeyer (3). Enolase (EC 4.2.1.11) was assayed by the method of Bergmeyer (3) with minor modifications. The assay mixture contained 100 mM triethanolamine-HCl buffer (pH 8.0), 1.5 mM MgSO4, 0.15 mM NADH, 10 mM ADP, 26.3 U of pyruvate kinase (Sigma) · ml−1, 11.3 U of lactate dehydrogenase (Boehringer) · ml−1, and cell extract. The reaction was started with 1 mM 2-phosphoglycerate. Pyruvate kinase (EC 2.7.1.40) was assayed by the method of de Jong-Gubbels et al. (7) with minor modifications. The assay mixture contained 100 mM cacodylic acid-KOH (pH 6.2), 100 mM KCl, 10 mM ADP, 1 mM fructose-1,6-diphosphate, 25 mM MgCl2, 0.15 mM NADH, 11.25 U of lactate dehydrogenase (Boehringer) · ml−1, and cell extract. The reaction was started with 2 mM phosphoenolpyruvate. Pyruvate decarboxylase (EC 4.1.1.1) and alcohol dehydrogenase (EC 1.1.1.1) were assayed by the method of Postma et al. (20).
Protein determinations.
The protein content of whole cells was estimated by a modified biuret method (33). Protein concentrations in cell extracts were determined by the Lowry method. Dried bovine serum albumin (fatty-acid free; Sigma) was used as a standard.
RESULTS
Growth and metabolite formation in glucose-limited chemostat cultures.
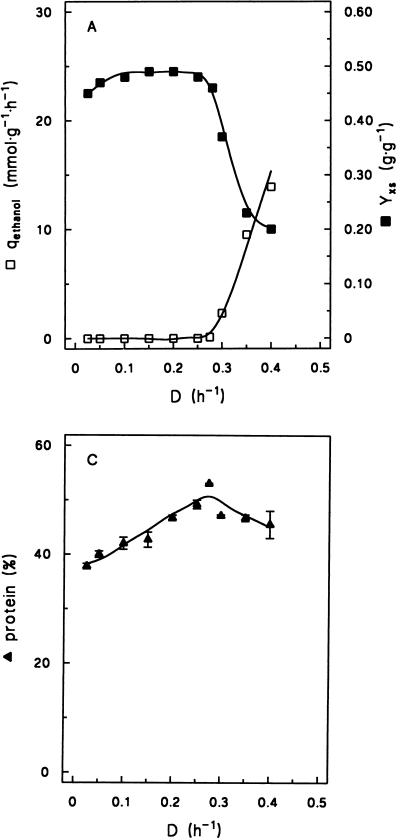
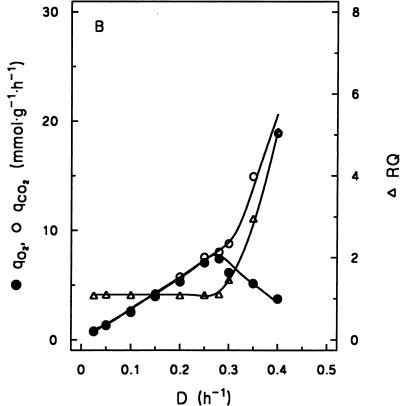
In batch cultures, the maximum specific growth rate of the industrial baker’s yeast strain DS28911 on glucose in a defined mineral medium with vitamins was 0.42 h−1 (30). Steady-state chemostat cultures were studied at dilution rates ranging from 0.025 to 0.40 h−1. In contrast to cultures of many laboratory yeast strains (13, 17), this strain did not exhibit spontaneous synchronization of the cell cycle with associated metabolic oscillations. At dilution rates between 0.025 and 0.28 h−1, ethanol and other typical fermentation products were absent from the culture supernatants and glucose carbon could be quantitatively recovered as biomass and carbon dioxide (Table 1; Fig. 1A and B). The fully respiratory metabolism of these cultures was further evident from the fact that the respiratory coefficient (RQ; ratio of specific rates of CO2 production and O2 consumption) was close to unity (Fig. 1B).
TABLE 1.
