Abstract
Background: The SARS-CoV-2 Delta variant (B.1.617.2) has been associated with more severe disease, particularly when compared to the Alpha variant. Most of this data, however, is from high income countries and less is understood about the variant’s disease severity in other settings, particularly in an African context, and when compared to the Beta variant.
Methods: A novel proxy marker, RNA-dependent RNA polymerase (RdRp) target delay in the Seegene Allplex TM 2019-nCoV (polymerase chain reaction) PCR assay, was used to identify suspected Delta variant infection in routine laboratory data. All cases diagnosed on this assay in the public sector in the Western Cape, South Africa, from 1 April to 31 July 2021, were included in the dataset provided by the Western Cape Provincial Health Data Centre (PHDC). The PHDC collates information on all COVID-19 related laboratory tests, hospital admissions and deaths for the province. Odds ratios for the association between the proxy marker and death were calculated, adjusted for prior diagnosed infection and vaccination status.
Results: A total of 11,355 cases with 700 deaths were included in this study. RdRp target delay (suspected Delta variant) was associated with higher mortality (adjusted odds ratio [aOR] 1.45; 95% confidence interval [CI]: 1.13-1.86), compared to presumptive Beta infection. Prior diagnosed infection during the previous COVID-19 wave, which was driven by the Beta variant, was protective (aOR 0.32; 95%CI: 0.11-0.92) as was vaccination (aOR [95%CI] 0.15 [0.03-0.62] for complete vaccination [≥28 days post a single dose of Ad26.COV2.S or ≥14 days post second BNT162b2 dose]).
Conclusion: RdRp target delay, a proxy for infection with the Delta variant, is associated with an increased risk of mortality amongst those who were tested for COVID-19 in our setting.
Keywords: SARS-CoV-2, Delta, B.1.617.2, clinical severity, RdRp target delay, South Africa
Introduction
To date, the Western Cape Province of South Africa, has experienced four large waves of SARS-CoV-2 infections. The first wave caused by early ancestral clades of SARS-CoV-2 peaked in June 2020, the second wave with the Beta variant (B.1.351) peaked in December 2020, the third wave caused by the Delta variant (B.1.617.2) peaked in August 2021 and the fourth most recent wave was caused by the Omicron variant peaked in December 2021 1 .
Prior to the arrival of Omicron, Delta had been the dominant variant globally, its increased clinical severity particularly when compared to Alpha is well documented 2– 6 . Most of the severe disease in these studies occurred in those who were unvaccinated. The concern is that in these settings with well-established vaccination programmes, there are differences between the vaccinated and unvaccinated population, largely driven by socioeconomic disparities 7 , that might contribute to the clinical severity seen and confound the association between variant and disease severity. In addition, there is only limited data on Delta disease severity within a sub-Saharan African setting.
The start of the third wave of COVID-19 in the Western Cape Province was characterized by a rapid transition from the previously dominant Beta variant to Delta 8 . Using a novel proxy marker for Delta, namely RNA-dependent RNA polymerase (RdRp) target delay (RTD) in (polymerase chain reaction) PCR positive samples, our objective was to assess mortality associated with Delta, compared to Beta, in our population which had relatively low levels of vaccine coverage as well as a high prevalence of comorbidities, including HIV and tuberculosis.
Methods
RdRP target delay
RTD, defined as a difference in cycle threshold (Ct) value of >3.5 in the RdRp relative to E gene target in the Seegene Allplex TM 2019-nCoV PCR assay (Seegene Inc, USA), has recently been described 9 . This phenomenon is due to the G15451A mutation found in Delta, resulting in a RdRp primer mismatch 9 . When evaluated against genomic data, this method had a sensitivity of 93.6% and specificity of 89.7% in detecting Delta 9 . RTD has been used to assess variant disease severity in our setting during the fourth wave 10 .
