Abstract
Arabidopsis (Arabidopsis thaliana) high-affinity NITRATE TRANSPORTER2.1 (NRT2.1) plays a dominant role in the uptake of nitrate, the most important nitrogen (N) source for most terrestrial plants. The nitrate-inducible expression of NRT2.1 is regulated by NIN-LIKE PROTEIN (NLP) family transcriptional activators and NITRATE-INDUCIBLE GARP-TYPE TRANSCRIPTIONAL REPRESSOR1 (NIGT1) family transcriptional repressors. Phosphorus (P) availability also affects the expression of NRT2.1 because the PHOSPHATE STARVATION RESPONSE1 transcriptional activator activates NIGT1 genes in P-deficient environments. Here, we show a biology-based mathematical understanding of the complex regulation of NRT2.1 expression by multiple transcription factors using 2 different approaches: a microplate-based assay for the real-time measurement of temporal changes in NRT2.1 promoter activity under different nutritional conditions, and an ordinary differential equation (ODE)-based mathematical modeling of the NLP- and NIGT1-regulated expression patterns of NRT2.1. Both approaches consistently reveal that NIGT1 stabilizes the amplitude of NRT2.1 expression under a wide range of nitrate concentrations. Furthermore, the ODE model suggests that parameters such as the synthesis rate of NIGT1 mRNA and NIGT1 proteins and the affinity of NIGT1 proteins for the NRT2.1 promoter substantially influence the temporal expression patterns of NRT2.1 in response to nitrate. These results suggest that the NLP–NIGT1 feedforward loop allows a precise control of nitrate uptake. Hence, this study paves the way for understanding the complex regulation of nutrient acquisition in plants, thus facilitating engineered nutrient uptake and plant response patterns using synthetic biology approaches.
Luciferase-based analysis and mathematical modeling suggest that a transcriptional network structure stabilizes the expression of the NITRATE TRANSPORTER2.1 under various concentrations of nitrate.
Introduction
Plants need to absorb most of the elements required for their growth from the soil because of their sessile nature. Among the 17 indispensable elements, nitrogen (N) and phosphorus (P) absorbed at the root surface by plasma membrane-localized transporters are required in large amounts and, therefore, are especially closely related to plant growth rates (Ågren and Weih 2012). One of the primary N sources, nitrate , is taken up and distributed throughout the plant body by nitrate transporter (NRT) family proteins, each of which plays distinct but vital roles under different nitrate concentrations in various tissues, contributing to robust nitrate uptake and optimum growth in changing N environments (Kiba and Krapp 2016). Another major N source for plants, ammonium , is taken up by ammonium transporter family proteins (von Wirén et al. 2000). Owing to the oxidative soil environment, a large part of the N source on land is present as nitrate, which renders nitrate as a predominantly accessible form of N for terrestrial plants (Crawford and Glass 1998; Miller et al. 2007). Indeed, plants overexpressing NRTs exhibit enhanced growth in the soil (Fan et al. 2016), demonstrating the importance of nitrate for plant growth and productivity.
Plant NRTs belong to 1 of 2 gene families, NRT1 or NRT2, which encode structurally distinguishable proteins. In Arabidopsis (Arabidopsis thaliana), 53 and 7 members constitute the NRT1 and NRT2 gene family, respectively (Orsel et al. 2002; Corratgé-Faillie and Lacombe 2017). One of the NRT2 family genes, NRT2.1, which encodes a high-affinity NRT functional at the micromolar range, plays an essential role in the absorption of soil nitrate by roots (Okamoto et al. 2003). NRT2.1 is highly expressed in roots, and its expression level is positively correlated with the actual nitrate uptake rate after nitrate provision, while the expression of other NRT2 and NRT1 genes involved in nitrate uptake seems to exert a comparatively less influence on nitrate uptake rates, suggesting that NRT2.1 plays a dominant role in the absorption of environmental nitrate (Okamoto et al. 2003; Remans et al. 2006; Li et al. 2007). Consistently, an Arabidopsis knockout mutant carrying a deletion of NRT2.1 and the 3ʹ region of NRT2.2 adjacent to NRT2.1 shows severe growth defects, especially when the nitrate concentration in the surrounding environment is low (Li et al. 2007).
Nitrate basically promotes the growth of plants. However, nitrate uptake and assimilation require a large amount of energy (Bloom et al. 1985), and excessive accumulation of N-containing compounds may reduce the pathogen resistance of plants (Fagard et al. 2014). Therefore, the nitrate uptake rate must be fine-tuned to maintain the internal nitrate status of plants. Several studies have revealed that plants possess an intrinsic mechanism to absorb nitrate efficiently, while avoiding excess nitrate absorption. For instance, in one study, it was found that NRT2.1 expression was initially induced in Arabidopsis seedlings upon 1 mm nitrate application but then declined over time (Zhuo et al. 1999). In another study, it was found that 0.2 mm nitrate concentration maximized the expression of NRT2.1, while higher concentrations weakened its steady–state expression level (Nazoa et al. 2003). These examples demonstrate that plants regulate the NRT2.1 expression level in time- and nitrate concentration–dependent manners to optimize nitrate uptake in changing environments.
In the past decade, many transcription factors (TFs) involved in the positive or negative regulation of NRT2.1 in response to changes in nutrient availability have been identified in Arabidopsis. NIN-LIKE PROTEIN (NLP) family proteins, which are activated through direct nitrate binding and nitrate-induced phosphorylation in nuclei (Liu et al. 2017, 2022), bind to 2 DNA sequences called nitrate-responsive cis-elements (NREs) in the NRT2.1 promoter and induce NRT2.1 expression (Maeda et al. 2018). Another group of TFs classified into the GARP family, comprising NITRATE-INDUCIBLE GARP-TYPE TRANSCRIPTIONAL REPRESSOR 1 (NIGT1) family proteins encoded by NLP-dependent nitrate-inducible genes, repress the expression of NRT2.1 (Maeda et al. 2018; Ueda et al. 2020b). The NLP and NIGT1 TFs regulate NRT2.1 in an antagonistic manner (Maeda et al. 2018) and together form the incoherent Type I feedforward loop (Shen-Orr et al. 2002; Mangan and Alon 2003), which is frequently found in bacteria and yeast (Mangan et al. 2006). In the incoherent Type I feedforward loop, Factor A positively regulates Factors B and C, and Factor B negatively regulates Factor C (Mangan et al. 2006). The incoherent Type I feedforward loop has been suggested to enable pulse-like output (Basu et al. 2004), nonmonotonic output (i.e. dose–response curve peaks at a certain strength of the input signal) (Ishihara et al. 2005; Entus et al. 2007; Kaplan et al. 2008), faster response (Rosenfeld et al. 2002), and less noisy output (Bleris et al. 2011; Osella et al. 2011). Therefore, the incoherent Type I feedforward loop consisting of NLP and NIGT1 TFs in the nitrate-responsive gene expression network may entail some functions that increase the adaptability of plants to the natural environment (Ueda and Yanagisawa 2019). This hypothesis is further corroborated by the strong conservation of NLP and NIGT1 family proteins in vascular plants (Chardin et al. 2014; Kiba et al. 2018; Sakuraba et al. 2022). However, experimental evidence supporting the physiological importance of the feedforward loop involving NLP and NIGT1 TFs has yet to be reported.
In this study, we elucidate the functions of the NLP–NIGT1 incoherent feedforward loop via experimental monitoring and mathematical modeling of NRT2.1 promoter activity, in turn demonstrating that the nitrate response network is increasingly complex and sophisticated in vascular plants. The results of the current study indicate that the presence of the NLP–NIGT1 incoherent-type feedforward loop stabilizes the expression of NRT2.1 over a wide range of soil nitrate concentrations. Moreover, these data suggest that the response pattern of NRT2.1 expression could be engineered by altering the synthesis rate of NIGT1 protein or NIGT1 mRNA or by modifying the affinity of NIGT1 for the NRT2.1 promoter.
Results
Development of an NRT2.1 expression monitoring system in planta
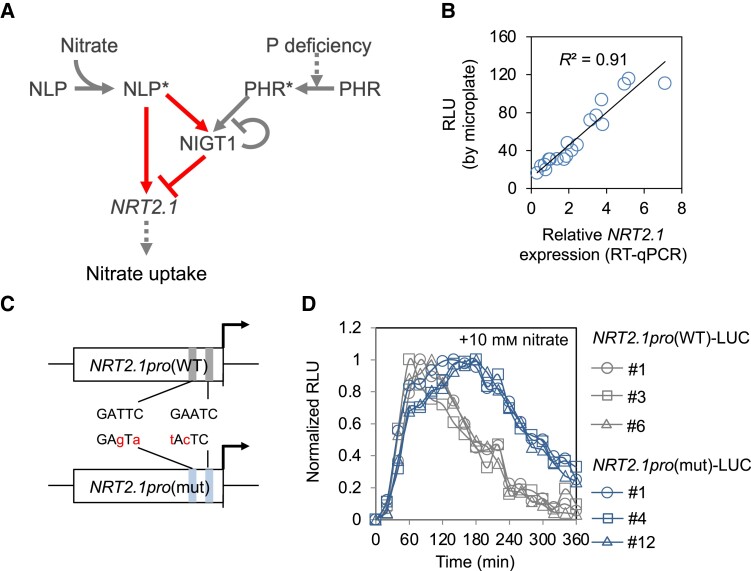
NRT2.1 expression is regulated by NLP, NIGT1, and the master regulators of P deficiency response, i.e. PHOSPHATE STARVATION RESPONSE (PHR) family TFs, in response to variations in N and P nutrient conditions (Fig. 1A). We developed a microplate-based assay system for monitoring nutrient signal-induced changes in NRT2.1 promoter activity over time in living plants using a similar approach adopted for other genes in previous studies (e.g. Dixon et al. 2014; Urquiza-García and Millar 2019). Transgenic Arabidopsis plants expressing the firefly luciferase (LUC) gene under the control of the 1.3 kb NRT2.1 promoter (Maeda et al. 2018) were grown under the nitrate-starved condition in a 96-well microplate for 3 d and then supplemented with nitrate at a final concentration of 10 mm. Nitrate-induced changes in LUC activity in planta were monitored with chemiluminescence over time and compared with changes in nitrate-induced NRT2.1 expression by reverse transcription quantitative PCR (RT-qPCR) analysis (Fig. 1B). LUC activity was highly correlated with the expression level of NRT2.1 (R2 > 0.90, P < 0.0001). This result indicated that this microplate-based assay is a reliable system for reporting nitrate-induced changes in NRT2.1 expression in living plants.
Figure 1.