Cell yields, metabolic fluxes, and carbon recovery as a function of the dilution rate in aerobic, glucose-limited chemostat cultures of S. cerevisiae DS28911a
| D (h−1) | Yield (g · g−1) | qO2 | qCO2 | qglucose | qethanol | qacetate | qpyruvate | qglycerol | Carbon recovery (%)b |
|---|---|---|---|---|---|---|---|---|---|
| 0.025 | 0.45 | 0.8 | 0.8 | 0.3 | 0c | 0 | 0 | 0 | 98.9 |
| 0.05 | 0.47 | 1.3 | 1.4 | 0.6 | 0 | 0 | 0 | 0 | 95.0 |
| 0.10 | 0.48 | 2.5 | 2.7 | 1.1 | 0 | 0 | 0 | 0 | 96.0 |
| 0.15 | 0.49 | 3.9 | 4.2 | 1.7 | 0 | 0 | 0 | 0 | 102.4 |
| 0.20 | 0.48 | 5.3 | 5.7 | 2.3 | 0 | 0 | 0 | 0 | 100.9 |
| 0.25 | 0.48 | 7.0 | 7.5 | 2.8 | 0 | 0 | 0 | 0 | 102.6 |
| 0.28 | 0.46 | 7.4 | 8.0 | 3.4 | 0.11 | 0.08 | 0.01 | 0 | 97.0 |
| 0.30 | 0.37 | 6.1 | 8.8 | 4.5 | 2.3 | 0.41 | 0.01 | 0 | 99.1 |
| 0.35 | 0.23 | 5.1 | 14.9 | 8.6 | 9.5 | 0.62 | 0.03 | 0.05 | 99.4 |
| 0.40 | 0.20 | 3.7 | 18.9 | 11.1 | 13.9 | 0.60 | 0.05 | 0.15 | 97.9 |
Fluxes (q) are expressed as millimoles per gram of dry yeast biomass per hour.
Carbon recoveries were based on a carbon content of dry yeast biomass of 48%.
0, below detection limit.
FIG. 1.
Physiology of S. cerevisiae DS28911 in aerobic, glucose-limited chemostat cultures grown at various specific growth rates (i.e., dilution rate [D]). Data are presented as the means and standard deviations of results from duplicate assays at different time points in the same steady-state chemostat cultures. (A) Biomass yield (Ysx; grams of dry yeast biomass per gram of glucose) and specific rate of ethanol production (qethanol; millimoles per gram of dry yeast biomass per hour). (B) Specific rates of oxygen consumption (qO2; millimoles per gram of dry yeast biomass per hour), carbon dioxide production (qCO2, millimoles per gram of dry yeast biomass per hour), and the ratio of qO2 to qCO2 (RQ). (C) Protein content (percentage of dry yeast biomass).
The biomass yield of 0.49 g · (g of glucose)−1 found at dilution rates between 0.10 and 0.25 h−1 (Fig. 1A) is a typical value for respiratory S. cerevisiae cultures (34). The slight decrease at even lower dilution rates is probably due to the increased relative contribution of the maintenance energy requirement to the overall energy budget (19).
At D = 0.28 h−1, the specific oxygen consumption rate reached a maximum of 7.4 mmol · g of dry yeast biomass−1 · h−1 (Fig. 1B). At higher dilution rates, a respirofermentative metabolism occurred, as is evident from the production of ethanol by the cultures (Fig. 1A) and an increase of the RQ (Fig. 1B). The increase of the RQ was due mainly to a sharp increase of the specific rate of CO2 production (due to the onset of alcoholic fermentation) but also to a decrease of the specific O2 consumption rate (Fig. 1B). At dilution rates above 0.28 h−1, the biomass yield on glucose decreased sharply due to the lower ATP yield from fermentative glucose metabolism (Table 1; Fig. 1A).
The protein content of the biomass increased linearly with increasing specific growth rate from 38% at D = 0.025 h−1 to 53% at D = 0.28 h−1 (Fig. 1C). At higher dilution rates, the protein content decreased again, to 46% at D = 0.40 h−1.
Effect of specific growth rate on fermentative capacity.
