Abstract
Background
Poly (ADP-ribose) polymerase (PARP) inhibitors are a new maintenance therapy option for patients with ovarian cancer (OC).
Objective
To evaluate the efficacy and influencing factors of the novel PARP inhibitor niraparib for maintenance treatment of Chinese patients with advanced OC.
Patients and Methods
In this retrospective multicenter real-world study patients with advanced OC from 15 hospitals throughout China were enrolled. The primary endpoint was progression-free survival (PFS) and the secondary endpoints included the time to treatment discontinuation and safety. Least Absolute Shrinkage and Selection Operator (LASSO) regression was used to identify possible risk factors for PFS, after which a prediction model was established to evaluate the likelihood of achieving an 18-month PFS. The relationship between the dose of niraparib and PFS was also evaluated.
Results
The PFS rates of 199 patients at 6, 12, 18, 24, and 30 months were 87.4%, 75.9%, 63.6%, 56.1%, and 51.8%, respectively. LASSO regression model revealed that only age < 65 years (P = 0.011), BRCA mutations (P < 0.001), and R0 status after cytoreductive surgery (P = 0.01) were significant factors associated with prolonged PFS times. Based on the LASSO logistic regression analysis, a clinical prediction formula was developed: − 2.412 + 1.396Age≥65yr + 2.374BRCAwt + 1.387R1 + 0.793Interval≥12w + 0.178BMI>24kg/m2 which yielded a cut-off value of 0.091, an area under the curve (AUC) of 0.839 (0.763–0.916), a sensitivity of 94.3%, and an accuracy of 78.5%. A nomogram was then built to visualize the results. The major treatment-emergent adverse events of ≥ grade 3 included a platelet count decrease (19.1%), white blood cell count decrease (15.1%), neutrophil count decrease (13.1%), and anemia (18.6%). The 18-month PFS rates in patients treated with 200 mg niraparib were somewhat higher than in patients treated with 100 mg after 3-months of therapy.
Conclusions
For Chinese OC patients, niraparib, particularly at a 200 mg individual starting dose, was an effective therapy with easily manageable safety.
Plain Language Summary
Maintenance therapy with poly (ADP-ribose) polymerase inhibitors is a new option for patients with ovarian cancer (OC) after they have received platinum-based chemotherapy to reduce the recurrence or relapse rates, but it remains unclear whether there are any changes in efficacy and safety when different starting doses of niraparib are administrated to Chinese patients, who typically have a bodyweight < 77 kg. We found that niraparib exhibited satisfactory efficacy with tolerable safety during maintenance therapy for advanced OC whether administered at 100 mg or 200 mg doses. We believe these regimens can serve as a valuable addition to the previous results of randomized controlled trials.
Key Points
| Niraparib at an individualized starting dose strategy of 200 mg with dose adjustments from 200 to 100 mg was a satisfactory treatment regimen. |
| A prediction model was established to evaluate the likelihood of achieving 18-month progression-free survival in Chinese ovarian cancer (OC) patients. |
| Niraparib exhibited tolerable safety during maintenance therapy for advanced OC whether administered at 100 mg or 200 mg doses. |
Introduction
From 2005, ovarian cancer (OC) has become the second main gynecological cause of cancer-related deaths in China, replacing uterine cancer [1]. According to the GLOBOCAN Global Cancer Statistics, the number of new OC cases in China was 313,959, with a related mortality rate of 2.1% (207,252 patients) in 2020 [2]. It is expected that the incidence of OC in China will continue to rise during the next decade to a higher rate than that predicted globally [3]. In particular, advanced OC has a recurrence rate as high as 80%, with resistance to chemotherapy being a limiting factor for the success rates of relapsed OC treatments [4].
Maintenance treatment (MT) with poly (ADP-ribose) polymerase (PARP) inhibitors is a novel option for patients with OC who had previously received platinum-based chemotherapy treatment to decrease the rates of recurrence or relapse [5, 6]. Recently, trials and real-world studies found that olaparib with and without bevacizumab was an effective and safe MT for patients with OC [7–12].