Real-time monitoring of NRT2.1 promoter activity. A) Signaling cascades that modulate NRT2.1 expression and nitrate uptake upon nitrate treatment and phosphorus deficiency. The arrows and “T” signs indicate positive and negative regulations, respectively. The dotted lines indicate indirect effects. The asterisks indicate the active forms of TFs. The network motif representing the incoherent Type I feedforward loop is highlighted. B) Correlation between NRT2.1 promoter activity levels and NRT2.1 expression levels determined by performing the microplate-based LUC assay and RT-qPCR, respectively. Seedlings were treated with 10 mm nitrate. C) Structure of the WT and mutant NRT2.1 promoters (NRT2.1pro(WT) and NRT2.1pro(mut), respectively). Two functional NIGT1-binding sites, GATTC and GAATC, were changed to GAgTa and tAcTC sequences in the mutant NRT2.1 promoter, with modified bases indicated with small cases. D) Nitrate-induced temporal changes in LUC activity in 3 independent transgenic lines obtained using each construct, NRT2.1pro(WT)-LUC and NRT2.1pro(mut)-LUC. The lowest and highest LUC activities in each line within 6 h were set to 0 and 1, respectively, and the remaining values in each line were expressed as relative values. Similar results were obtained in at least one repeated experiment. In B) and D), data represent the mean of biological replicates (n = 2 for RT-qPCR analysis and 8 for LUC assay). RLU, relative luminescence unit.
To validate the practicality of the developed monitoring system, we examined the effects of NIGT1 binding on the kinetics of NRT2.1 promoter activity. Since we previously showed that the NRT2.1 promoter possesses 2 functional NIGT1-binding sites (Maeda et al. 2018; Ueda et al. 2020b) (Fig. 1C), we compared the kinetic pattern of NRT2.1 promoter activity in transgenic Arabidopsis lines expressing LUC under the control of the wild-type (WT) NRT2.1 promoter (NRT2.1pro(WT)-LUC) or a mutant NRT2.1 promoter harboring mutations within both NIGT1-binding sites (NRT2.1pro(mut)-LUC). The NRT2.1pro(WT)-LUC and NRT2.1pro(mut)-LUC lines were created in the same genetic background; however, the latter lacked the NIGT1 ⊣NRT2.1 pathway. Highly consistent temporal response patterns were observed among 3 independent lines of each transgenic genotype, although the pattern was different between the NRT2.1pro(WT)-LUC and the NRT2.1pro(mut)-LUC lines (Fig. 1D). This result is consistent with previous studies, which showed that NIGT1-binding sites in the NRT2.1 promoter play a vital role in the temporal regulation of NRT2.1 expression (Maeda et al. 2018; Ueda et al. 2020b) and suggested the practicality of the developed monitoring system. Therefore, we performed further analyses using representative transgenic lines (NRT2.1pro(WT)-LUC #6 and NRT2.1pro(mut)-LUC #1).
Kinetic patterns of nitrate-responsive NRT2.1 expression in nlp6 nlp7-1, nigt1.1/1.2-2/1.3/1.4, and phr1 phl1 mutants
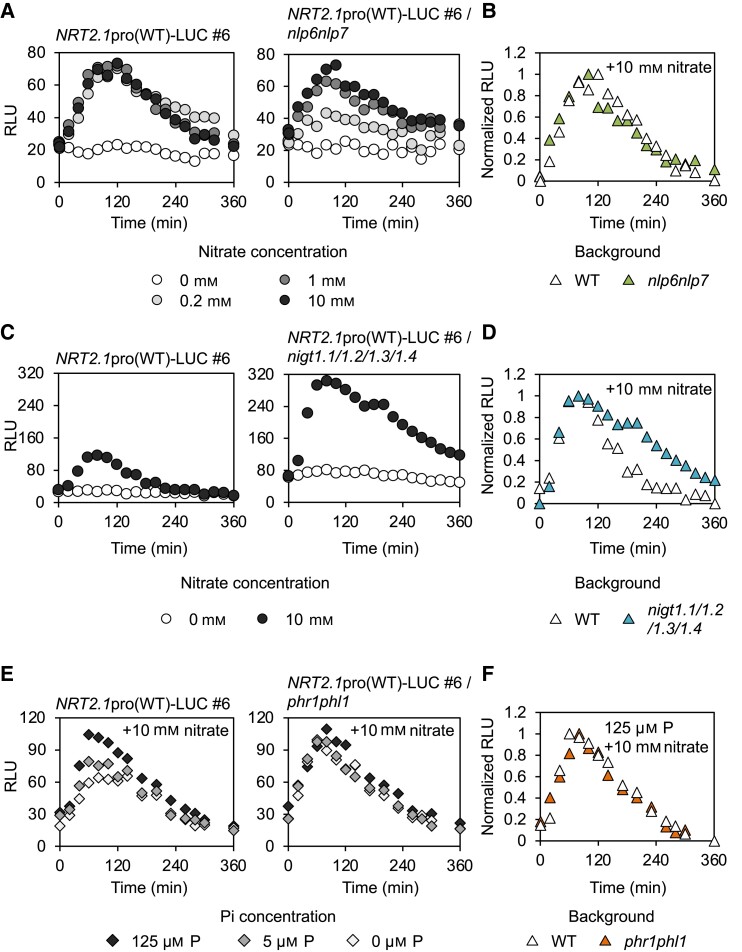
The effects of eliminating NRT2.1-regulating NLP or NIGT1 TFs on the kinetic pattern of the nitrate response of the NRT2.1 promoter were investigated using the developed monitoring system. Although Arabidopsis possesses 9 NLP TFs (NLP1 to 9), the NRT2.1 promoter is mainly under the control of NLP6 and NLP7 (Konishi and Yanagisawa 2013; Marchive et al. 2013; Guan et al. 2017; Maeda et al. 2018; Konishi et al. 2021; Cheng et al. 2023). Therefore, we introduced the NRT2.1pro(WT)::LUC construct into the nlp6 nlp7-1 double mutant (Guan et al. 2017; Maeda et al. 2018) by crossbreeding and then compared the kinetic patterns of the WT NRT2.1 promoter-driven LUC activity between the WT and nlp6 nlp7-1 mutant backgrounds (Fig. 2A). In the WT background, LUC activity peaked at 80 to 100 min after the onset of the nitrate treatment, regardless of the nitrate concentration, and then gradually decreased to the initial level. In contrast, in the nlp6 nlp7-1 background, the height of LUC activity peak achieved upon nitrate treatment depended on the nitrate concentration, and a low nitrate concentration (0.2 mm) failed to fully activate the NRT2.1 promoter (Fig. 2A). However, the loss of NLP6 and NLP7 did not affect the kinetics of NRT2.1 promoter activity (Fig. 2B), probably because other NLP TFs compensated for the loss of NLP6 and NLP7 under high nitrate concentrations, but not under low nitrate concentrations similar to those found in the natural environment, illustrating the essential roles of NLP6 and NLP7.
Figure 2.
Nitrate-induced temporal changes in NRT2.1 promoter activity in different genetic backgrounds and under different nutrient conditions. A, B) Raw values A) and relative values B) of nitrate-induced temporal changes in NRT2.1 promoter activity in the nlp6 nlp7 background. Seedlings were treated with 0, 0.2, 1, and 10 mm KNO3 in A), and data obtained in the presence of 10 mm KNO3 are shown for B). C, D) Raw values C) and relative values D) of nitrate-induced temporal changes in NRT2.1 promoter activity in the nigtQ background. Seedlings were treated with 10 mm KNO3. E, F) Raw values E) and relative values F) of the effects of P status on nitrate-induced temporal changes in NRT2.1 promoter activity in the WT and phr1 phl1 backgrounds. Seedlings were treated with 10 mm KNO3 in all cases. Data represent the mean of biological replicates (n = 7 to 8). Similar results were obtained in at least one repeated experiment. RLU, relative luminescence unit.
Next, we examined the effect of the loss of NIGT1 proteins on the kinetics of LUC activity upon nitrate treatment. Arabidopsis possesses 4 NIGT1 proteins (NIGT1.1, 1.2, 1.3, and 1.4). Therefore, we introduced the NRT2.1pro(WT)::LUC construct into the nigt1.1 1.2-2 1.3 1.4 quadruple mutant (designated as nigtQ) (Maeda et al. 2018) by crossbreeding and then compared the kinetics of nitrate-induced LUC activity between the WT and nigtQ backgrounds. The amplitude of LUC activity in the nigtQ background was greater than that in the WT background (Fig. 2C). Furthermore, the quadruple mutation affected the kinetics of the nitrate response. When LUC signal intensity was expressed as relative values, it was clear that NRT2.1 promoter activity, after reaching the peak, declined less steeply in the nigtQ background than in the WT background (Fig. 2D).
The PHR1 transcriptional activator, which is activated by P deficiency posttranslationally and then induces the P deficiency response (Puga et al. 2014; Ried et al. 2021), is another regulator of the NRT2.1 expression, because it promotes the expression of NIGT1 family genes (Maeda et al. 2018). Therefore, we analyzed the effects of P deficiency and the deletion of PHR1 and its homolog PHR1-LIKE (PHL1) (49.9% protein sequence similarity) (Bustos et al. 2010) on the kinetics of NRT2.1 promoter activity upon nitrate treatment using the monitoring system. We introduced the NRT2.1pro(WT)::LUC construct into the phr1 phl1 double mutant (Maeda et al. 2018) by crossbreeding. To examine the kinetics of NRT2.1 promoter activity under P-deficient conditions, seedlings harboring NRT2.1pro(WT)::LUC construct in the WT and phr1 phl1 backgrounds were grown in nutrient media containing different concentrations of phosphate and then treated with 10 mm nitrate. The amplitude of NRT2.1 promoter activity observed after the application of 10 mm nitrate was reduced by P deficiency in the WT background, while P deficiency-dependent reduction in the amplitude of NRT2.1 promoter activity was hardly detected in the phr1 phl1 mutant background (Fig. 2E). Furthermore, by representing the signal intensity obtained under the P-sufficient condition as relative values, we confirmed that the absence of PHR1 and PHL1 did not affect the temporal changes of the response curve (Fig. 2F), consistent with the previously reported finding that PHR1 and PHL1 are inactive under P-sufficient conditions (Puga et al. 2014; Ried et al. 2021).
These observed dynamics of NRT2.1 promoter activity under varying nutritional conditions can be qualitatively accountable by the previously proposed regulatory model of the NRT2.1 promoter (Fig. 1A), verifying the accuracy of the model.