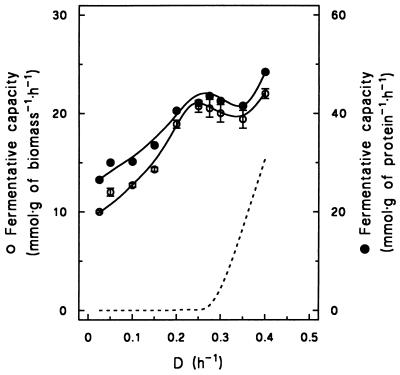
Despite the absence of alcoholic fermentation in chemostat cultures grown at dilution rates below D = 0.28 h−1, a substantial fermentative capacity became apparent when cells were incubated with excess glucose under anaerobic conditions. The fermentative capacity increased linearly with increasing dilution rate, from 10.0 mmol of ethanol · g of dry yeast biomass−1 · h−1 at D = 0.025 h−1 to 20.7 mmol of ethanol · g of dry yeast biomass−1 · h−1 at D = 0.28 h−1 (Fig. 2). Above D = 0.28 h−1, the fermentative capacity slightly decreased to 19.4 mmol · g of dry yeast biomass−1 · h−1 at D = 0.35 h−1. Only at the highest dilution rate studied (D = 0.40 h−1) was a higher fermentative capacity of 22.0 mmol · g of dry yeast biomass−1 · h−1 observed. This result is unlikely to be due to experimental variation, since a similarly high fermentative capacity (25.3 mmol of ethanol · g of dry yeast biomass−1 · h−1) was observed in exponentially growing batch cultures of strain DS28911, which exhibit a specific growth rate of 0.42 h−1 (30). Qualitatively, the pattern of fermentative capacity versus specific growth rate did not change when the specific activity was expressed per amount of yeast protein rather than per amount of dry yeast biomass (Fig. 2).
FIG. 2.
Effect of specific growth rate on the fermentative capacity of S. cerevisiae DS28911, expressed as millimoles of ethanol produced per gram of dry yeast biomass and expressed per gram of cell protein. Fermentative capacity was assayed anaerobically under a CO2 atmosphere in complete mineral medium supplemented with 2% (wt/vol) glucose. The dashed line indicates the specific rate of ethanol production (qethanol; millimoles per gram of dry yeast biomass per hour) in chemostat cultures (Fig. 1A). Data are presented as the means and standard deviations of results from duplicate assays at different time points in the same steady-state chemostat cultures.
Correlation of enzyme levels in steady-state cultures with fermentative capacity.
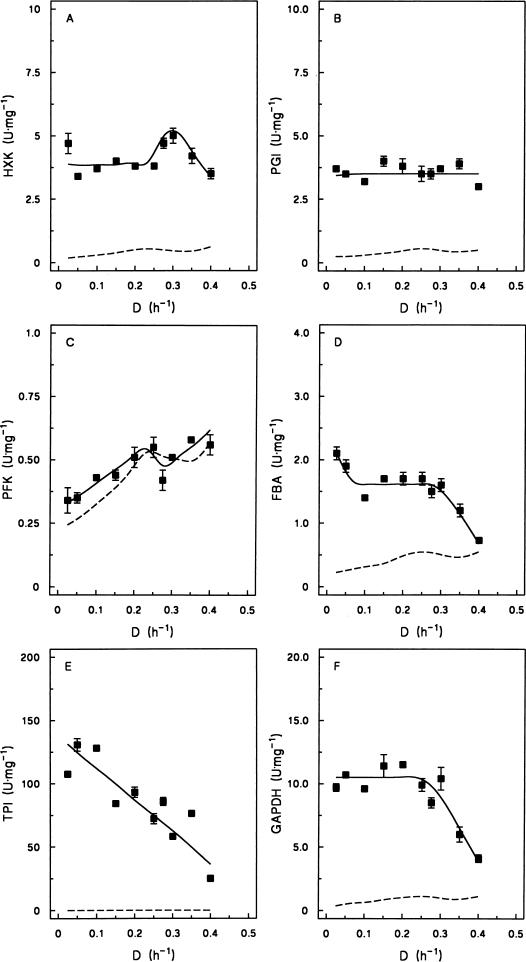
To investigate whether the profile of fermentative capacity versus specific growth rate shown in Fig. 2 could be correlated with the levels of key enzymes of glucose catabolism, the activities of all glycolytic enzymes as well as those of the fermentative key enzymes pyruvate decarboxylase and alcohol dehydrogenase were determined in cell extracts (Fig. 3). By taking into account the soluble-protein content of S. cerevisiae cells, specific enzyme activities in cell extracts (expressed as micromoles of substrate converted per minute per milligram of protein) could be compared with the fermentative capacity in the off-line assays (which was expressed as millimoles of ethanol per hour per gram of dry yeast biomass). This comparison revealed that in almost all cases, the enzyme activities measured in cell extracts were sufficiently high to explain the flux through the glycolytic pathway found in the off-line assays (Fig. 3).