Population and statistical analysis
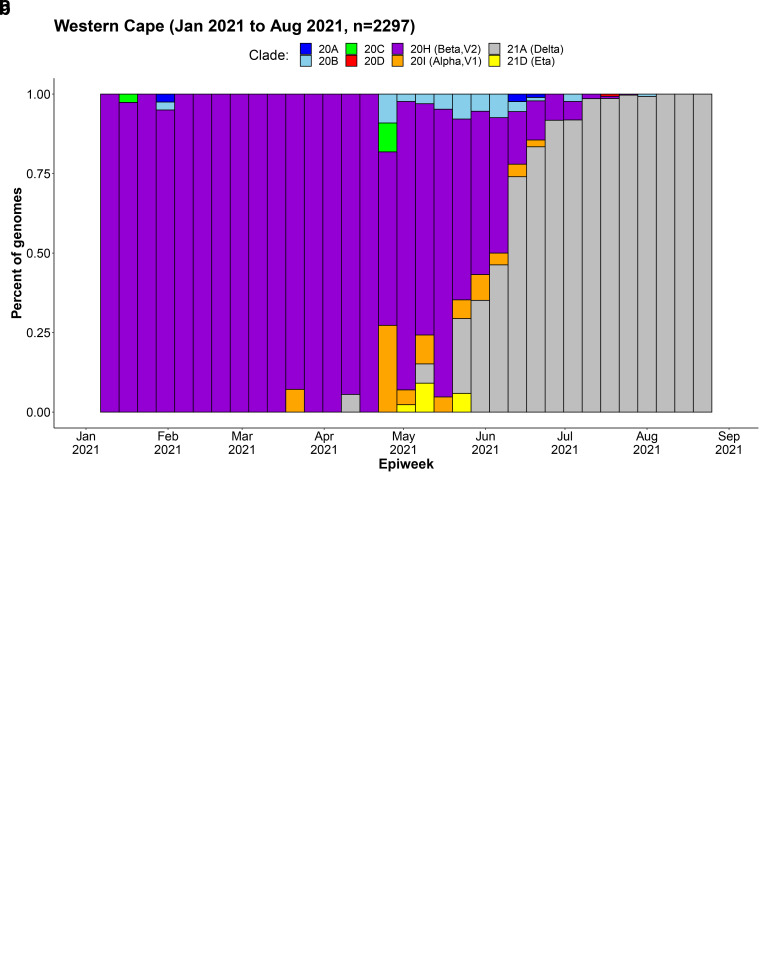
We included all COVID-19 cases diagnosed on the Seegene Allplex TM 2019-nCoV diagnostic PCR assay in the Western Cape public sector from 1 April to 31 July 2021, a period when both Beta and Delta were co-circulating ( Appendix Figure 1) All available cases were included without sampling. Alpha and non-variant of concern lineages accounted for a negligible number of infections at this time 8 . Follow-up ended on 31 August 2021, at which point most of the expected COVID-19 related outcomes would have occurred. Approximately 70% of the Western Cape population uses the public sector for health services and the Western Cape Provincial Health Data Centre (PHDC) collates all available electronic health data on these patients 11 . The PHDC combines laboratory data on COVID-19 tests with hospital admission and death data, as well as information on known comorbidities and where available, vaccination status. Use of de-identified linked data provided by the PHDC in COVID-19 analyses has been previously described 12 . COVID-19 deaths were defined analytically as deaths within 28 days of diagnosis of COVID-19 or 14 days after discharge from hospital (where hospital admission occurred within 21 days of the COVID-19 diagnosis), without a non-COVID-19 cause of death recorded by the health facility or COVID-19 case managers. Out-of-facility deaths were determined by civil identifier linkage of patients with COVID-19 diagnoses to the national population register.
Figure 1.
Weekly frequency and distribution of SARS-CoV-2 variants circulating in the Western Cape Province, South Africa, 1 January to 31 August 2021: a) absolute count of genomes sequenced, b) proportion of genomes sequenced 9 .
We undertook logistic regression using Stata 13.1 (Stata corp, 2013) (RRID:SCR_012763) to determine the association between RTD and mortality, adjusted for age, sex, known comorbidities, prior diagnosed infection, vaccination status at time of diagnosis, sub-district of residence, month of diagnosis and hospital admission pressure (number of public sector admissions in the calendar week of diagnosis, categorized into four [weekly admission of ≤350, ≤700, ≤1000 and >1000]). All variables were included as binary or categorical variables. Prior diagnosed infections were defined as a positive COVID-19 test more than 90 days prior to the current test and classified into those with their first infection in the first wave (1 March – 30 September 2020) or second wave (1 October 2020 – 31 March 2021). Vaccination status at time of COVID-19 diagnosis was determined by the PHDC prior to de-identification by linking COVID-19 cases with the national Electronic Vaccination Data System (EVDS) through national civil identifiers in both the PHDC and EVDS databases. EVDS, a national clinical data system that is not publicly accessible, is one of the data sources integrated into the PHDC 11 . For the purposes of this study, we defined complete vaccination analytically as ≥28 days post-vaccination with Janssen/Johnson & Johnson (Ad26.COV2.S), or ≥14 days post second dose of Pfizer–BioNTech (BNT162b2). Patients were deemed partially vaccinated from the day after their (first) vaccine dose until meeting criteria for complete vaccination.