Description of the network motif by ordinary differential equations
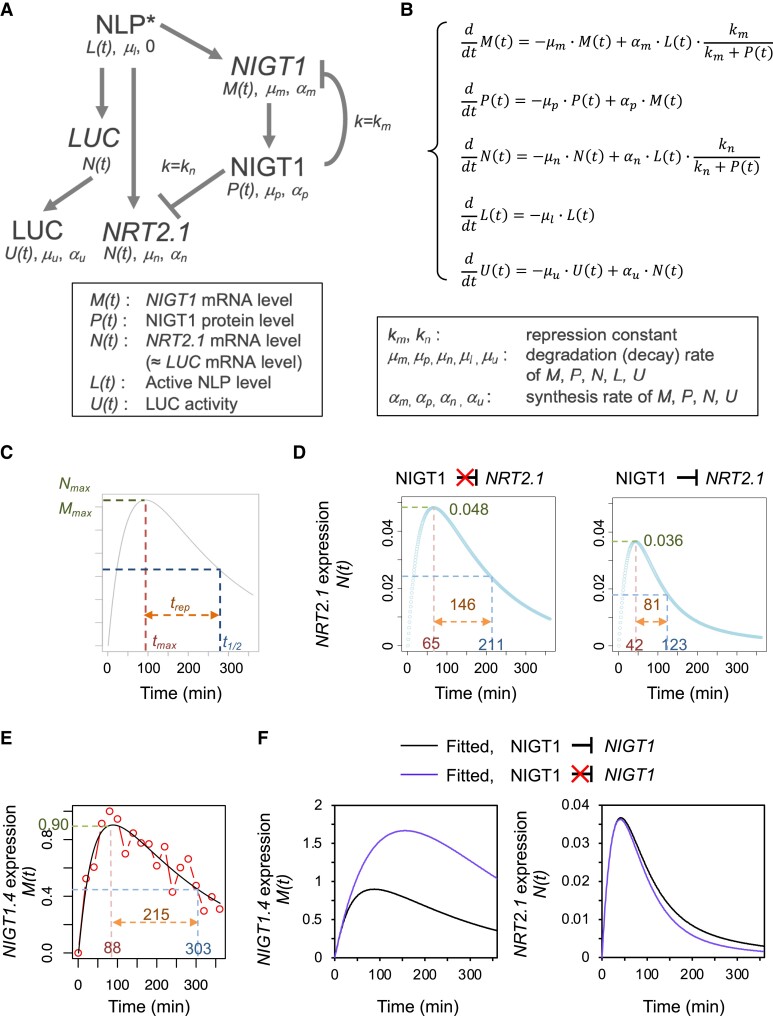
The results of microplate-based LUC assays confirmed that our current molecular understanding of the nitrate response is valid. To quantitatively describe the temporal changes in NRT2.1 expression, we built an ordinary differential equation (ODE) model (Fig. 3, A and B; Supplemental Note S1). Given that the promoter activity of NRT2.1 declined over time in the nigtQ genetic background (Fig. 2, C and D), we assumed a model in which NLP activity declines over time at a constant rate. Even though LUC activity was a good approximation of NRT2.1 promoter activity (Fig. 1B), we also included an ODE describing the synthesis of LUC protein and decay of LUC activity, as in a previous study (Finkenstädt et al. 2008) (Fig. 3B; Supplemental Note S1). To estimate the Hill coefficient for the NIGT1 ⊣NRT2.1 and NIGT1 ⊣NIGT1 regulatory pathways, we carried out protoplast-based transient expression assays by introducing mixtures containing the plasmid encoding NIGT1.1 and an empty plasmid in different ratios, together with a reporter construct; (see Materials and methods). The experimentally determined Hill coefficient was close to 1 for both pathways (Supplemental Fig. S1). Therefore, both Hill coefficients were assumed to be 1. Because NIGT1.4 is specifically expressed in roots and has a strong negative influence on NRT2.1 expression in roots (Maeda et al. 2018), we selected NIGT1.4 as the representative NIGT1 gene for further experiments. The temporal expression pattern of NIGT1.4 was experimentally determined by RT-qPCR using plants grown under the same conditions as those used for the microplate-based assays (Supplemental Fig. S2). The use of NIGT1.4 as a representative NIGT1 family member was also supported by the observation that the temporal expression patterns of NIGT1.1 to NIGT1.4 were similar (Supplemental Fig. S2). The other parameters were obtained by fitting the equations to the temporal changes in LUC activity (see Materials and methods; Supplemental Note S1).
Figure 3.
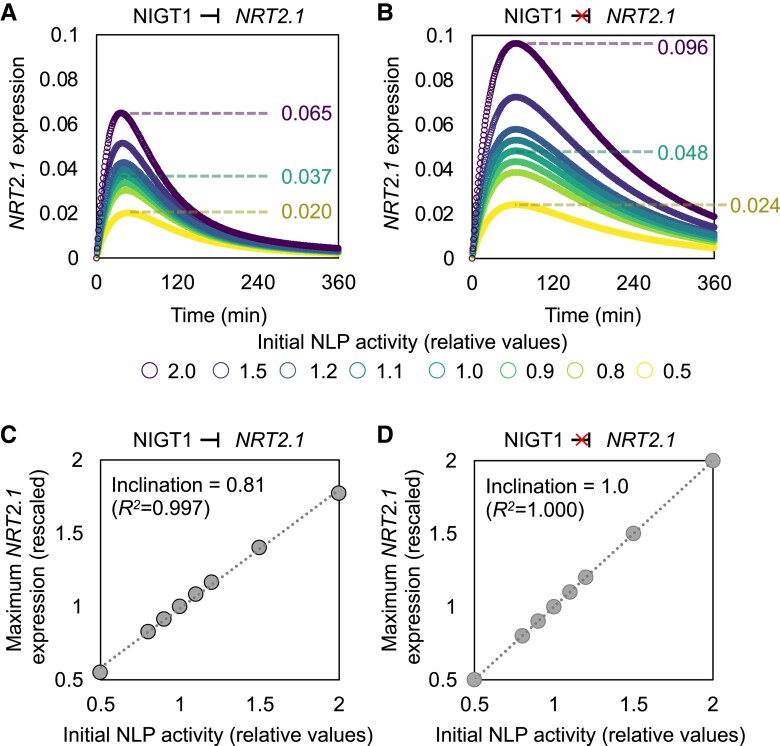
Mathematical modeling for the regulation of NIGT1 and NRT2.1 expression. A) Simplified model of the nitrate-responsive expression of NIGT1 and NRT2.1. The arrows and “T” signs indicate positive and negative regulations, respectively. Repression constants for the NIGT1 ⊣NIGT1 and NIGT1 ⊣NRT2.1 pathways are also shown. B) Formula and parameters to describe the model in A). C) Definition of parameters used to describe the kinetic patterns of NIGT1 and NRT2.1 expression levels. D) Simulated expression levels of NRT2.1 in the WT (right panel) and nigtQ (left panel) backgrounds. E) Observed (dots and lines) and fitted (smooth line) expression levels of NIGT1.4.F) Fitted expression levels of NIGT1.4 (left panel) and NRT2.1 (right panel) in the presence or the absence of the NIGT1 ⊣NIGT1 pathway.
The nitrate response curves of NRT2.1 and NIGT1 were quantitatively described using the following parameters: Nmax and Mmax, the maximum values of NRT2.1 and NIGT1 expression, respectively; tmaxN and tmaxM, time points coinciding with the maximum expression of NRT2.1 and NIGT1, respectively; t1/2N and t1/2M, time points coinciding with 50% of Nmax and Mmax after the peak, respectively; and trepN and trepM, the difference between t1/2 and tmax for NRT2.1 and NIGT1, respectively (Fig. 3C). The model fitting successfully simulated the expression level of NIGT1.4 as well as LUC activity originating from WT and mutant NRT2.1 promoter-driven LUC, which also yielded a simulated expression pattern of NRT2.1 in WT and nigtQ backgrounds (Fig. 3, D and E; Supplemental Fig. S3). Furthermore, we simulated a situation in which only the NIGT1 ⊣NIGT1 auto-repressive regulation is eliminated. The elimination of the auto-repressive pathway drastically changed the response pattern of NIGT1.4 but only minimally altered the expression pattern of NRT2.1 (Fig. 3F), suggesting a limited contribution of this pathway to the regulation of NRT2.1 expression within the adopted parameter range. The model further suggested that around 90% of the NLP activity is diminished after 6 h, while the NIGT1 protein level peaks at around 3.5 h (Supplemental Fig. S4), predicting the behavior of unobserved parameters.