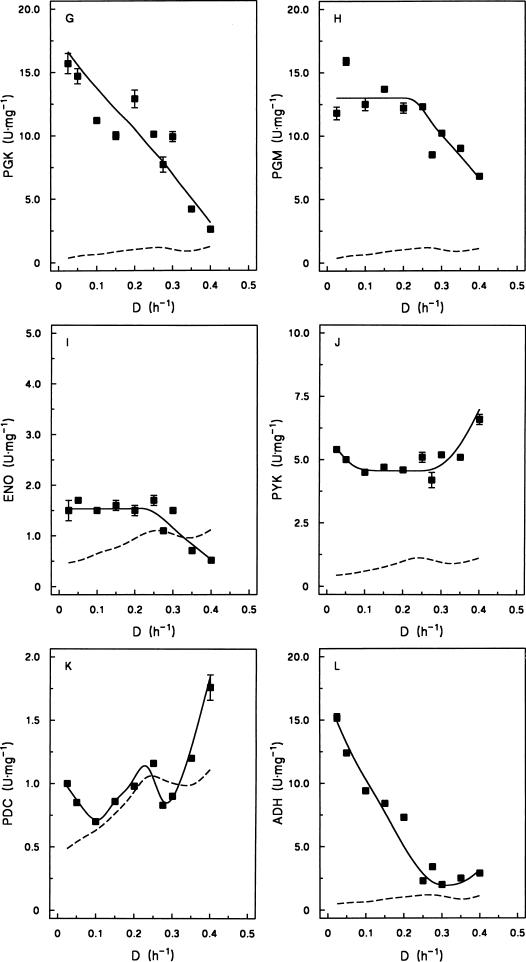
FIG. 3.
Effect of specific growth rate on the specific activities of enzymes involved in alcoholic fermentation of glucose in cell extracts of S. cerevisiae DS28911 grown under aerobic, glucose-limited conditions. The dashed lines represent the calculated specific in vivo activities of the enzymes in the off-line fermentation assay (Fig. 2), based on a soluble protein content of dry yeast biomass of 33% (21). Standard deviations are based on duplicate enzyme assays on samples taken at different time points in the same steady-state chemostat cultures. Note that the y axes represent different activity scales in different panels. The in vivo activities of the enzymes in panels E to L (C3-converting enzymes) are twice as high as those of the enzymes in panels A to D (hexose-converting enzymes). Abbreviations: HXK, hexokinase (A); PGI, phosphoglucose isomerase (B); PFK, phosphofructokinase (C); FBA, fructose-1,6-diphosphate aldolase (D); TPI, triosephosphate isomerase (E); GAPDH, glyceraldehyde-3-phosphate dehydrogenase (F); PGK, phosphoglycerate kinase (G); PGM, phosphoglycerate mutase (H); ENO, enolase (I); PYK, pyruvate kinase (J); PDC, pyruvate decarboxylase (K); ADH, alcohol dehydrogenase (L).
The relationship between specific growth rate and in vitro enzyme activity differed markedly among the enzyme activities investigated. The activities of triosephosphate isomerase, phosphoglycerate kinase, and alcohol dehydrogenase decreased with increasing growth rate over the range of dilution rates investigated. Glyceraldehyde phosphate dehydrogenase, phosphoglycerate mutase, fructose bisphosphate aldolase, and enolase activities remained constant at low dilution rates but decreased above D = 0.28 h−1 when respirofermentative metabolism occurred. At the highest dilution rate studied (D = 0.40 h−1), the levels of fructose bisphosphate aldolase, glyceraldehyde-3-phosphate dehydrogenase, phosphoglycerate kinase, enolase, and alcohol dehydrogenase were close to the calculated enzyme activity required to sustain the observed rate of glucose catabolism in the off-line fermentative capacity assays (Fig. 3). The in vitro activity of phosphoglucose isomerase was essentially independent of the dilution rate, whereas hexokinase and pyruvate kinase activities remained constant at low specific growth rates but increased above the critical dilution rate of 0.28 h−1. Phosphofructokinase and pyruvate decarboxylase stood out as enzymes whose in vitro activity increased with increasing specific growth rate and which thus exhibited a clear positive correlation with fermentative capacity.