Niraparib is a potent selective PARP-1/2 inhibitor with excellent bioavailability when administered orally and is up to 100 times more active against these enzymes than other PARP family members [13]. It was approved in the EU and USA in 2017 [14, 15], and in China in 2019 [16] for MT of recurrent OC. However, in several clinical trials, which analyzed outcomes of niraparib for patients with advanced OC (NOVA, QUADRA, PRIMA, NORA, and PRIME), treatment-emergent adverse events (TEAEs) were reported in 99% of the patients with 51–74% being grade ≥ 3 [17]. It is noteworthy that niraparib increased the progression-free survival (PFS) times significantly compared with placebo [18–21] as well as eliciting clinically relevant activity in heavily pretreated OC patients [22] irrespective of their homologous recombination deficiency (HRD) status [23]. In addition, the dosage of niraparib as an individualized starting dose (ISD) was determined by the patient’s baseline bodyweight and platelet count. If a patient’s platelet count was < 150,000/μL or their bodyweight was < 77 kg, they received a daily dose of 200 mg. For other patients, the daily dose was 300 mg. Considering that the majority of Chinese patients have a bodyweight < 77 kg, we were particularly interested in the present study to investigate whether it was more beneficial for Chinese patients to have treatment initiated at 200 mg/day compared with 100 mg/day. We also aimed to determine whether reducing the dosage of niraparib from 200 to 100 mg, following ISD dosing principles, affected treatment efficacy [24].
To the best of our knowledge, after niraparib was covered by Chinese health insurance from 2021, multicenter studies on Chinese real-world experience with niraparib as first-line MT for OC have not been thoroughly investigated. The present large-scale observational study used real-world data to assess the efficacy and safety profile of niraparib as first-line MT in patients with newly diagnosed advanced OC, to investigate the clinical benefits associated with prolonged niraparib treatment, and to evaluate the relationship between different doses of niraparib and PFS of patients who were given a reduced dosage due to the occurrence of TEAEs.
Methods
Patients
Patients with advanced OC treated with niraparib as first-line MT in 15 Chinese hospitals between January 2019 and December 2021 were retrospectively enrolled in the study.
Inclusion Criteria
The inclusion criteria for the study were: patients ≥ 18 years old diagnosed with histologically confirmed OC [Federation International of Gynecology and Obstetrics (FIGO) stage II–IV], had underwent surgery and received first-line chemotherapy, and patients being treated with niraparib as first-line MT. Patients who exhibited disease progression or died during the follow-ups were also eligible, i.e., from the date of commencing niraparib MT to the last follow-up or death.
Exclusion Criteria
The exclusion criteria were: patients who had other malignancies ≤ 5 years before the study, were enrolled in previous clinical trials, and had been diagnosed with myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML).
Study Design
This multicenter observational retrospective study collected the data from comprehensive medical records of patients with advanced OC, who were treated with niraparib as first-line MT. The patients enrolled came from 15 hospitals across China, with at least 10 patients per hospital. In clinical practice, these patients received different oral doses of niraparib until disease progression, severe toxicity, or death occurred. The study was conducted from January 2019 to December 2021 (the index date was the date of initiating MT with niraparib) and the database was locked on 31 December 2022.
Study Endpoints
The primary endpoint was PFS, defined as the time from when a patient first received MT with niraparib to the date of disease progression. Clinical and objective radiology assessments were carried out according to Response Evaluation Criteria in Solid Tumors (RECIST ver. 1.1) guidelines. The secondary endpoints were the time to discontinuation (TTD) of therapy, which was defined as the time from the date of initiation of MT with niraparib to TTD and safety, which were assessed according to the incidence of TEAEs, laboratory test findings, vital signs, and physical examinations. TEAEs were graded following Common Terminology Criteria for Adverse Events (CTCAE) guidelines (ver. 5).