Contribution of each ODE parameter to NRT2.1 promoter activity
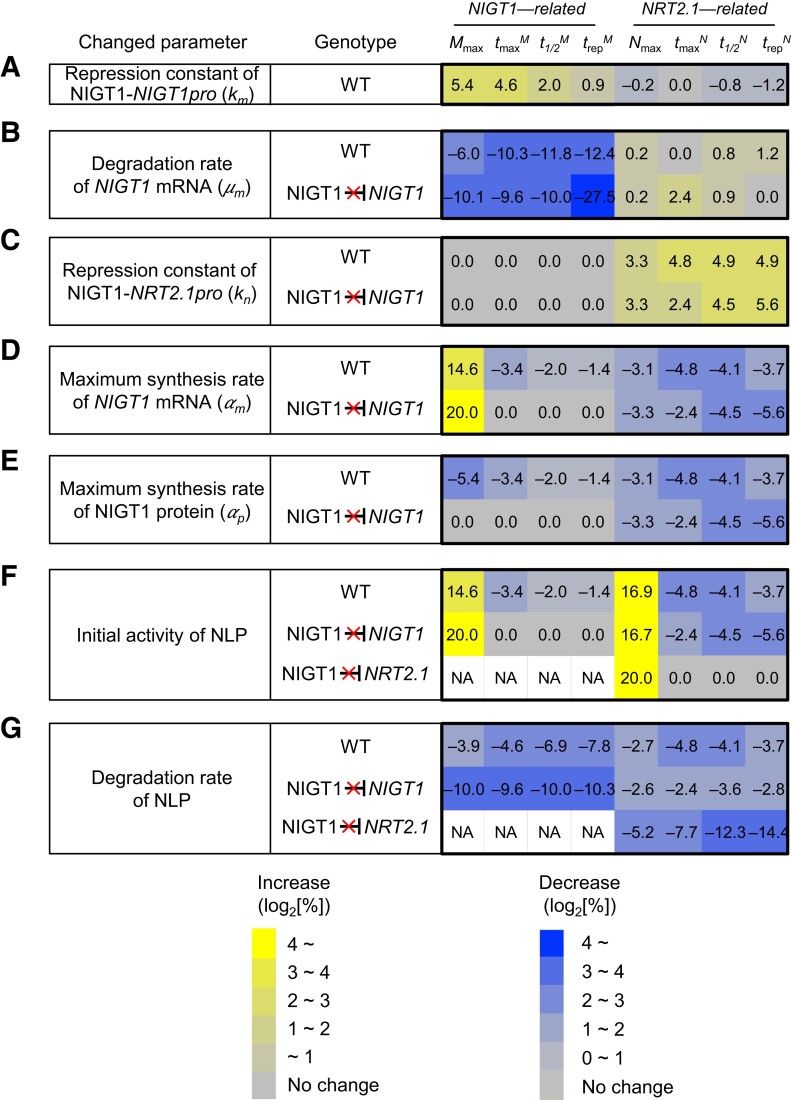
We performed sensitivity analysis to identify critical parameters that affect the temporal expression patterns of NIGT1 and NRT2.1 during the nitrate response using a similar approach adopted in previous studies (e.g. Pokhilko et al. 2012). Each parameter in the ODE model was separately increased or reduced by 10%, and the relative change in parameters used to describe the curve (i.e. maximum expression of NRT2.1 and NIGT1 [Nmax, Mmax], tmax, t1/2, and trep) was evaluated. In WT plants possessing NIGT1 ⊣NRT2.1 regulation, reducing the affinity of NIGT1 for its own promoter (NIGT1 promoter; i.e. increased km) was predicted to result in an increase in the value of all parameters including Mmax, tmaxM, t1/2M, and trepM (Fig. 4A). Increasing the degradation rate of NIGT1 mRNA was shown to exert a much stronger effect on NIGT1-related parameters, but its effect on the response patterns of NRT2.1 was limited (Fig. 4B). Similarly, decreasing the affinity of NIGT1 for the NRT2.1 promoter (i.e. increasing kn) increased the value of all parameters including Nmax, tmaxN, t1/2N, and trepN (Fig. 4C). Changing the synthesis or degradation rate of NIGT1 mRNA or NIGT1 protein more strongly reflected some parameters describing the expression pattern of NIGT1 and NRT2.1 in the nigtQ mutant than in WT plants; for instance, a 20% increase in the maximum synthesis rate of NIGT1 mRNA or NIGT1 protein reduced trepN by 3.7% in the WT but by 5.6% in the absence of NIGT1 ⊣NIGT1 auto-repression (Fig. 4, D and E). Changes in the initial activity and degradation rate of NLP caused greater changes in Nmax in the nigtQ mutant than in WT plants (Fig. 4, F and G), suggesting that NIGT1 proteins dampen the changes in NLP activity or active NLP degradation rate. Similar sensitivity analyses were performed by changing the input parameters to 0.1×, 0.5×, 2×, and 10× of the original values (Supplemental Fig. S5). Some of the parameters affected the nitrate response curves of NIGT1 and NRT2.1 to a greater extent when the value was changed by 10-fold; however, generally, greater changes were observed in the model where NIGT1 ⊣NIGT1 auto-repression or the NIGT1 ⊣NRT2.1 regulatory pathway is lacking. Overall, these results suggest that the presence of the NIGT1 ⊣NIGT1 and NIGT1 ⊣NRT2.1 regulatory pathways increases the robustness of NRT2.1 regulation by increasing stability against changes in the parameters of network components.
Figure 4.
Sensitivity analysis of each kinetic parameter. A to G) Results of the sensitivity analysis of kmA), μmB), knC), αmD), αpE), initial NLP activity (F), and degradation rate of NLP (G). The analysis was performed by changing each input parameter by 20% (10% increase and 10% decrease). The number in each cell indicates the relative changes in kinetic parameters (%). In addition to the analysis of the WT model, models in which the NIGT1 ⊣NIGT1 or NIGT1 ⊣NRT2.1 pathway is eliminated were also considered. Each cell is colored according to the values in the cell. NA, not analyzed.
Since the NIGT1 ⊣NRT2.1 pathway stabilized NRT2.1 expression, we further simulated the response of NRT2.1 to a broader range of initial NLP activity both in the WT background and a genetic background lacking the NIGT1 ⊣NRT2.1 regulatory pathway. When the initial NLP activity was changed from the original level (1) to 0.5 to 2, the value of Nmax (maximum NRT2.1 expression) in WT plants changed from 0.020 to 0.065, which corresponds to 122% of the variation in the original value (0.037) (Fig. 5A). On the other hand, in plants lacking the NIGT1 ⊣NRT2.1 pathway, Nmax changed from 0.024 to 0.096, corresponding to 150% of the original value (0.048) (Fig. 5B). Furthermore, the slope of linear regression between NLP and Nmax was 0.81 in the presence of the NIGT1 ⊣NRT2.1 regulatory pathway and 1.00 in the absence of that pathway (Fig. 5, C and D). This mathematical modeling analysis led us to hypothesize that shifts in initial NLP activity are dampened by the NIGT1 ⊣NRT2.1 pathway, which provides stability in the peak value of NRT2.1 expression under a broad range of nitrate concentrations.
Figure 5.
Buffering of NLP-dependent changes in NRT2.1 promoter activity by NIGT1 proteins. A, B) Simulated expression levels of NRT2.1 assuming different strengths of the original NLP activity in the presence A) and absence B) of the NIGT1 ⊣NRT2.1 pathway. The maximum NRT2.1 expression levels under the initial NLP activity of 0.5 (bottom), 1 (middle), and 2 (top) are also indicated. C, D) Relationship between the initial NLP activity and maximum NRT2.1 expression in the presence C) and absence D) of the NIGT1 ⊣NRT2.1 pathway.
NIGT1 proteins buffer changes in NLP activity and stabilize output
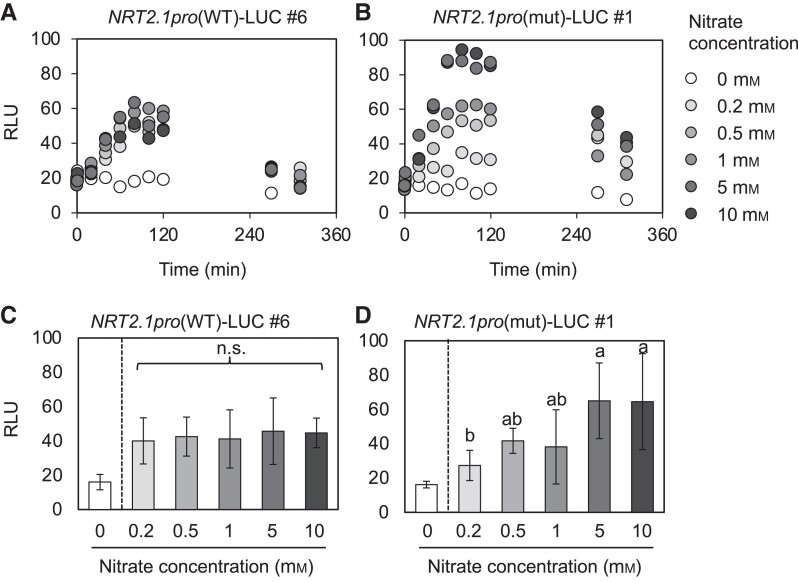
Using the microplate-based assay, we experimentally validated the hypothesis that the NIGT1 ⊣NRT2.1 regulation stabilizes NRT2.1 expression. Although the peak activity of the WT NRT2.1 promoter was surprisingly consistent under a wide range of nitrate concentrations, that of the mutant NRT2.1 promoter varied substantially (Fig. 6, A and B). ANOVA test also revealed that nitrate concentration did not significantly affect the peak activity of the WT NRT2.1 promoter, whereas the mutant NRT2.1 promoter showed significantly higher peak activity at nitrate concentrations greater than 0.2 mm (Fig. 6, C and D). These results are coherent with our hypothesis and confirm the physiological relevance of the NIGT1 ⊣NRT2.1 pathway in stabilizing NRT2.1 expression upon perturbations in environmental nitrate concentrations.
Figure 6.
Nitrate concentration–dependent changes in NRT2.1 promoter activity. A, B) Temporal changes of activity of the WT A) and mutant (B) NRT2.1 promoters in response to different nitrate concentrations. Changes in the NRT2.1pro(WT)-LUC (#6) and NRT2.1pro(mut)-LUC (#1) seedlings were subjected to the analysis. C, D) LUC signal intensity derived from the WT C) and mutant D) NRT2.1 promoters in the NRT2.1pro(WT)-LUC (#6) and NRT2.1pro(mut)-LUC (#1) seedlings, respectively, treated with different concentrations of nitrate for 80 min. In A) and B), data represent mean values (n = 8). In C) and D), data represent mean ± Sd (n = 6) after removing the highest and lowest values at each time point. One-way ANOVA was conducted among the data obtained in the 0.2 to 10 mm nitrate treatments, and different letters indicate significant differences. In A) to D), similar results were obtained in at least one repeated experiment. n.s., not significant; RLU, relative luminescence unit.
Conservation of the NLP–NIGT1–NRT2 network motif in Arabidopsis ecotypes and rice cultivars
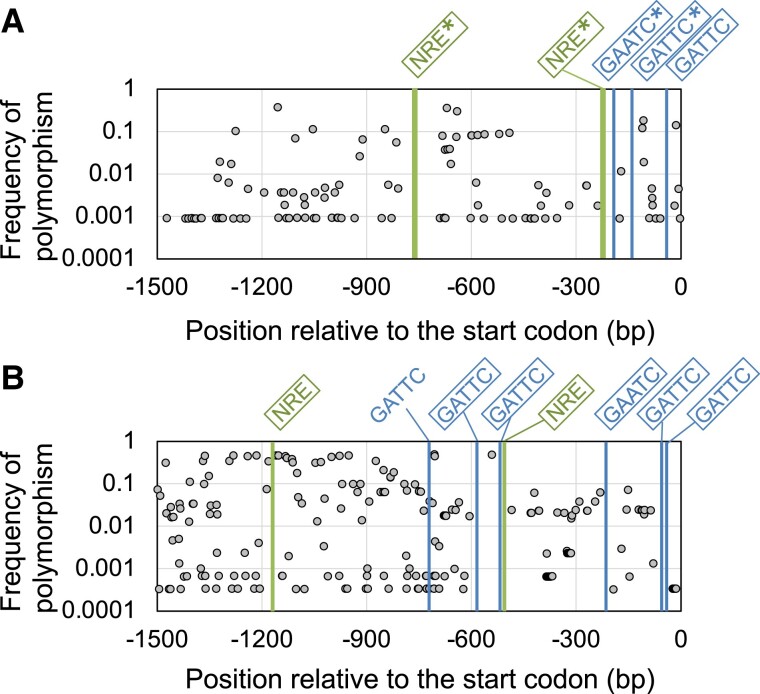
Previous phylogenetic analyses revealed that NLP, NIGT1, and NRT2 family proteins are widely conserved among vascular plants (Chardin et al. 2014; von Wittgenstein et al. 2014; Kiba et al. 2018). Considering the physiological relevance of the network comprising NLP, NIGT1, and NRT2 proteins, we hypothesized that the structure of this network is conserved across different Arabidopsis ecotypes and other plant species. To test this hypothesis, we compared the sequences of the NRT2.1 promoter among 1,135 Arabidopsis ecotypes (Alonso-Blanco et al. 2016). The results showed that 2 experimentally confirmed functional NIGT1-binding sites in the 1.5 kb NRT2.1 promoter sequence were completely conserved among all 1,135 ecotypes (Fig. 7A). Furthermore, 2 functional NLP-binding sites experimentally identified in the 1.5 kb NRT2.1 promoter sequence (Maeda et al. 2018) were also completely conserved among these accessions (Fig. 7A). This result suggests that the loss of this network structure would have compromised the adaptability of plants to natural habitats.