DISCUSSION
Physiology of S. cerevisiae DS28911 in aerobic chemostat cultures.
The threshold dilution rate at which aerobic fermentation sets in in aerobic, glucose-limited chemostat cultures of S. cerevisiae (Dcrit) appears to be a strain-dependent property: reported values range from 0.16 h−1 (18) to 0.38 h−1 (21) for different laboratory strains. The Dcrit of 0.28 h−1 found for the industrial strain DS28911 (Table 1; Fig. 1A and B) was close to that observed for a number of other strains (1, 24, 37).
Qualitatively, the patterns of biomass production and metabolite formation as a function of the specific growth rate of the industrial strain used in this study are similar to those reported for other S. cerevisiae strains (1, 21, 24, 37). A notable difference involved the production of acetate and pyruvate, which has been reported to occur at dilution rates slightly below Dcrit in laboratory strains (1, 21). In strain DS28911, acetate and pyruvate production were detected above D = 0.28 h−1 (Table 1) only when ethanol production also became apparent (Table 1; Fig. 1A).
A decline of the specific oxygen consumption rate (qO2) at dilution rates above Dcrit (Fig. 1B) was also found in earlier studies and was attributed to glucose repression of the synthesis of respiratory enzymes (1, 9, 37). It has been reported that this repression may be overcome by long-term adaptation of respirofermentative cultures. During this long-term adaptation, which may take more than 100 generations, specific rates of oxygen uptake eventually reach constant values at dilution rates above Dcrit, although metabolism remains respirofermentative (1, 21). Since this long-term adaptation seems of little relevance for the dynamic industrial fed-batch process, which involves only about 12 to 20 h of cultivation (4), the definition of steady-state conditions given in Materials and Methods was used throughout the present study.
Effect of cultivation conditions on fermentative capacity.
The fermentative capacity of the industrial strain S. cerevisiae DS28911 was strongly affected by the specific growth rate of the glucose-limited, aerobic chemostat cultures (Fig. 2). Surprisingly, no clear correlation was observed between the fermentative capacity found under anaerobic conditions in the presence of 2% (wt/vol) glucose and the in situ rate of alcoholic fermentation in the aerobic, glucose-limited chemostat cultures (Fig. 2). A substantial fermentative capacity was expressed in cultures grown at low dilution rates, which exhibited a completely respiratory glucose metabolism (Fig. 1 and 2). In its natural environment, this may allow S. cerevisiae to respond rapidly to fluctuations of oxygen availability. The fermentative capacity showed an almost linear correlation with specific growth rates in respiratory cultures where the specific growth rate (μ) was less than μcrit. A slight further increase was observed only at specific growth rates close to the maximum growth rate (μmax), where vigorous aerobic fermentation was observed in the cultures. These results indicate that optimization of baker’s yeast production with respect to fermentative capacity does not necessarily interfere with the aim of optimizing biomass productivity. With the industrial strain used in this study, both goals can be met by maximizing the specific growth rate during the process but without exceeding the specific growth rate at which aerobic fermentation sets in.
Other factors as well as specific growth rate are likely to affect the fermentative capacity. Of particular importance in this respect is medium composition. Most industrial baker’s yeast processes use complex feeds (mostly containing molasses) instead of defined mineral media. Our results demonstrate that in studies of the optimization of medium composition and other process parameters, the specific growth rate should be controlled. This implies that batch cultivation cannot be used for this purpose, since, for example, medium composition may affect the specific growth rate in such cultures.
As mentioned above, oxygen transfer necessitates a progressive decrease of the specific growth rate during the fed-batch production process, leading to average specific growth rates of around 0.15 h−1 during fed-batch processes for baker’s yeast production (23). In particular, during the final phase of the industrial process, where the specific growth rate decreases continuously, the relationship between specific growth rate and fermentative capacity is bound to differ from that under the steady-state conditions in chemostat cultures. Accurate prediction of fermentative capacity under such dynamic conditions requires studies of its regulation under transient conditions.
With respect to attempts to improve fermentative capacity by genetic engineering, the main challenge is to improve the fermentative capacity at low specific growth rates. It is clear that evaluation of the success of such genetic attempts should involve measurement not only of fermentative capacity but also of other important characteristics of the engineered strains. In particular, it will be of interest to see whether increased fermentative capacity at low specific growth rates may negatively affect the specific growth rate at which aerobic fermentation sets in.