For exploratory outcomes, patients were stratified according to: their age when given the first dose (< 65 years versus ≥ 65 years), international FIGO stage II–IV, Eastern Cooperative Oncology Group (ECOG) performance status (0–2), chronic disease (yes versus no), breast cancer (BRCA) genes mutational status [mutated (BRCAm), wild type (BRCAwt), or unknown], HRD status (positive, negative, or unknown), CA-125 elimination rate constant K (KELIM) score (> 1, ≤ 1, or unknown), postoperative status of cytoreductive surgery (R0, R1), best response to first-line chemotherapy [complete response (CR) versus partial response (PR), or if there were any stabilizations], starting dose (100 mg, 200 mg, or 300 mg), after 3 months (100 mg, 200 mg, or 300 mg), neoadjuvant chemotherapy (yes versus no), BMI standards for Chinese people (≤ 24 kg/m2 or > 24 kg/m2), and the interval (≤ 12 weeks versus > 12 weeks) between completion of first-line chemotherapy and the start of niraparib MT.
Data Collection
Data from the medical records of all participating patients were collected from the hospital information system, including demographics, clinicopathological features, degree of residual disease after primary surgery, genetic-testing results, and other relevant information. Any missing data were obtained by telephone follow-ups or direct patient interviews (if they were alive and coherent). The rate of occurrence of TEAEs, dose reductions, dose interruptions, and discontinuation of treatment because of unacceptable TEAEs were also carefully documented. All criteria for efficacy and safety assessments were sent to the participating hospitals using uniform criteria.
Ethical Considerations
The study was conducted strictly following the guiding principles of the Declaration of Helsinki, the International Conference on Harmonization of Good Clinical Practice and appropriate legal guidelines for non-interventional and observational studies. As the study was retrospective in nature and data collection anonymized, the informed consent of patients was not required. The study was approved by the Ethics Committee of Zhongda Hospital, School of Medicine, Southeast University on 9 September 2022 (no: 2022040092). This study was registered with ClinicalTrials.gov (registration number: NCT05734911).
Statistical Analysis
Statistical analyses were carried out using R software (ver. 4.2.2). Categorical variables are reported as frequencies or a percentage, and continuous variables as the median plus range. No sample size calculation was made because of the exploratory nature of the study. The Kaplan–Meier method and the log-rank test were used for analysis of survival. The 95% confidence intervals (95% CIs) were evaluated using the Clopper–Pearson test. Safety data were summarized with descriptive statistics (numbers and percentages).
Univariate logistic regression analysis was used to identify possible risk factors for an 18-month PFS. Variables with significance (P < 0.05) from the univariate logistic regression analysis and those of clinical interest (e.g., the interaction of surgery and chemotherapy outcome) were analyzed using the Least Absolute Shrinkage and Selection Operator (LASSO) logistic regression model, which minimizes the influence from multicollinearity. Following this, a novel nomogram incorporating all independent prognostic factors was developed for predicting the 18-month PFS in advanced OC patients [25].
To assess the effectiveness of the model, a receiver operating characteristic (ROC) curve was used to evaluate the discriminative ability of the nomogram. A calibration curve was used to determine the level of consistency between predicted probabilities and the observed outcomes. Missing data were designated as unknown for both analyses and a P-value < 0.05 was deemed to be significant.
Results
Baseline Demographics and Clinical Features of the Enrolled Patients
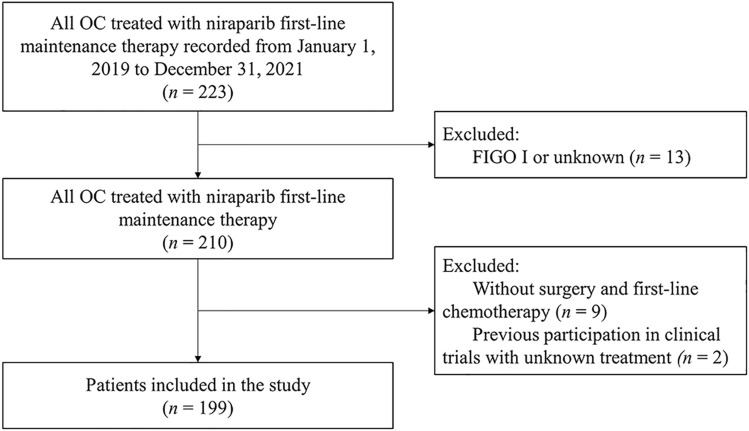
In total, 223 eligible patients underwent screening and 199 patients’ data were added to a centralized database (Fig. 1). Thirteen patients with FIGO stage I or an unknown status and cases with missing initial tumor surgery and first-line chemotherapy were excluded. Two patients were excluded because they had previously participated in clinical trials with unknown therapeutic regimens.