Figure 7.
Conservation of NIGT1-binding sites in the NRT2.1 promoter in Arabidopsis and rice. A, B) Frequency of nucleotide polymorphisms in 1,500 bp sequence upstream of the translational start codon of Arabidopsis NRT2.1A) and rice OsNRT2.1B) among 1,135 and 3,024 accessions, respectively. The bars labeled with “GAATC/GATTC” and “NRE” indicate the positions of NIGT1- and NLP-binding sites, respectively. The squares indicate motifs showing 100% conservation among the examined accessions. The asterisk (*) in A) indicates experimentally validated functional binding sites.
We also analyzed polymorphisms in the promoter sequence of OsNRT2.1, a nitrate-inducible gene and a close rice (Oryza sativa) homolog of Arabidopsis NRT2.1 (82.1% protein sequence similarity) (Katayama et al. 2009; von Wittgenstein et al. 2014). A total of 3,024 rice accessions reported previously (Mansueto et al. 2017; Wang et al. 2018) showed substantial sequence variation in the OsNRT2.1 promoter region, especially in the region located more than 600 bp upstream of the transcription start site (Fig. 7B). A total of 6 putative NIGT1-binding sites were found in the 1.5 kb OsNRT2.1 promoter sequence. In addition, we also found 2 putative NREs (TGCCCCTT and AAAGGCCA) that matched the TGNC(C/T)(C/T)TT sequence, which was previously suggested as the consensus sequence of NREs and NLP7-binding sites in Arabidopsis (Konishi and Yanagisawa 2014; O’Malley et al. 2016). All putative NREs and NIGT1-binding sites were completely conserved, with the only exception being an NIGT1-binding site carrying a 1 nt polymorphism (GAATC → AAATC) at −719 bp.
Theoretically, the extremely high degree of sequence conservation in both plant species would be predicted to occur at a very low rate if polymorphisms were assumed to be evenly distributed along the 1.5 kb promoter sequence. Since polymorphisms were observed at 108 sites within the 1.5 kb NRT2.1 promoter sequence in Arabidopsis, the probability that all 3 NIGT1-binding sites (total 15 bp) and 2 NREs (total 21 bp) are entirely conserved by chance is 6.8%. Similarly, the 1.5 kb OsNRT2.1 promoter sequence in rice contained polymorphisms at 211 locations, suggesting that the theoretical probability that 5 of the 6 NIGT1-binding sequences (30 bp total) are completely conserved is 1.1%, which further decreases to 0.09% if the 2 putative NREs are also considered. Hence, the NLP–NIGT1–NRT2 network motif is likely conserved in various plant species because of its physiological relevance.
Discussion
Functional relevance of the network motif involving NIGT1 proteins
The relationship among NLP, NIGT1, and NRT2 is referred to as the incoherent Type I feedforward loop, and similar network structures have been reported to be involved in several physiological processes of plants, such as secondary metabolism (Binkert et al. 2014) and cell wall formation (Taylor-Teeples et al. 2015). Here, we experimentally demonstrated that the NLP–NIGT1 incoherent feedforward loop provides stability to the output signal (i.e. NRT2.1 expression) under various strengths of input signals (i.e. different nitrate concentrations) and serves as a sophisticated device that can fine-tune genetic circuits.
The importance of the NLP–NIGT1 incoherent feedforward loop could also be inferred from the complete sequence conservation of the previously validated Arabidopsis NREs and NIGT1-binding sites in the NRT2.1 promoter and the conservation of most of the putative NREs and NIGT1-binding sites in rice OsNRT2.1 promoter. This essentiality is further supported by the possibility that the OsNIGT1 locus has been under artificial selection during the domestication of Asian rice (Zhao et al. 2019). These facts may suggest that plants have evolved the NLP–NIGT1 incoherent feedforward loop as a network motif to enable the fine-tuning of biologically important physiological processes, the precise regulation of which is critical for the survival and generation of offspring in both natural habitats and human-controlled agricultural fields.
In the natural habitat, soil nitrate concentration is affected by several factors, including temperature, water and oxygen availability, and microbial activity, over time (Miller et al. 2007; Li et al. 2016). However, since natural soils are generally deficient in N, plants seldom encounter toxic nitrate levels. Therefore, the priority for plants is likely absorbing sufficient nitrate when it is available rather than avoiding an excess of nitrate. Thus, under temporarily fluctuating nitrate conditions, it is advantageous for plants to first fully activate the nitrate uptake mechanism upon exposure to nitrate and then gradually reduce the uptake activity to prevent excessive nitrate accumulation or futile energy loss. In the absence of the NIGT1 ⊣NRT2.1 regulation, the amplitude of NRT2.1 expression and probably the maximum uptake capacity of nitrate would depend on the concentration of external nitrate, and low nitrate concentrations would fail to fully activate the nitrate uptake capacity of plants. It has also been suggested that the induction of the output signal upon the receipt of an input signal is much faster when a network motif is adopted rather than when a simple regulatory pathway is adopted (Mangan et al. 2006), because negative regulation allows the use of a stronger promoter for reaching the desired level of expression. Furthermore, a faster response is suggested to increase the fitness of an organism when the input signal fluctuates over time (Ramsey et al. 2006). Hence, given the advantages conferred by network motifs, the NLP–NIGT1 incoherent feedforward loop likely plays a substantial role in controlling nitrate uptake in plants.
Implications of mathematical models for revealing complex regulatory patterns in plants
The advantages of incoherent Type I feedforward loops, such as output-stabilizing effects, have been reported in various organisms (Bleris et al. 2011; Osella et al. 2011). However, in plants, temporary changes in the expression levels of genes constituting a feedforward loop have rarely been quantitatively described, and experimental evidence supporting the gene expression-stabilizing function of incoherent Type I feedforward loops is minimal. In the current study, the utilization of plants harboring a promoter–reporter construct facilitated the acquisition of temporal changes in NRT2.1 expression, which further enabled the construction of an ODE model. ODE-based mathematical modeling is beneficial for analyzing the response patterns of systems involving multiple factors, in which intuitive approaches are of limited use. Furthermore, mathematical models allow simulations of different situations, some of which are difficult to validate experimentally. For example, our study suggested that changes in the degradation rate of active NLP proteins or the repression constant of NIGT1 (calculated against its target gene promoters) greatly influence the nitrate response pattern of NRT2.1 (Fig. 4), which may be cumbersome to verify experimentally. Such ODE-based mathematical models have been adopted to describe temporal changes in several plant processes, such as ethylene production (Van de Poel et al. 2014), flowering (Pokhilko et al. 2013), lateral root formation (Chen et al. 2015), metabolite content variation (Pokhilko et al. 2015), and P response (Ajmera et al. 2018). These modeling studies enabled the simulation of network behavior under different parameter settings and suggested hidden molecular mechanisms or key network elements, greatly enhancing our understanding of the related molecular framework.
Limitations in our current model may include incomplete simulation. For instance, even though the simulation suggested that the presence of the NIGT1 ⊣NRT2.1 regulatory pathway dampens the changes in NLP activity to some extent (Fig. 4), a much stronger dampening effect was observed in real plants (Fig. 6). This discrepancy could partly be due to the simplification of our model. For instance, the expression of NRT2.1 is also modulated by TFs other than NLPs and NIGT1s, such as basic leucine zipper family proteins (Alvarez et al. 2014; Chen et al. 2016), as well as by non-TF proteins such as BTB AND TAZ DOMAIN PROTEIN 2 (Araus et al. 2016) and CBL-INTERACTING PROTEIN KINASE 8 (Hu et al. 2009). In addition, application of N-containing metabolites, such as glutamine, also represses NRT2.1 expression through an as-yet-unknown mechanism (Nazoa et al. 2003). Furthermore, multiple feedback loops have been suggested to further stabilize the output signal in yeast (Ramsey et al. 2006). Hence, revealing the molecular connections among these components and developing monitoring systems for the expression of other related genes as well as for N-containing metabolites would enable the construction of a more complex mathematical model and would account for the stability of NRT2.1 induction by nitrate.
Implications of designing nutrient response patterns using a synthetic biology approach
Sensitivity analysis indicated that the response patterns of NIGT1 and NRT2.1 mRNAs are certainly affected by the degradation and synthesis rates of the respective mRNAs (Fig. 4; Supplemental Fig. S5). In addition, changing the parameters, such as the maximum synthesis rate of NIGT1 mRNA and the affinity of NIGT1 protein for the NRT2.1 promoter, also affected the temporal response pattern of NRT2.1 expression (Fig. 4; Supplemental Fig. S5), suggesting that the behavior of the network can be altered by genetic engineering. The affinity of TFs for their target promoters could be modified in several ways. For instance, Arabidopsis AUXIN RESPONSE FACTOR 1/5 (ARF1/5) TFs form dimers and bind to palindromic recognition sites separated by 7 bp (Boer et al. 2014). Mutation of either of the binding sites, changes in nucleotide sequence length between the 2 recognition sites, and mutation of amino acid residues forming the dimerization surface compromised the binding of ARF1/5 TFs to the target sequences, which increased the dissociation constants from 3- to more than 40-fold (Boer et al. 2014). Another study showed that PHR1 proteins strongly bind to target promoters as tetramers, but substitutions of amino acid residues responsible for oligomerization greatly impaired the affinity of PHR1 proteins for the target promoters and increased the dissociation constant (Ried et al. 2021). Since the presence of multiple binding sites and the interaction between NIGT1 monomers are vital for the strong binding of NIGT1 to the NRT2.1 promoter (Ueda et al. 2020b), modification of the promoter elements or monomerization of NIGT1 proteins likely increases km or kn, which affects the kinetics of NRT2.1 expression. Through such changes, it might be possible to “design” genetic circuits to obtain the desired output signal behavior. For instance, a previous study employed Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR)/CRISPR-associated 9 (Cas9)-mediated genome editing to introduce mutations into the binding sequence of transcriptional activators to increase tolerance to pathogens (Xu et al. 2019). Such genetic engineering could be applied to the NRT2.1 promoter through synthetic biology approaches to alter its temporal expression patterns.