A secondary analysis was done, using the same methodology as above, but stratifying the cases into those aged less than 50 years and those aged 50 years and above.
Ethical approval
The study was approved by the University of Cape Town Research Ethics Committee (HREC 460/2020).
Results
We included 11,355 cases tested using the Seegene Allplex TM assay, which were 22% of all positive test results in the province in that time period ( Appendix Table 1). The median age was 43 years (interquartile range [IQR] 32-55) and 44% were male. RTD was present in 9106 (80%) of the cases included ( Table 1). Patient characteristics were similar in those with and without RTD. There was, however, a difference in Ct values (average of the E and N gene targets); those with RTD had lower median Ct values (26.1, IQR 22.2-30.9, vs 32.7 IQR 25.6-37.6).
Table 1. Characteristics of all COVID-19 cases (Seegene Allplex TM 2019-nCoV assay positive) in the Western Cape, April – July 2021, by presence of RNA-dependent RNA polymerase (RdRP) target delay (RTD).
| All cases
(n=11,355; 700 deaths) |
RdRP Target
Delay (n=9,106; 570 deaths) |
No RdRP Target
(n=2,249; 130 deaths) |
|||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| RTD | Absent | 2249 | 19.8 | ||||
| Present | 9106 | 80.2 | |||||
| Sex | Female | 6411 | 56.5 | 5143 | 56.5 | 1268 | 56.4 |
| Male | 4944 | 43.5 | 3963 | 43.5 | 981 | 43.6 | |
| Age category | 20–29 years | 2243 | 19.8 | 1810 | 19.9 | 433 | 19.3 |
| 30–39 years | 2623 | 23.1 | 2149 | 23.6 | 474 | 21.1 | |
| 40–49 years | 2427 | 21.4 | 1956 | 21.5 | 471 | 20.9 | |
| 50–59 years | 2113 | 18.6 | 1701 | 18.7 | 412 | 18.3 | |
| 60–69 years | 1177 | 10.4 | 899 | 9.9 | 278 | 12.4 | |
| ≥70 years | 772 | 6.8 | 591 | 6.5 | 181 | 8.1 | |
| Comorbidities | HIV positive | 726 | 6.4 | 560 | 6.2 | 166 | 7.4 |
| Diabetes | 1546 | 13.6 | 1218 | 13.4 | 328 | 14.6 | |
| Current tuberculosis | 94 | 0.8 | 66 | 0.7 | 28 | 1.2 | |
| Hypertension | 2475 | 21.8 | 1963 | 21.6 | 512 | 22.8 | |
| Chronic Kidney Disease | 374 | 3.3 | 299 | 3.3 | 75 | 3.3 | |
|
Chronic Obstructive Pulmonary
Disease |
876 | 7.7 | 685 | 7.5 | 191 | 8.5 | |
|
Prior diagnosed
infection |
None | 11107 | 97.8 | 8914 | 97.9 | 2193 | 97.5 |
| First infection in first wave | 64 | 0.6 | 46 | 0.5 | 18 | 0.8 | |
| Second infection in second wave | 184 | 1.6 | 146 | 1.6 | 38 | 1.7 | |
|
Vaccination status at
time of diagnosis ¶ |
Unvaccinated | 10422 | 91.8 | 8358 | 91.8 | 2064 | 91.8 |
| Partially vaccinated | 694 | 6.1 | 544 | 6.0 | 150 | 6.7 | |
| Completely vaccinated | 239 | 2.1 | 204 | 2.2 | 35 | 1.6 | |
¶ Fully vaccinated was defined as ≥28 days post-vaccination with Janssen/Johnson & Johnson or ≥14 days post second dose of Pfizer–BioNTech. Patients were deemed partially vaccinated from the day after their (first) vaccine dose until meeting criteria for complete vaccination.