Fig. 1.
Flowchart of the study. FIGO Federation International of Gynecology and Obstetrics, OC ovarian cancer
The median age of the study patients was 57.0 years [interquartile range (IQR) 26.0–78.0 years], and the median duration of the follow-up times was 15.3 months (range 0.9–34.5). The clinical characteristics and demographics of the study patients are listed in Table 1. Among them, 89.4% were diagnosed with high-grade plasmacytoma and 88.4% at FIGO stage III or IV. Of the included cases, 65.3% had no macroscopic residual disease (R0) after primary surgery and 54 (27.1%) had coexisting chronic diseases. A total of 40 patients (20.1%) had a BRCA mutation, while 131 (65.8%) had BRCAwt. The results of HRD testing revealed that 66 (33.2%) patients were positive, 52 (26.1%) negative, and 81 (40.7%) not known. It is noteworthy that 87.9% of the patients achieved CR and 12.1% PR after platinum-based chemotherapy, based on RECIST 1.1 and Gynecological Cancer InterGroup (GCIG) criteria (Table 1).
Table 1.
Baseline demographics and clinical characteristics of the enrolled patients
| Characteristics | Patients |
|---|---|
| Age (years), median [IQR] | 57.0 [26.0, 78.0] |
| Age, n (%) | |
| < 65 years | 154 (77.4) |
| ≥ 65 years | 45 (22.6) |
| BMI (kg/m2), median [IQR] | 23.0 [21.0, 25.1] |
| BMI (kg/m2), n (%) | |
| < 18.5 | 8 (4.0) |
| 18.5–24 | 117 (58.8) |
| > 24 | 74 (37.2) |
| Primary tumor site, n (%) | |
| Ovaries | 179 (89.9) |
| Fallopian tube | 13 (6.5) |
| Peritoneum | 5 (2.5) |
| Other | 2 (1.0) |
| Histological subtypes, n (%) | |
| High-grade plasmacytoma | 178 (89.4) |
| Endometrioid carcinoma | 7 (3.5) |
| Clear cell carcinoma | 6 (3.0) |
| Mucinous carcinoma | 2 (1.0) |
| Low-grade plasmacytoma | 1 (0.5) |
| Other | 5 (2.5) |
| FIGO staging, n (%) | |
| II | 23 (11.6) |
| III | 136 (68.3) |
| IV | 40 (20.1) |
| ECOG, n (%) | |
| 0 | 165 (82.9) |
| 1 | 30 (15.1) |
| 2 | 4 (2.0) |
| Family history, n (%) | |
| OC | 8 (4.0) |
| Breast cancer | 2 (1.0) |
| Other tumors | 15 (7.5) |
| None | 174 (87.4) |
| Chronic diseases, n (%) | |
| No | 145 (72.9) |
| Yes | 54 (27.1) |
| Whether received neoadjuvant chemotherapy, n (%) | |
| No | 126 (63.3) |
| Yes | 73 (36.7) |
| Outcome of surgical treatment, n (%) | |
| R0 | 130 (65.3) |
| R1 | 69 (34.7) |
| Bevacizumab use in first-line chemotherapy, n (%) | |
| Yes | 8 (4.0) |
| No | 191 (96.0) |
| First-line chemotherapy regimens, n (%) | |
| Paclitaxel + carboplatin/cisplatin | 179 (89.9) |
| Paclitaxel + carboplatin/cisplatin + bevacizumab | 8 (4.0) |
| Liposomal doxorubicin + carboplatin/cisplatin | 4 (2.0) |
| Doxorubicin + carboplatin/cisplatin | 3 (1.5) |
| Other | 5 (2.5) |