This study was performed with a pure culture of an industrial baker’s yeast strain. We have recently obtained evidence that regulation of the fermentative capacity in industrial strains may differ from that in typical laboratory strains of S. cerevisiae (30). Indeed, preliminary studies with a laboratory strain suggested that the fermentative capacity was constant below Dcrit and increased upon the onset of respirofermentative metabolism (29). Detailed comparative studies of laboratory strains and industrial strains may provide further insight in the molecular mechanisms that control fermentative capacity in S. cerevisiae.
Relation between fermentative capacity and enzyme levels.
In theory, control of fermentative capacity can reside in any (combination) of three processes: (i) sugar uptake, (ii) catabolism of sugars via glycolysis and alcoholic fermentation, and (iii) regeneration of ADP from the ATP produced in glycolysis. In this study, attention was focused on the effect of the specific growth rate on the levels of enzymes involved in glycolysis and alcoholic fermentation.
There are a number of potential pitfalls in attempts to correlate enzyme activities in cell extracts with metabolic processes occurring in intact cells. For example, the concentrations of substrates and effectors in in vitro enzyme assays have generally been optimized to give maximum activity. Provided that the environmental conditions in the assay resemble those in the yeast cell, data from such assays can provide an indication of the maximum capacity of an enzymatic reaction (without, in most cases, discriminating between the activities of isoenzymes [12]). Based on the assumption that the activities of key enzymes measured in cell extracts accurately reflected their in vivo capacity (maximum rate of metabolism [Vmax]), many enzymes appeared to operate substantially below their Vmax in the fermentative-capacity assays when cells were pregrown at low specific growth rates (Fig. 3). Conversely, at high specific growth rates, enzyme activity in cell extracts was in many cases close to the in vivo glycolytic flux (Fig. 3). This observation suggests that these enzymes operate close to saturation in cells growing at μmax in batch cultures. This may, at least in part, explain the observation that overexpression of individual glycolytic enzymes in exponentially growing batch cultures of S. cerevisiae does not increase their rate of alcoholic fermentation (25).
It is not possible to identify rate-controlling reactions based on the data presented in Fig. 3. Moreover, two processes that have been proposed in the literature to contribute to controlling the glycolytic flux, namely, glucose uptake (11, 16) and ADP regeneration (14), should also be taken into account. Nevertheless, our data strongly suggest that a substantial improvement of fermentative capacity will at least require the combined overexpression of pyruvate decarboxylase and phosphofructokinase. Only these two enzymes, whose in vitro activities were measured in the physiological direction, exhibited a positive correlation with fermentative capacity at low specific growth rates, while their in vitro activities were close to their estimated activities in the whole-cell fermentative-capacity assay (Fig. 3).
ACKNOWLEDGMENTS
We thank our colleagues of the Delft-Leiden Yeast Group and André Terwisscha van Scheltinga, Lex de Boer, and Rutger van Rooijen from Gist-Brocades B.V., Delft, The Netherlands, for many stimulating discussions.
This work was financially supported by Gist-Brocades B.V. and by the Dutch Ministry of Economic Affairs (EET program). Research in our group is supported by the European Community (via the research project “From Gene to Product in Yeast: a Quantitative Approach,” which is part of the EC Framework IV Cell Factory Program).