Amongst the Seegene Allplex TM cases in our study, there were 700 deaths meeting the above definition for COVID-19-associatedness, with a case fatality rate of 6.3% in those with RTD, compared to 5.8% in those without. After adjusting for all covariates, we found that RTD was associated with death among cases (aOR 1.45 [95% CI 1.13-1.86]) and among those admitted to hospital (aOR 1.39 [95% CI 1.03-1.88]) ( Table 2).
Prior diagnosed infection with a first infection in the Beta dominated second wave (vs no prior diagnosed infection), was protective against death (aOR 0.32 [95% CI 0.11-0.92]), whereas prior diagnosed infection with a first infection in the first wave was not (aOR: 1.56 [95% CI 0.50-4.92]). Vaccination was protective against death. The aORs (95% CI) for partial and full vaccination were 0.59 (0.45-0.77) and 0.15 (0.03-0.62) respectively.
Table 2. Logistic regression for outcome of death in a) all positive Seegene Allplex TM cases; b) restricted to those admitted to hospital only; c) stratified by age into those aged under 50 and over 50 years.
| Adjusted § OR (95%CI) for outcome of death | |||||
|---|---|---|---|---|---|
| All cases
(n=11,355; 700 deaths) |
Only admitted
cases * (n=1856; 612 deaths) |
All cases < 50 years
(n=7293; 113 deaths) |
All cases ≥ 50
years (n=4062; 587 deaths) |
||
| RTD | absent | Ref | Ref | Ref | Ref |
| present | 1.45 (1.13-1.86) | 1.39 (1.03-1.88) | 1.32 (0.77-2.26) | 1.44 (1.09-1.91) | |
| Sex | female | Ref | Ref | Ref | Ref |
| male | 1.50 (1.25-1.80) | 1.19 (0.95-1.47) | 1.16 (0.78-1.72) | 1.63 (1.33-2.00) | |
| Age category | 20–29 years | Ref | Ref | Ref | |
| 30–39 years | 2.75 (1.19-6.31) | 2.19 (0.81-5.93) | 2.49 (1.07-5.78) | ||
| 40–49 years | 6.89 (3.15-15.05) | 5.91 (2.27-15.54) | 6.48 (2.92-14.37) | ||
| 50–59 years | 11.49 (5.31 - 24.88) | 6.86 (2.66-17.67) | Ref | ||
| 60–69 years | 24.64 (11.36-53.42) | 11.75 (4.56-30.28) | 2.16 (1.68-2.79) | ||
| ≥70 years | 72.81 (33.54-158.06) | 25.93 (10.03-67.01) | 6.43 (4.96-8.33) | ||
| Comorbidities † | HIV positive | 1.66 (1.11-2.47) | 1.14 (0.69-1.88) | 2.21 (1.27-3.85) | 0.99 (0.53-1.85) |
| Diabetes | 2.60 (2.14-3.16) | 1.19 (0.95-1.49) | 2.58 (1.55-4.32) | 2.61 (2.11-3.22) | |
| Current tuberculosis | 3.62 (1.76-7.42) | 1.57 (0.68-3.61) | 4.90 (1.90-12.65) | 2.38 (0.78-7.20) | |
| Hypertension | 1.13 (0.93-1.38) | 0.95 (0.76-1.20) | 1.07 (0.63-1.82) | 1.16 (0.93-1.43) | |
| Chronic Kidney Disease | 2.17 (1.65-2.86) | 1.76 (1.29-2.42) | 9.33 (4.20-20.72) | 1.87 (1.41-2.50) | |
|
Chronic Obstructive
Pulmonary Disease |
1.27 (1.00-1.62) | 0.91 (0.69-1.19) | 1.95 (1.08-3.53) | 1.17 (0.90-1.52) | |
|
Prior diagnosed
infection |
None | Ref | Ref | Ref | Ref |
|
First infection in first
wave |
1.56 (0.50-4.92) | 2.63 (0.51-13.64) | 1.11 (0.14-78.87) | 2.12 (0.49-9.17) | |
|
First infection in
second wave |
0.32 (0.11-0.92) | 0.34 (0.10-1.09) | 0.68 (0.15-3.19) | 0.14 (0.03-0.67) | |
|
Vaccination status at
time of diagnosis ¶ |
Unvaccinated | Ref | Ref | Ref | Ref |
| Partially vaccinated | 0.59 (0.45-0.77) | 0.79 (0.57-1.09) | 1.47 (0.32-6.66) | 0.57 (0.44-0.75) | |
| Completely vaccinated | 0.15 (0.03-0.62) | 0.14 (0.02-1.20) | (omitted) | 0.22 (0.05-0.96) | |
§ Adjusted for all variables in the model, as well as month of diagnosis, sub-district of residence and number of COVID-19 admissions in week of diagnosis.