Materials and methods
Plant materials and growth conditions
Arabidopsis (A. thaliana) ecotype Columbia (Col-0) was used as the WT throughout the study. The NRT2.1pro(WT)-LUC and NRT2.1pro(mut)-LUC lines have been described previously (Maeda et al. 2018). A representative NRT2.1pro(WT) line (#6) was crossed with nlp6 nlp7-1 (SALK_036557/SALK_026134) (Guan et al. 2017), phr1 phl1 (SALK_067629C/SAIL_731_B09) (Bustos et al. 2010), and nigtQ-1 (=nigt1.1 1.2-2 1.3 1.4; GABI_267G03/SALK_044835C/SAIL_28_D08/SALK_067074) (Maeda et al. 2018). Another NIGT1 quadruple mutant nigtQ-2 (=nigt1.1 1.2-1 1.3 1.4; GABI_267G03/SALK_070096/SAIL_28_D03/SALK_067074) (Maeda et al. 2018) was used for protoplast transient assays. The nlp6, nlp7-1, phr1, phl1, nigt1.1, nigt1.2, nigt1.3, and nigt1.4 are all T-DNA insertion mutants. Therefore, to establish the intended lines, seedlings that survived on ammonium glufosinate-containing medium, owing to the presence of the bar gene next to NRT2.1pro(WT)::LUC construct in their genome, were selected, and the presence of T-DNA insertions at the target loci was confirmed in these lines by PCR-based genotyping using sequence-specific primers (Supplemental Table S1). Seeds obtained from a single plant homozygous for the NRT2.1pro(WT)::LUC construct and all the intended T-DNA insertions were used for subsequent analyses. Seeds were sterilized in 0.7% NaClO (w/v) solution for 5 min and rinsed with deionized water. To perform the microplate-based LUC assay, plants were aseptically grown in a black 96-well plate (Greiner, F-bottom, #655077). Each well contained 4 seeds and 100 µL of 1/10× modified MS solution supplemented with 0.5 mm ammonium succinate and 125 µm KH2PO4 as the N and P sources, respectively. The gaps between wells were filled with deionized water to prevent evaporation of the nutrient solution. The plates were covered with transparent lids and placed under continuous illumination (60 µE) at 23 °C. To apply the P deficiency treatment, the nutrient solution was replaced with fresh medium containing 0, 5, or 125 µm P after 2 d.
Plants for protoplast transient assays were grown in 1/10 MS hydroponic solution, supplemented with 0.5 mm MES (pH 5.7), under continuous light (60 µE) at 23 °C. To minimize the effects of TFs, protoplasts were prepared from the previously reported nigtQ-2 plants, which lack all 4 NIGT1 proteins (Maeda et al. 2018).
Microplate-based in vivo LUC assay
Nitrate-induced temporal changes in NRT2.1 expression were analyzed using 3-d-old seedlings. Prior to the analysis, 1/10× modified MS solution in the wells of the 96-well plate was replaced with new 1/10× modified MS solution containing 100 µm luciferin potassium. After 1 h, the indicated concentration of KNO3 was added to each well and mixed by pipetting. Plants were exposed to light, except during chemiluminescence recording. Luminescence was recorded every 20 min using a microplate reader (Infinite M1000, TECAN).
Transient expression assays in protoplasts
Isolation of mesophyll protoplasts and transfection with various plasmids were carried out as reported previously (Yoo et al. 2007). Approximately 2 × 104 cells were cotransfected with 3 µg of a reporter plasmid (pJD301 containing the NRT2.1 or NIGT1.1 promoter-driven LUC gene), 2 µg of an internal calibrator (pUBQ10pro::GUS), 1.5 µg of an effector plasmid carrying NLP7, and various amounts of a titrated effector plasmid carrying NIGT1.1. The total amount of titrated effector plasmids and an empty plasmid was adjusted to 12 µg. The above-mentioned plasmids have been described previously (Maeda et al. 2018; Ueda et al. 2020a). After transfection, protoplasts were incubated in washing and incubation solution, supplemented with 1 mm KNO3, in the dark. Protoplasts were harvested 16 h after the transfection and lysed with 1× Cell Culture Lysis Reagent (Promega). LUC and β-glucuronidase (GUS) activities were measured as reported previously (Maeda et al. 2018).
RNA extraction and RT-qPCR
The harvested seedlings were pulverized in liquid N2, and total RNA was extracted from the ground seedlings using the ISOSPIN Plant RNA Kit (Nippon Genetics, Tokyo, Japan), according to the manufacturer's instructions. Total RNA (50 ng) was reverse-transcribed using SuperScript II (Invitrogen, Carlsbad, CA, USA), and RT-qPCR was conducted using the KAPA SYBR Fast qPCR Kit (KAPA Biosystems) using gene-specific primers (Supplemental Table S1). UBQ10 was used as the internal reference gene. The expression level of each gene was calculated using a standard curve generated with a cDNA dilution series.
Statistical analysis
One-way ANOVA was conducted using the anova function in the CAR package of the R software (R core team 2017), with Tukey's honestly significant difference as the post hoc test.
Determination of coefficients
The Hill coefficient for the dose–response curve of each regulatory pathway was determined using the “nls” command of the R package nlstools (Baty et al. 2015). Coefficients for the ODE model were determined using the “function,” “ode,” and “optim” functions of the R package deSolve, with method=“ode45” and 1,000 iterations (Soetaert et al. 2010). Successful curve fitting was ensured by “convergence = 0” output. First, we considered a model where the NIGT1 ⊣NRT2.1 regulation was absent (i.e. P(t) = 0) and fitted the ODE model (Fig. 3, A and B; Supplemental Note S1) to the time-course changes in the activity of LUC deriving from the mutant NRT2.1 promoter (Fig. 1D) after rescaling to 0 to 1 (i.e. maximum value = 1, minimum value = 0). Parameters were estimated using the following equation to minimize the objective function:
where and represent simulated and observed LUC activity levels, respectively, at time t. This yielded μn, μl, and αn, which represent the degradation rate of NRT2.1 mRNA, the reduction rate of active NLP, and synthesis rate of NRT2.1 mRNA, respectively (Fig. 3; Supplemental Note S1). Next, these parameters were used to estimate other parameters by fitting the equation to the temporal changes in the LUC activity deriving from the WT NRT2.1 promoter (Fig. 1D) and the NIGT1.4 expression level (Supplemental Fig. S2). NIGT1.4 is the NIGT1 gene family member having the strongest influence on NRT2.1 expression in roots (Maeda et al. 2018). The expression of NIGT1.4 was rescaled to 0 to 1 prior to curve fitting. Based on a few experiments, we assumed that the maximum LUC activity deriving from the WT NRT2.1 promoter in the response to 10 mm nitrate is 2/3 of that deriving from the mutant NRT2.1 promoter. Thus, the LUC activity deriving from the WT NRT2.1 promoter was rescaled to 0 to 0.67. The parameters were estimated using the following equation to minimize the objective function:
where and represent simulated and observed NIGT1 mRNA levels, respectively, at time t.
Numerical simulation of ODE
Based on the constructed model and parameters, we simulated the behavior of NIGT1 and NRT2.1 using CellDesigner (version 4.4.2) (Funahashi et al. 2003; Kitano et al. 2005). Sensitivity analysis was conducted by running simulations after manually increasing and decreasing each input parameter in CellDesigner.
Accession numbers
Sequence data from this article can be found in The Arabidopsis Information Resource (TAIR) and The Rice Annotation Project (RAP) databases under the following accession numbers: UBQ10 (At4g05320), NRT2.1 (At1g08090), NIGT1.1 (At1g25550), NIGT1.2 (At1g68670), NIGT1.3 (At3g25790), NIGT1.4 (At1g13300), NLP6 (At1g64530), NLP7 (At4g24020), PHR1 (At4g28610), PHL1 (At5g29000), and OsNRT2.1 (Os02g0112100).
Supplementary Material
Contributor Information
Yoshiaki Ueda, Crop, Livestock and Environment Division, Japan International Research Center for Agricultural Sciences, Ohwashi 1-1, Tsukuba, Ibaraki 305-8686, Japan; Plant Functional Biotechnology, Agro-Biotechnology Research Center, Graduate School of Agricultural and Life Sciences, The University of Tokyo, Yayoi 1-1-1, Bunkyo-ku, Tokyo 113-8657, Japan.
Shuichi Yanagisawa, Plant Functional Biotechnology, Agro-Biotechnology Research Center, Graduate School of Agricultural and Life Sciences, The University of Tokyo, Yayoi 1-1-1, Bunkyo-ku, Tokyo 113-8657, Japan.
Author contributions
S.Y. conceived the project; Y.U. and S.Y. designed the research plans; Y.U. performed the experiments and analyzed the data; Y.U. and S.Y. wrote the manuscript.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Determination of the Hill coefficient for each regulatory pathway.
Supplemental Figure S2. Temporal expression patterns of NIGT1 family genes in response to nitrate supply.
Supplemental Figure S3. Mathematical modeling for the regulation of LUC activity.
Supplemental Figure S4. Predicted temporal changes in NLP activity and NIGT1 abundance.
Supplemental Figure S5. Sensitivity analysis of each kinetic parameter under a wider range of input values.
Supplemental Table S1. Primers used in this study.
Supplemental Note S1. Model description.
Funding
This study was supported, in part, by the Core Research for Evolutional Science and Technology, Japan Science and Technology Agency (grant no. JPMJCR15O5), and the Japan Society for the Promotion of Science KAKENHI (grant no. 22H04977).