REFERENCES
- 1.Barford J P, Hall R J. An examination of the Crabtree effect in Saccharomyces cerevisiae: the role of respiratory adaptation. J Gen Microbiol. 1979;114:267–275. [Google Scholar]
- 2.Benitez B, Gasent-Ramirez J M, Castrejon F, Codon A C. Development of new strains for the food industry. Biotechnol Prog. 1996;12:149–163. [Google Scholar]
- 3.Bergmeyer H U. Methods of enzymatic analysis. 2nd ed. Vol. 1. New York, N.Y: Academic Press, Inc.; 1974. pp. 501–502. [Google Scholar]
- 4.Beudeker R F, van Dam H W, van der Plaat J B, Vellega K. Developments in bakers’ yeast production. In: Verachtert H, De Mot R, editors. Yeast biotechnology and biocatalysis. New York, N.Y: Marcel Dekker, Inc.; 1990. pp. 103–146. [Google Scholar]
- 5.Chen S L, Chiger M. Production of bakers’ yeast. In: Moo-Young M, editor. Comprehensive biotechnology. Vol. 3. Oxford, United Kingdom: Pergamon Press; 1985. pp. 429–455. [Google Scholar]
- 6.De Deken R H. The Crabtree effect: a regulatory system in yeast. J Gen Microbiol. 1966;44:149–156. doi: 10.1099/00221287-44-2-149. [DOI] [PubMed] [Google Scholar]
- 7.de Jong-Gubbels P, Vanrolleghem P, Heijnen J J, van Dijken J P, Pronk J T. Regulation of carbon metabolism in chemostat cultures of Saccharomyces cerevisiae grown on mixtures of glucose and ethanol. Yeast. 1995;11:407–418. doi: 10.1002/yea.320110503. [DOI] [PubMed] [Google Scholar]
- 8.Enfors S O, Hedenberg J, Olsson K. Simulation of the dynamics in the bakers’ yeast process. Bioprocess Eng. 1990;5:191–198. [Google Scholar]
- 9.Käppeli O, Gschwend-Petrik M, Fiechter A. Transient responses of Saccharomyces uvarum to a change of the growth-limiting nutrient in continuous culture. J Gen Microbiol. 1985;131:47–52. [Google Scholar]
- 10.Kristiansen B. Integrated design of fermentation plant. The production of bakers’ yeast. Weinheim, Germany: VCH Verlagsgesellschaft mbH; 1994. pp. 1–83. [Google Scholar]
- 11.Lagunas R, Dominguez C, Busturia A, Sáez M J. Mechanisms of appearance of the Pasteur effect in Saccharomyces cerevisiae: inactivation of the sugar transport system. J Bacteriol. 1982;152:19–25. doi: 10.1128/jb.152.1.19-25.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mewes H W, et al. The yeast genome directory. Nature. 1997;387:35–66. [PubMed] [Google Scholar]
- 13.Münch T. Zellzyklusdynamik von Saccharomyces cerevisiae in Bioprozessen. Ph.D. thesis. Zurich, Switzerland: Technische Hochschule Zürich; 1992. [Google Scholar]
- 14.Navas M A, Cerdán S, Gancedo J M. Futile cycles in Saccharomyces cerevisiae strains expressing gluconeogenic enzymes during growth on glucose. Proc Natl Acad Sci USA. 1993;90:1290–1294. doi: 10.1073/pnas.90.4.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noorman H J, Baksteen J, Heijnen J J, Luyben K C A M. The bioreactor overflow device: an undesired selective separator in continuous cultures? J Gen Microbiol. 1991;13:2171–2177. [Google Scholar]
- 16.Oehlen L J W M, Scholte M E, de Koning W, van Dam K. Decrease in glycolytic flux in Saccharomyces cerevisiae cdc35-1 cells at restrictive temperature correlates with a decrease in glucose transport. Microbiology. 1994;140:1891–1898. doi: 10.1099/13500872-140-8-1891. [DOI] [PubMed] [Google Scholar]
- 17.Parulekar S J, Semones G B, Rolf M J, Lievense J C, Lim H C. Induction and elimination of oscillations in continuous cultures of Saccharomyces cerevisiae. Biotechnol Bioeng. 1986;28:700–710. doi: 10.1002/bit.260280509. [DOI] [PubMed] [Google Scholar]
- 18.Petrik M, Käppeli O, Fiechter A. An expanded concept for glucose effect in the yeast Saccharomyces uvarum: involvement of short- and long-term regulation. J Gen Microbiol. 1983;129:43–49. [Google Scholar]
- 19.Pirt S J. The maintenance energy of bacteria in growing cultures. Proc R Soc London Ser B. 1965;163:224–231. doi: 10.1098/rspb.1965.0069. [DOI] [PubMed] [Google Scholar]