* Date of hospital admission within 21 days of COVID-19 date of diagnosis.
† The reference group here is the absence of that specific comorbidity.
¶ Fully vaccinated was defined as ≥28 days post-vaccination with Janssen/Johnson & Johnson or ≥14 days post second dose of Pfizer–BioNTech. Patients were deemed partially vaccinated from the day after their (first) vaccine dose until meeting criteria for complete vaccination.
In a stratified analysis the association between RTD and death was similar in both those aged <50 and ≥50 years (aOR [95%CI] of 1.32 [0.77-2.26] and 1.44 [1.09-1.91] respectively).
Discussion
RTD, a proxy marker for the Delta variant, was associated with an increased risk for death compared to presumptive Beta variant infection, while prior diagnosed infection (with first infection in the second wave) and vaccination were strongly protective. These findings are corroborated by a recent study from the Western Cape, which is the only other study to assess mortality with the Delta variant to compared to Beta. In that analysis, the wave itself was used as a proxy for variant, and the third wave (“Delta”: 26 May - 23 June 2021) had a higher aOR for mortality when compared to the second wave (“Beta”: 25 October - 21 November 2020) of 1.79 (95%CI 1.41-2.27) 13 , which is slightly higher than this analysis. Although the confidence intervals do overlap, the possible difference might be explained by the inclusion of cases after testing restrictions were in place in this analysis.
The increased transmissibility of Delta is well established, and may be due to its higher viral load 6 . In our analysis, specimens with RTD had lower Ct values than those without, suggesting a higher viral load. This, along with immune evasion by Delta, could contribute to the increased disease severity seen 14 . Nonetheless, our results need to be interpreted in the context of our setting where not all COVID-19 cases would have accessed testing. Delta often results in mild symptoms and during wave surges, as was the case from 15 June 2021 until the end of the analysis period, in the public sector only those aged ≥45 years or with comorbidities or requiring hospital admission would be eligible for all SARS-CoV-2 testing 15 . The interpretation of our results is therefore, that among those who access a PCR test, the Delta variant is associated with worse outcomes. While it is unclear if this finding can be extrapolated to all those with COVID-19 in our setting, similar findings from countries with more widespread testing support the increased clinical severity of Delta 2– 6 .
Hospital admission was more likely with Delta compared to Alpha in England (aHR 2.26 [95%CI 1.32-3.89]) 2 and Denmark (RR 2.83 [95%CI 2.02-3.98]) 3 . In Singapore, infection with Delta and Beta vs. wild-type SARS-CoV-2 were both associated with a composite outcome of oxygen use, intensive care admission and death (aOR 4.9 [95%CI 1.43-30.78] and 1.69 [95%CI 0.19-14.69] respectively) 4 . Similarly, a recent study from Qatar found that Delta compared to Beta had an aOR of 3.61 (1.65-7.91) for severe-critical disease, which was defined as intensive care unit admission, use of high-flow oxygen, mechanical ventilation, or death 16 . Delta (vs wild-type) was associated with mortality in Canada (aOR 2.32 [95% CI 1.47-3.30]) as were N501Y-positive variants (Alpha, Beta, Gamma) (aOR 1.51 [95%CI 1.30-1.74]) 5 .
However, most of these countries had relatively high COVID-19 vaccination coverage rates by the time the Delta variant became dominant there 17 . Since most severe COVID-19 cases would be in unvaccinated people, differences between the vaccinated and the unvaccinated population might confound associations with variant infection and disease severity. By contrast in the Western Cape by 31 July 2021, only 5.0% of all adults, mostly in older age groups, were fully vaccinated 17 . In addition, most of these studies compared the outcomes of Delta to Alpha. The Alpha variant has been shown to have worse clinical outcomes compared to wild-type, with an aHR for death of 1.73 (95%CI 1.41 - 2.13) 18 .