Data availability
All relevant data supporting results presented are provided in supplemental files.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
References
- Ågren GI, Weih M. Plant stoichiometry at different scales: element concentration patterns reflect environment more than genotype. New Phytol. 2012:194(4):944–952. 10.1111/j.1469-8137.2012.04114.x [DOI] [PubMed] [Google Scholar]
- Ajmera I, Shi J, Giri J, Wu P, Stekel DJ, Lu C, Hodgman TC. Regulatory feedback response mechanisms to phosphate starvation in rice. NPJ Syst Biol Appl. 2018:4:4. 10.1038/s41540-017-0041-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Blanco C, Andrade J, Becker C, Bemm F, Bergelson J, Borgwardt KMM, Cao J, Chae E, Dezwaan TMM, Ding W, et al. 1,135 genomes reveal the global pattern of polymorphism in Arabidopsis thaliana. Cell. 2016:166(2):481–491. 10.1016/j.cell.2016.05.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez JM, Riveras E, Vidal EA, Gras DE, Contreras-López O, Tamayo KP, Aceituno F, Gómez I, Ruffel S, Lejay L, et al. Systems approach identifies TGA1 and TGA4 transcription factors as important regulatory components of the nitrate response of Arabidopsis thaliana roots. Plant J. 2014:80(1):1–13. 10.1111/tpj.12618 [DOI] [PubMed] [Google Scholar]
- Araus V, Vidal EA, Puelma T, Alamos S, Mieulet D, Guiderdoni E, Gutiérrez RA. Members of BTB gene family of scaffold proteins suppress nitrate uptake and nitrogen use efficiency. Plant Physiol. 2016:171(2):1523–1532. 10.1104/pp.15.01731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Mehreja R, Thiberge S, Chen M, Weiss R. Spatiotemporal control of gene expression with pulse-generating networks. Proc Natl Acad Sci U S A. 2004:101(17):6355–6360. 10.1073/pnas.0307571101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baty F, Ritz C, Charles S, Brutsche M, Flandrois JP, Delignette-Muller ML. A toolbox for nonlinear regression in R: the package nlstools. J Stat Softw. 2015:66(5):1–21. 10.18637/jss.v066.i05 [DOI] [Google Scholar]
- Binkert M, Kozma-Bognár L, Terecskei K, De Veylder L, Nagy F, Ulm R. UV-B-responsive association of the Arabidopsis bZIP transcription factor ELONGATED HYPOCOTYL5 with target genes, including its own promoter. Plant Cell. 2014:26(10):4200–4213. 10.1105/tpc.114.130716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleris L, Xie Z, Glass D, Adadey A, Sontag E, Benenson Y. Synthetic incoherent feedforward circuits show adaptation to the amount of their genetic template. Mol Syst Biol. 2011:7:519. 10.1038/msb.2011.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AJ, Chapin FS, Mooney HA. Resource limitation in plants-an economic analogy. Annu Rev Ecol Evol Syst. 1985:16(1):363–392. 10.1146/annurev.es.16.110185.002051 [DOI] [Google Scholar]
- Boer DR, Freire-Rios A, Van Den Berg WAM, Saaki T, Manfield IW, Kepinski S, López-Vidrieo I, Franco-Zorrilla JM, De Vries SC, Solano R, et al. Structural basis for DNA binding specificity by the auxin-dependent ARF transcription factors. Cell. 2014:156(3):577–589. 10.1016/j.cell.2013.12.027 [DOI] [PubMed] [Google Scholar]
- Bustos R, Castrillo G, Linhares F, Puga MI, Rubio V, Pérez-Pérez J, Solano R, Leyva A, Paz-Ares J. A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis. PLoS Genet. 2010:6(9):e1001102. 10.1371/journal.pgen.1001102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardin C, Girin T, Roudier F, Meyer C, Krapp A. The plant RWP-RK transcription factors: key regulators of nitrogen responses and of gametophyte development. J Exp Bot. 2014:65(19):5577–5587. 10.1093/jxb/eru261 [DOI] [PubMed] [Google Scholar]
- Chen Q, Liu Y, Maere S, Lee E, Van Isterdael G, Xie Z, Xuan W, Lucas J, Vassileva V, Kitakura S, et al. A coherent transcriptional feed-forward motif model for mediating auxin-sensitive PIN3 expression during lateral root development. Nat Commun. 2015:6:8821. 10.1038/ncomms9821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yao Q, Gao X, Jiang C, Harberd NP, Fu X. Shoot-to-root mobile transcription factor HY5 coordinates plant carbon and nitrogen acquisition. Curr Biol. 2016:26(5):640–646. 10.1016/j.cub.2015.12.066 [DOI] [PubMed] [Google Scholar]
- Cheng Y-H, Durand M, Brehaut V, Hsu F-C, Kelemen Z, Texier Y, Krapp A, Tsay Y-F. Interplay between NIN-LIKE PROTEINs 6 and 7 in nitrate signaling. Plant Physiol. 2023:192(4):3049–3068. 10.1093/plphys/kiad242 [DOI] [PubMed] [Google Scholar]
- Corratgé-Faillie C, Lacombe B. Substrate (un)specificity of Arabidopsis NRT1/PTR family (NPF) proteins. J Exp Bot. 2017:68(12):3107–3113. 10.1093/jxb/erw499 [DOI] [PubMed] [Google Scholar]
- Crawford NM, Glass ADM. Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci. 1998:3(10):389–395. 10.1016/S1360-1385(98)01311-9 [DOI] [Google Scholar]
- Dixon LE, Hodge SK, van Ooijen G, Troein C, Akman OE, Millar AJ. Light and circadian regulation of clock components aids flexible responses to environmental signals. New Phytol. 2014:203(2):568–577. 10.1111/nph.12853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entus R, Aufderheide B, Sauro HM. Design and implementation of three incoherent feed-forward motif based biological concentration sensors. Syst Synth Biol. 2007:1(3):119–128. 10.1007/s11693-007-9008-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagard M, Launay A, Clément G, Courtial J, Dellagi A, Farjad M, Krapp A, Soulié MC, Masclaux-Daubresse C. Nitrogen metabolism meets phytopathology. J Exp Bot. 2014:65(19):5643–5656. 10.1093/jxb/eru323 [DOI] [PubMed] [Google Scholar]
- Fan X, Tang Z, Tan Y, Zhang Y, Luo B, Yang M, Lian X, Shen Q, Miller AJ, Xu G. Overexpression of a pH-sensitive nitrate transporter in rice increases crop yields. Proc Natl Acad Sci U S A. 2016:113(26):7118–7123. 10.1073/pnas.1525184113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkenstädt B, Heron EA, Komorowski M, Edwards K, Tang S, Harper CV, Davis JRE, White MRH, Millar AJ, Rand DA. Reconstruction of transcriptional dynamics from gene reporter data using differential equations. Bioinformatics. 2008:24(24):2901–2907. 10.1093/bioinformatics/btn562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi A, Morohashi M, Kitano H, Tanimura N. CellDesigner: a process diagram editor for gene-regulatory and biochemical networks. Biosilico. 2003:1(5):159–162. 10.1016/S1478-5382(03)02370-9 [DOI] [Google Scholar]
- Guan P, Ripoll JJ, Wang R, Vuong L, Bailey-Steinitz LJ, Ye D, Crawford NM. Interacting TCP and NLP transcription factors control plant responses to nitrate availability. Proc Natl Acad Sci U S A. 2017:114(9):2419–2424. 10.1073/pnas.1615676114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HC, Wang YY, Tsay YF. AtCIPK8, a CBL-interacting protein kinase, regulates the low-affinity phase of the primary nitrate response. Plant J. 2009:57(2):264–278. 10.1111/j.1365-313X.2008.03685.x [DOI] [PubMed] [Google Scholar]
- Ishihara S, Fujimoto K, Shibata T. Cross talking of network motifs in gene regulation that generates temporal pulses and spatial stripes. Genes Cells. 2005:10(11):1025–1038. 10.1111/j.1365-2443.2005.00897.x [DOI] [PubMed] [Google Scholar]
- Kaplan S, Bren A, Dekel E, Alon U. The incoherent feed-forward loop can generate non-monotonic input functions for genes. Mol Syst Biol. 2008:4:203. 10.1038/msb.2008.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama H, Mori M, Kawamura Y, Tanaka T, Mori M, Hasegawa H. Production and characterization of transgenic rice plants carrying a high-affinity nitrate transporter gene (OsNRT2.1). Breed Sci. 2009:59(3):237–243. 10.1270/jsbbs.59.237 [DOI] [Google Scholar]
- Kiba T, Inaba J, Kudo T, Ueda N, Konishi M, Mitsuda N, Takiguchi Y, Kondou Y, Yoshizumi T, Ohme-Takagi M, et al. Repression of nitrogen starvation responses by members of the Arabidopsis GARP-type transcription factor NIGT1/HRS1 subfamily. Plant Cell. 2018:30(4):925–945. 10.1105/tpc.17.00810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiba T, Krapp A. Plant nitrogen acquisition under low availability: regulation of uptake and root architecture. Plant Cell Physiol. 2016:57(4):707–714. 10.1093/pcp/pcw052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano H, Funahashi A, Matsuoka Y, Oda K. Using process diagrams for the graphical representation of biological networks. Nat Biotechnol. 2005:23(8):961–966. 10.1038/nbt1111 [DOI] [PubMed] [Google Scholar]
- Konishi M, Okitsu T, Yanagisawa S. Nitrate-responsive NIN-like protein transcription factors perform unique and redundant roles in Arabidopsis. J Exp Bot. 2021:72(15):5735–5750. 10.1093/jxb/erab246 [DOI] [PubMed] [Google Scholar]
- Konishi M, Yanagisawa S. Arabidopsis NIN-like transcription factors have a central role in nitrate signalling. Nat Commun. 2013:4:1617. 10.1038/ncomms2621 [DOI] [PubMed] [Google Scholar]
- Konishi M, Yanagisawa S. Emergence of a new step towards understanding the molecular mechanisms underlying nitrate-regulated gene expression. J Exp Bot. 2014:65(19):5589–5600. 10.1093/jxb/eru267 [DOI] [PubMed] [Google Scholar]