- 20.Postma E, Scheffers W A, van Dijken J P. Adaptation of the kinetics glucose transport to environmental conditions in the yeast Candida utilis CBS 621: a continuous-culture study. J Gen Microbiol. 1988;134:1109–1116. [Google Scholar]
- 21.Postma E, Verduyn C, Scheffers W A, van Dijken J P. Enzymatic analysis of the Crabtree effect in glucose-limited chemostat cultures of Saccharomyces cerevisiae. Appl Environ Microbiol. 1989;53:468–477. doi: 10.1128/aem.55.2.468-477.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reed G. Prescott & Dunn’s industrial microbiology. 4th ed. Westport, Conn: AVI Publishing Co., Inc.; 1982. pp. 593–633. [Google Scholar]
- 23.Reed G, Nagodawithana T W. Yeast technology. 2nd ed. 1991. pp. 261–313. and 315–368. Van Nostrand Reinhold, New York, N.Y. [Google Scholar]
- 24.Rieger M, Käppeli O, Fiechter A. The role of limited respiration in the incomplete oxidation of glucose by Saccharomyces cerevisiae. J Gen Microbiol. 1983;129:653–661. [Google Scholar]
- 25.Schaaff I, Heinisch J, Zimmermann F K. Overproduction of glycolytic enzymes in yeast. Yeast. 1989;5:285–290. doi: 10.1002/yea.320050408. [DOI] [PubMed] [Google Scholar]
- 26.Schulze U. Anaerobic physiology of Saccharomyces cerevisiae. Ph.D. thesis. Lyngby: Technical University of Denmark; 1995. [Google Scholar]
- 27.Trivedi N B, Jacobson G K, Tesch W. Bakers’ yeast. Crit Rev Biotechnol. 1986;24:75–109. [Google Scholar]
- 28.van Dijken J P, Harder W, Beardsmore A J, Quayle J R. Dihydroxyacetone: an intermediate in the assimilation of methanol by yeasts? FEMS Microbiol Lett. 1978;4:97–102. [Google Scholar]
- 29.van Dijken J P, Jonker R, Houweling-Tan G B, Bruinenberg P M, Meijer J, Scheffers W A. The Crabtree effect in Saccharomyces cerevisiae and its significance for the rising power of bakers’ yeast. In: Houwink E H, van der Meer R R, Scheffers W A, editors. Proceedings of the Symposium of the Netherlands Society of Biotechnology. 1983. p. 171. [Google Scholar]
- 30.van Hoek, P., and R. van Rooijen. Unpublished data.
- 31.van Urk H, Mak P R, Scheffers W A, van Dijken J P. Metabolic responses of Saccharomyces cerevisiae CBS 8066 and Candida utilis CBS 621 upon transition from glucose limitation to glucose excess. Yeast. 1988;4:283–291. doi: 10.1002/yea.320040406. [DOI] [PubMed] [Google Scholar]
- 32.Verduyn C, van Dijken J P, Scheffers W A. Colorimetric alcohol assays with alcohol oxidase. J Microbiol Methods. 1984;2:15–25. [Google Scholar]
- 33.Verduyn C, Postma E, Scheffers W A, van Dijken J P. Physiology of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J Gen Microbiol. 1990;136:395–403. doi: 10.1099/00221287-136-3-395. [DOI] [PubMed] [Google Scholar]
- 34.Verduyn C, Stouthamer A H, Scheffers W A, van Dijken J P. A theoretical evaluation of growth yields of yeasts. Antonie Leeuwenhoek. 1991;59:49–63. doi: 10.1007/BF00582119. [DOI] [PubMed] [Google Scholar]
- 35.Verduyn C, Postma E, Scheffers W A, van Dijken J P. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous study on the regulation of respiration and alcoholic fermentation. Yeast. 1992;8:501–517. doi: 10.1002/yea.320080703. [DOI] [PubMed] [Google Scholar]
- 36.Verduyn C. Energetic aspects of metabolic fluxes in yeast. Ph.D. thesis. Delft, The Netherlands: Delft University of Technology; 1992. [Google Scholar]
- 37.Von Meyenburg H K. Energetics of the budding cycle of Saccharomyces cerevisiae during glucose-limited aerobic growth. Arch Mikrobiol. 1969;66:289–303. doi: 10.1007/BF00414585. [DOI] [PubMed] [Google Scholar]
- 38.Weusthuis R A, Luttik M A H, Scheffers W A, van Dijken J P, Pronk J T. Is the Kluyver effect in yeast caused by product inhibition? Microbiology. 1994;140:1723–1729. doi: 10.1099/13500872-140-7-1723. [DOI] [PubMed] [Google Scholar]
- 39.Weusthuis R A, Pronk J T, van den Broek J A, van Dijken J P. Chemostat cultivation as a tool for studies on sugar transport in yeasts. Microbiol Rev. 1994;58:616–630. doi: 10.1128/mr.58.4.616-630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]