Despite Delta no longer being the dominant variant, it continues to circulate globally and understanding and quantifying the disease severity associated with it and its spectrum of mutations remains critical. A Delta resurgence cannot be excluded, particularly with the recent emergence of the Omicron-Delta recombinant strain (BA.1 x AY.4) that the World Health Organisation has declared to be a variant under monitoring 19 . In addition, disease severity of dominant circulating variants is often calculated relative to the previously dominant variants 20 . The Omicron variant, for instance, has been found to cause less disease than the Delta variant, but this has to be understood in the context of the Delta variant already being associated with more severe disease compared to Alpha or Beta, hence quantifying the clinical severity of different variants is important for such comparisons 10, 13 .
This study has several limitations. First, we could only include cases tested on the Seegene Allplex TM 2019-nCoV assay, excluding cases diagnosed by other PCR methods or antigen testing. However, the included cases are mostly representative of all diagnosed PCR cases in the Western Cape ( Appendix Table 1). Patients tested on this assay were similar in age, sex, known comorbidities, prior diagnosed infection and vaccination status to those who tested positive using other PCR assays. As different laboratories service different geographical regions there was a difference, however, in the district of residence. Patients who tested positive on antigen tests tended to be younger, have fewer comorbidities and fewer were admitted to hospital. This is in accordance with the Western Cape’s testing guidelines where PCR was preferred for hospital patients, while antigen testing was promoted at primary health care facilities 15 .
Second, while RTD is a reliable proxy marker for Delta, it is not as accurate as whole genome sequencing, and misclassification may have diluted the effect of Delta. Third, we could only assess the effect of prior laboratory-confirmed COVID-19 infection and seroprevalence studies suggest considerably higher numbers were infected, even after the first wave, and that prior infection prevalence differed by sub-district of residence 21 . While we did adjust for sub-district, the absence of a protective effect of prior infection in the first wave may be due to insufficient numbers of those infections being diagnosed. Fourth, the inclusion of cases after testing restrictions were introduced is likely to result in an underestimate of Delta disease severity. However, in the stratified analysis, those younger than 50 years, who would be more likely to be affected by the testing restrictions, still had a similar findings to those >50 years. Fifth, although we adjusted for COVID-19 hospital admissions to account for escalating service pressure during the wave surge, we could not adjust for non-COVID-19 admissions that might have added to pressure on facilities and contributed to mortality.
And finally, there are innate limitations in using observational data from surveillance and routine health records to assess variant disease severity, particularly as potential biases around testing patterns cannot always be fully adjusted for 20.
Conclusion
In this study we found that RTD, a useful proxy for infection with the Delta variant, is associated with an increased risk of mortality amongst those who were tested for COVID-19 in our setting. This suggests that the Delta variant is associated with an increased risk of mortality when compared with other variants, and the Beta variant in particular.
Data availability
The Western Cape Provincial Health Data Centre collates all available electronic health data on public sector patients in the province for operational service use. The underlying data in this study are routinely collected patient records that have been de-identified and pseudo-anonymised in accordance with research ethics requirements. The patients have not consented to these data being part of publicly accessible repositories considering the inherent risks of re-identification. The Western Cape Department of Health and Wellness evaluates research proposals for all research in the public health sector in the province, subject to standard research ethics, government approval and data governance prescripts. This includes those that draw on routine datasets like the current study. For more information on conducting research in health services or on data managed by the Western Cape Government Department of Health and Wellness (WCGHW), please email health.research@westerncape.gov.za.
Funding Statement
This study was funded by the Grand Challenges ICODA pilot initiative delivered by Health Data Research UK and funded by the Bill & Melinda Gates and the Minderoo Foundations (INV-017293), and by a research Flagship grant from the South African Medical Research Council. Additional support was provided by the Francis Crick Institute which receives its core funding from Cancer Research UK (FC0010218), the UK Medical Research Council (FC0010218), and the Wellcome Trust (FC0010218) as well as Wellcome (203135, 222574).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
Appendix
Table 1. All positive SARS-CoV-2 tests in the Western Cape public sector, April to July 2021.