| Best response to first-line chemotherapy, n (%) | |
| Complete response | 175 (87.9) |
| Partial response | 24 (12.1) |
| BRCA mutation status, n (%) | |
| BRCAm | 40 (20.1) |
| BRCAwt | 131 (65.8) |
| Unknown | 28 (14.1) |
| HRD status, n (%) | |
| Positive | 66 (33.2) |
| Negative | 52 (26.1) |
| Unknown | 81 (40.7) |
| KELIM score, n (%) | |
| > 1 | 55 (27.6) |
| ≤ 1 | 23 (11.6) |
| Unknown | 121 (60.8) |
| Previous use of PARP inhibitor*, n (%) | |
| No | 196 (98.5) |
| Yes | 3 (1.5) |
| Time between the last chemotherapy treatment and the initiation of niraparib MT, n (%) | |
| 0 to ≤ 12 weeks | 147 (73.9) |
| > 12 weeks | 52 (26.1) |
| Niraparib monotherapy/combination therapy, n (%) | |
| Single drug | 174 (87.4) |
| Combination | 25 (12.6) |
HRD homologous recombination deficiency, KELIM elimination rate constant K, MT maintenance treatment, OC ovarian cancer, PARP poly (ADP-ribose) polymerase
*Three patients have received olaparib as first-line MT and then switched to niraparib as MT by patient-directed selection
Efficacy
Primary Endpoint for PFS
The data maturity was 36.2% and the median follow-up time for PFS was 17.8 months (95% CI 17.2–18.8), while the median PFS was not reached [95% CI 23.8–not estimable (NE)]. However, the probabilities of PFS at 6, 12, 18, 24, and 30 months for the entire cohort were 87.4%, 75.9%, 63.6%, 56.1%, and 51.8%, respectively (Fig. 2a).
Fig. 2.

Kaplan–Meier curves of PFS (a) and TTD (b). NE not estimable, NR not reached, PFS progression-free survival, TTD time to treatment discontinuation
Secondary Endpoint for TTD
In this study, the median follow-up time of TTD was 17.8 months (95% CI 17.1–18.8), while the median TTD was not reached (95% CI 22.9–NE). However, the proportion of patients on TTD at 6, 12, 18, 24, and 30 months for the entire cohort were 89.4%, 76.4%, 62.8%, 55.4%, and 51.2%, respectively (Fig. 2b).
Safety
Of the 199 patients in the study, 180 (90.5%) experienced TEAEs of any grade associated with niraparib therapy (Table 2). Hematologic grade ≥ 3 events included a platelet count decrease (19.1%), anemia (18.6%), white blood cell count decrease (15.1%), and neutrophil count decrease (13.1%). The platelet count decrease was the most frequently occurring drug-related grade ≥ 3 TEAE and nausea (10.1%) was the most common non-hematological grade ≥ 3 TEAE. Dosing was interrupted in 79 (39.7%) patients and 47 (23.6%) had dose reductions. A total of 11 (5.5%) patients had their medication discontinued due to TEAEs, including a platelet count decrease in 5, anemia in 3, white blood cell count decrease in 2 patients, and vomiting in 1 patient. No TEAE-related AML, MDS, or death were reported (Table 2).
Table 2.