- Li Y, Kronzucker HJ, Shi W. Microprofiling of nitrogen patches in paddy soil: analysis of spatiotemporal nutrient heterogeneity at the microscale. Sci Rep. 2016:6:27064. 10.1038/srep27064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wang Y, Okamoto M, Crawford NM, Siddiqi MY, Glass ADM. Dissection of the AtNRT2.1:AtNRT2.2 inducible high-affinity nitrate transporter gene cluster. Plant Physiol. 2007:143(1):425–433. 10.1104/pp.106.091223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K-H, Liu M, Liin Z, Wang Z-F, Chen B, Liu C, Guo A, Konishi M, Yanagisawa S, Wagner G, et al. NIN-like protein 7 transcription factor is a plant nitrate sensor. Science. 2022:377(6613):1419–1425. 10.1126/science.add1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K-H, Niu Y, Konishi M, Wu Y, Du H, Chung HS, Li L, Boudsocq M, McCormack M, Maekawa S, et al. Discovery of nitrate-CPK-NLP signalling in central nutrient-growth networks. Nature. 2017:545(7654):311–316. 10.1038/nature22077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Konishi M, Kiba T, Sakuraba Y, Sawaki N, Kurai T, Ueda Y, Sakakibara H, Yanagisawa S. A NIGT1-centred transcriptional cascade regulates nitrate signalling and incorporates phosphorus starvation signals in Arabidopsis. Nat Commun. 2018:9(1):1376. 10.1038/s41467-018-03832-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan S, Alon U. Structure and function of the feed-forward loop network motif. Proc Natl Acad Sci U S A. 2003:100(21):11980–11985. 10.1073/pnas.2133841100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan S, Itzkovitz S, Zaslaver A, Alon U. The incoherent feed-forward loop accelerates the response-time of the gal system of Escherichia coli. J Mol Biol. 2006:356(5):1073–1081. 10.1016/j.jmb.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Mansueto L, Fuentes RR, Borja FN, Detras J, Abrio-Santos JM, Chebotarov D, Sanciangco M, Palis K, Copetti D, Poliakov A, et al. Rice SNP-seek database update: new SNPs, indels, and queries. Nucleic Acids Res. 2017:45(D1):D1075–D1081. 10.1093/nar/gkw1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchive C, Roudier F, Castaings L, Bréhaut V, Blondet E, Colot V, Meyer C, Krapp A. Nuclear retention of the transcription factor NLP7 orchestrates the early response to nitrate in plants. Nat Commun. 2013:4:1713. 10.1038/ncomms2650 [DOI] [PubMed] [Google Scholar]
- Miller AJ, Fan X, Orsel M, Smith SJ, Wells DM. Nitrate transport and signalling. J Exp Bot. 2007:58(9):2297–2306. 10.1093/jxb/erm066 [DOI] [PubMed] [Google Scholar]
- Nazoa P, Vidmar JJ, Tranbarger TJ, Mouline K, Damiani I, Tillard P, Zhuo D, Glass ADM, Touraine B. Regulation of the nitrate transporter gene AtNRT2.1 in Arabidopsis thaliana: responses to nitrate, amino acids and developmental stage. Plant Mol Biol. 2003:52(3):689–703. 10.1023/A:1024899808018 [DOI] [PubMed] [Google Scholar]
- Okamoto M, Vidmar JJ, Glass ADM. Regulation of NRT1 and NRT2 gene families of Arabidopsis thaliana: responses to nitrate provision. Plant Cell Physiol. 2003:44(3):304–317. 10.1093/pcp/pcg036 [DOI] [PubMed] [Google Scholar]
- O’Malley RC, Huang SSC, Song L, Lewsey MG, Bartlett A, Nery JR, Galli M, Gallavotti A, Ecker JR. Cistrome and epicistrome features shape the regulatory DNA landscape. Cell. 2016:165(5):1280–1292. 10.1016/j.cell.2016.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsel M, Krapp A, Daniel-Vedele F. Analysis of the NRT2 nitrate transporter family in Arabidopsis. Structure and gene expression. Plant Physiol. 2002:129(2):886–896. 10.1104/pp.005280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osella M, Bosia C, Corá D, Caselle M. The role of incoherent microRNA-mediated feedforward loops in noise buffering. PLoS Comput Biol. 2011:7(3):e1001101. 10.1371/journal.pcbi.1001101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokhilko A, Bou-Torrent J, Pulido P, Rodríguez-Concepción M, Ebenhöh O. Mathematical modelling of the diurnal regulation of the MEP pathway in Arabidopsis. New Phytol. 2015:206(3):1075–1085. 10.1111/nph.13258 [DOI] [PubMed] [Google Scholar]
- Pokhilko A, Fernández AP, Edwards KD, Southern MM, Halliday KJ, Millar AJ. The clock gene circuit in Arabidopsis includes a repressilator with additional feedback loops. Mol Syst Biol. 2012:8:574. 10.1038/msb.2012.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokhilko A, Mas P, Millar AJ. Modelling the widespread effects of TOC1 signalling on the plant circadian clock and its outputs. BMC Syst Biol. 2013:7:23. 10.1186/1752-0509-7-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga MI, Mateos I, Charukesi B, Wang Z, Franco-Zorrilla JM, de Lorenzo L, Irigoyen ML, Masiero S, Bustos R, Rodriguez J, et al. SPX1 Is a phosphate-dependent inhibitor of PHOSPHATE STARVATION RESPONSE 1 in Arabidopsis. Proc Natl Acad Sci U S A. 2014:111(41):14947–14952. 10.1073/pnas.1404654111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey SA, Smith JJ, Orrell D, Marelli M, Petersen TW, De Atauri P, Bolouri H, Aitchison JD. Dual feedback loops in the GAL regulon suppress cellular heterogeneity in yeast. Nat Genet. 2006:38(9):1082–1087. 10.1038/ng1869 [DOI] [PubMed] [Google Scholar]
- R Core Team . R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2017. [accessed 2023 Jun]. https://www.r- proje ct. org/ [Google Scholar]
- Remans T, Nacry P, Pervent M, Girin T, Tillard P, Lepetit M, Gojon A. A central role for the nitrate transporter NRT2.1 in the integrated morphological and physiological responses of the root system to nitrogen limitation in Arabidopsis. Plant Physiol. 2006:140(3):909–921. 10.1104/pp.105.075721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ried MK, Wild R, Zhu J, Pipercevic J, Sturm K, Broger L, Harmel RK, Abriata LA, Hothorn LA, Fiedler D, et al. Inositol pyrophosphates promote the interaction of SPX domains with the coiled-coil motif of PHR transcription factors to regulate plant phosphate homeostasis. Nat Commun. 2021:12:384. 10.1038/s41467-020-20681-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld N, Elowitz MB, Alon U. Negative autoregulation speeds the response times of transcription networks. J Mol Biol. 2002:323(5):785–793. 10.1016/S0022-2836(02)00994-4 [DOI] [PubMed] [Google Scholar]
- Sakuraba Y, Zhuo M, Yanagisawa S. RWP-RK domain-containing transcription factors in the Viridiplantae: biology and phylogenetic relationships. J Exp Bot. 2022:73(13):4323–4337. 10.1093/jxb/erac229 [DOI] [PubMed] [Google Scholar]
- Shen-Orr SS, Milo R, Mangan S, Alon U. Network motifs in the transcriptional regulation network of Escherichia coli. Nat Genet. 2002:31(1):64–68. 10.1038/ng881 [DOI] [PubMed] [Google Scholar]
- Soetaert K, Petzoldt T, Setzer RW. Solving differential equations in R: package deSolve. J Stat Softw. 2010:33(9):1–25. 10.18637/jss.v033.i0920808728 [DOI] [Google Scholar]
- Taylor-Teeples M, Lin L, De Lucas M, Turco G, Toal TW, Gaudinier A, Young NF, Trabucco GM, Veling MT, Lamothe R, et al. An Arabidopsis gene regulatory network for secondary cell wall synthesis. Nature. 2015:517(7536):571–575. 10.1038/nature14099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Kiba T, Yanagisawa S. Nitrate-inducible NIGT1 proteins modulate phosphate uptake and starvation signalling via transcriptional regulation of SPX genes. Plant J. 2020a:102(3):448–466. 10.1111/tpj.14637 [DOI] [PubMed] [Google Scholar]
- Ueda Y, Nosaki S, Sakuraba Y, Miyakawa T, Kiba T, Tanokura M, Yanagisawa S. NIGT1 family proteins exhibit dual mode DNA recognition to regulate nutrient response-associated genes in Arabidopsis. PLoS Genet. 2020b:16(11):e1009197. 10.1371/journal.pgen.1009197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Yanagisawa S. Perception, transduction and integration of nitrogen and phosphorus nutritional signals in the transcriptional regulatory network in plants. J Exp Bot. 2019:70(15):3709–3717. 10.1093/jxb/erz148 [DOI] [PubMed] [Google Scholar]
- Urquiza-García U, Millar AJ. Expanding the bioluminescent reporter toolkit for plant science with NanoLUC. Plant Methods. 2019:15:68. 10.1186/s13007-019-0454-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Poel B, Bulens I, Hertog MLATM, Nicolai BM, Geeraerd AH. A transcriptomics-based kinetic model for ethylene biosynthesis in tomato (Solanum lycopersicum) fruit: development, validation and exploration of novel regulatory mechanisms. New Phytol. 2014:202(3):952–963. 10.1111/nph.12685 [DOI] [PubMed] [Google Scholar]
- von Wirén N, Gazzarrini S, Gojon A, Frommer WB. The molecular physiology of ammonium uptake and retrieval. Curr Opin Plant Biol. 2000:3(3):254–261. 10.1016/S1369-5266(00)00073-X [DOI] [PubMed] [Google Scholar]
- von Wittgenstein NJJB, Le CH, Hawkins BJ, Ehlting J. Evolutionary classification of ammonium, nitrate, and peptide transporters in land plants. BMC Evol Biol. 2014:14:11. 10.1186/1471-2148-14-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Mauleon R, Hu Z, Chebotarov D, Tai S, Wu Z, Li M, Zheng T, Fuentes RR, Zhang F, et al. Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature. 2018:557(7703):43–49. 10.1038/s41586-018-0063-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Xu X, Gong Q, Li Z, Li Y, Wang S, Yang Y, Ma W, Liu L, Zhu B, et al. Engineering broad-spectrum bacterial blight resistance by simultaneously disrupting variable TALE-binding elements of multiple susceptibility genes in rice. Mol Plant. 2019:12(11):1434–1446. 10.1016/j.molp.2019.08.006 [DOI] [PubMed] [Google Scholar]
- Yoo S-D, Cho Y-H, Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc. 2007:2(7):1565–1572. 10.1038/nprot.2007.199 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Qiang C, Wang X, Chen Y, Deng J, Jiang C, Sun X, Chen H, Li J, Piao W, et al. New alleles for chlorophyll content and stay-green traits revealed by a genome wide association study in rice (Oryza sativa). Sci Rep. 2019:9(1):2541. 10.1038/s41598-019-39280-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo D, Okamoto M, Vidmar JJ, Glass ADM. Regulation of a putative high-affinity nitrate transporter (Nrt2; 1At) in roots of Arabidopsis thaliana. Plant J. 1999:17(5):563–568. doi: 10.1046/j.1365-313X.1999.00396.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data supporting results presented are provided in supplemental files.