| Total positive tests
(n=50,768) |
Seegene AllplexTM PCR
(n=11,355; 22.4%) |
Other PCR tests
(n=25,042; 49.3%) |
Antigen tests
(n=14,371; 28%) |
||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Month of diagnosis | April | 165 | 1.5 | 900 | 3.6 | 106 | 0.7 |
| May | 302 | 2.7 | 1260 | 5.0 | 637 | 4.4 | |
| June | 2161 | 19.0 | 5223 | 20.9 | 2836 | 19.7 | |
| July | 8727 | 76.9 | 17659 | 70.5 | 10792 | 75.1 | |
| District of residence | Cape Winelands | 508 | 4.5 | 2449 | 9.8 | 3125 | 21.8 |
| Central Karoo | 246 | 2.2 | 431 | 1.7 | 302 | 2.1 | |
| City of Cape Town | 4975 | 43.8 | 12039 | 48.1 | 8954 | 62.3 | |
| Garden Route | 4642 | 40.9 | 4063 | 16.2 | 961 | 6.7 | |
| Overberg | 426 | 3.8 | 2448 | 9.8 | 522 | 3.6 | |
| West Coast | 557 | 4.9 | 3503 | 14.0 | 486 | 3.4 | |
| Unallocated | 1 | 0.0 | 109 | 0.4 | 21 | 0.2 | |
| Sex | Male | 4944 | 43.5 | 10602 | 42.3 | 6427 | 44.7 |
| Age category | 20–29 years | 2243 | 19.8 | 4805 | 19.2 | 2711 | 18.9 |
| 30–39 years | 2623 | 23.1 | 5497 | 22.0 | 3410 | 23.7 | |
| 40–49 years | 2427 | 21.4 | 5200 | 20.8 | 3025 | 21.1 | |
| 50-59 years | 2113 | 18.6 | 5104 | 20.4 | 3187 | 22.2 | |
| 60–69 years | 1177 | 10.4 | 2660 | 10.6 | 1241 | 8.6 | |
| ≥70 years | 772 | 6.8 | 1776 | 7.1 | 797 | 5.6 | |
| Comorbidities | HIV positive | 726 | 6.4 | 1586 | 6.3 | 405 | 2.8 |
| Diabetes | 1546 | 13.6 | 3433 | 13.7 | 1488 | 10.4 | |
| Current tuberculosis | 94 | 0.8 | 284 | 1.1 | 60 | 0.4 | |
| Hypertension | 2475 | 21.8 | 5357 | 21.4 | 2272 | 15.8 | |
| Chronic Kidney Disease | 374 | 3.3 | 702 | 2.8 | 270 | 1.9 | |
| Chronic Obstructive Pulmonary Disease | 876 | 7.7 | 2115 | 8.5 | 878 | 6.1 | |
| Prior diagnosed infection | None | 11107 | 97.8 | 24339 | 97.2 | 14245 | 99.1 |
| First infection in first wave | 64 | 0.6 | 202 | 0.8 | 43 | 0.3 | |
| First infection in second wave | 184 | 1.6 | 501 | 2.0 | 83 | 0.6 | |
| Vaccination status at time of diagnosis * | Unvaccinated | 10422 | 91.8 | 22930 | 91.6 | 13223 | 92.0 |
| Partially vaccinated | 694 | 6.1 | 1457 | 5.8 | 904 | 6.3 | |
| Completely vaccinated | 239 | 2.1 | 655 | 2.6 | 244 | 1.7 | |
| Admitted § | Case admitted to hospital | 1856 | 16.4 | 4964 | 19.8 | 1649 | 11.5 |
* Fully vaccinated was defined as ≥28 days post-vaccination with Janssen/Johnson & Johnson or ≥14 days post second dose of Pfizer–BioNTech. Patients were deemed partially vaccinated from the day after their (first) vaccine dose until meeting criteria for complete vaccination.
§ Admission with 21 days before or after COVID-19 diagnosis date
References
- 1. Network for Genomic Surveillance in South Africa (NGS-SA): SARS-CoV-2 Sequencing Update - 25 February 2022. n.d. Reference Source [Google Scholar]
- 2. Twohig KA, Nyberg T, Zaidi A, et al. : Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis. 2022;22(1):35–42. 10.1016/S1473-3099(21)00475-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bager P, Wohlfahrt J, Rasmussen M, et al. : Hospitalisation associated with SARS-CoV-2 delta variant in Denmark. Lancet Infect Dis. 2021;21(10):1351. 10.1016/S1473-3099(21)00580-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ong SWX, Chiew CJ, Ang LW, et al. : Clinical and virological features of SARS-CoV-2 variants of concern: a retrospective cohort study comparing B.1.1.7 (Alpha), B.1.315 (Beta), and B.1.617.2 (Delta). Clin Infect Dis. 2021;ciab721. 10.1093/cid/ciab721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fisman DN, Tuite AR: Progressive Increase in Virulence of Novel SARS-CoV-2 Variants in Ontario, Canada. MedRxiv. 2021; 2021.07.05.21260050. 10.1101/2021.07.05.21260050 [DOI] [Google Scholar]
- 6. Public Health England: 25 June 2021 Risk assessment for SARS-CoV-2 variant: Delta (VOC-21APR-02, B.1.617.2).2021. Reference Source [Google Scholar]
- 7. Caspi G, Dayan A, Eshal Y, et al. : Socioeconomic disparities and COVID-19 vaccination acceptance: a nationwide ecologic study. Clin Microbiol Infect. 2021;27(10):1502–1506. 10.1016/j.cmi.2021.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Network for Genomic Surveillance South Africa: Tracking SARS-COV-2 variants - 15 October 2021.2021. Reference Source [Google Scholar]
- 9. Valley-Omar Z, Marais G, Iranzadeh A, et al. : Reduced amplification efficiency of the RNA-dependent-RNA-polymerase target enables tracking of the Delta SARS-CoV-2 variant using routine diagnostic tests. J Virol Methods. 2022;302:114471. 10.1016/j.jviromet.2022.114471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hussey H, Davies MA, Heekes A, et al. : Assessing the clinical severity of the Omicron variant in the Western Cape Province, South Africa, using the diagnostic PCR proxy marker of RdRp target delay to distinguish between Omicron and Delta infections – a survival analysis. Int J Infect Dis. 2022;118:150–154. 10.1016/j.ijid.2022.02.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boulle A, Heekes A, Tiffin N, et al. : Data centre profile: The provincial health data centre of the western cape province, South Africa. Int J Popul Data Sci. 2019;4(2):1143. 10.23889/ijpds.v4i2.1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boulle A, Davies MA, Hussey H, et al. : Risk factors for Coronavirus Disease 2019 (COVID-19) death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2021;73(7):e2005–e2015. 10.1093/cid/ciaa1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davies MA, Kassanjee R, Rosseau P, et al. : Outcomes of laboratory-confirmed SARS-CoV-2 infection in the Omicron-driven fourth wave compared with previous waves in the Western Cape Province, South Africa. Trop Med Int Health. 2022;27(6):564–573. 10.1111/tmi.13752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tao K, Tzou PL, Nouhin J, et al. : The biological and clinical significance of emerging SARS-CoV-2 variants. Nat Rev Genet. 2021;22(12):757–773. 10.1038/s41576-021-00408-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Western Cape Government: Updated coronavirus PCR and antigen testing criteria during the third wave 2021. [Google Scholar]
- 16. Butt AA, Dargham SR, Chemaitelly H, et al. : Severity of Illness in Persons Infected With the SARS-CoV-2 Delta Variant vs Beta Variant in Qatar. JAMA Intern Med. 2022;182(2):197–205. 10.1001/jamainternmed.2021.7949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mathieu E, Ritchie H, Ortiz-Ospina E, et al. : A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5(7):947–953. 10.1038/s41562-021-01122-8 [DOI] [PubMed] [Google Scholar]
- 18. Grint DJ, Wing K, Williamson E, et al. : Case fatality risk of the SARS-CoV-2 variant of concern B.1.1.7 in England, 16 November to 5 February. Euro Surveill. 2021;26(11):2100256. 10.2807/1560-7917.ES.2021.26.11.2100256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization: Tracking SARS-CoV-2 variants 2022. (accessed March 22, 2022). Reference Source [PubMed] [Google Scholar]
- 20. Cevik M, Mishra S: SARS-CoV-2 variants and considerations of inferring causality on disease severity. Lancet Infect Dis. 2021;21(11):1472–1474. 10.1016/S1473-3099(21)00338-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hsiao M, Davies MA, Kalk E, et al. : SARS-CoV-2 seroprevalence in the Cape Town metropolitan sub-districts after the peak of infections. Covid-19 Spec Public Heal Surveill Bull. 2020;18. [Google Scholar]