Summary of adverse events
| Adverse events | Patients (n, %) |
|---|---|
| Any TEAEs | 180 (90.5) |
| CTCAE grade ≥ 3 TEAEs | 113 (56.8) |
| TEAEs led to interruption of medication | 79 (39.7) |
| TEAEs led to reductions in dose | 47 (23.6) |
| TEAEs led to drug discontinuation | 11 (5.5) |
| TEAEs led to death | 0 |
| Any grade (n, %) | Grade ≥ 3 (n, %) | |
|---|---|---|
| Hematological TEAE | ||
| Platelet count decreased | 77 (38.7) | 38 (19.1) |
| White blood cell count decreased | 83 (41.7) | 30 (15.1) |
| Neutrophil count decreased | 63 (31.7) | 26 (13.1) |
| Anemia | 94 (47.2) | 37 (18.6) |
| Non-hematological TEAE | ||
| Nausea | 77 (38.7) | 20 (10.1) |
| Vomiting | 23 (11.6) | 9 (4.5) |
| Fatigue | 53 (26.6) | 13 (6.5) |
| Constipation | 37 (18.6) | 12 (6.0) |
| Elevated ALT | 31 (15.6) | 13 (6.5) |
| Elevated AST | 31 (15.6) | 11 (5.5) |
| Elevated GGT | 36 (18.1) | 10 (5.0) |
| Insomnia | 58 (29.1) | 16 (8.0) |
| Abdominal pain | 12 (6.0) | 7 (3.5) |
| Palpitations | 24 (12.1) | 8 (4.0) |
| Other | 7 (3.5) | 3 (1.5) |
ALT alanine aminotransferase, AST aspartate aminotransferase, GGT glutamyl transferase, TEAEs treatment-emergent adverse events
Exploratory Endpoints
PFS Analysis of Stratified Populations
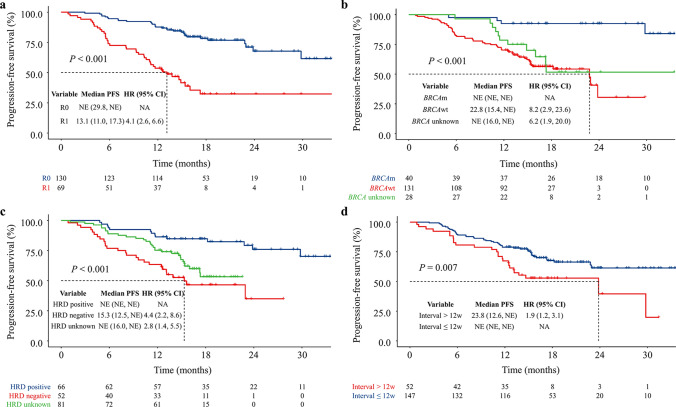
Analysis of PFS in various subgroups of the population revealed that the median PFS (95% CI) for the subgroups were significantly different for surgical treatment R0 versus R1, BRCAm versus BRCAwt, and HRD-negative versus HRD-positive, as well as the interval time ≤ 12 weeks versus > 12 weeks between completion of first-line chemotherapy and the initiation of niraparib MT, indicating that a starting niraparib MT time < 12 weeks would achieve higher benefits of PFS for patients (Fig. 3). In addition, the rates of PFS at 6, 12, 18, 24, and 30 months were 93.9%, 86.4%, 84.8%, 76.0%, and 70.1% for HRD-positive patients, and 97.5% for patients with BRCA mutations at 6 months, respectively. In contrast, patients with an HRD-negative status had a lower disease progression-free probability of 76.9%, 63.5%, 46.6%, and 34.9% at 6, 12, 18, and 24–30 months, respectively, which was also the case for BRCAwt cases with PFS rates of 82.4–30.5% at 6–30 months (Table 3). Analyses of TTD in subgroups of the population showed that patients with different postoperative status of cytoreductive surgery, those with different BRCA mutation status, as well as patients with different HRD status, exhibited different TTD periods (all P < 0.001) (Fig. 4).
Fig. 3.
PFS of stratified population by using Kaplan–Meier method and the log-rank test. a PFS of patients with different postoperative status after cytoreductive surgery. b PFS of patients with different BRCA mutational status. c PFS of patients with different HRD status. d PFS of patients with different interval times from the end of chemotherapy until niraparib MT. HRD homologous recombination deficiency, MT maintenance treatment, PFS progression-free survival
Table 3.
Probability of PFS at 6, 12, 18, 24, and 30 months
| Parameters | Total number of people (N = 199) |
BRCA mutation (N = 40) |
BRCA wild type (N = 131) |
BRCA unknown (N = 28) |
HRD positive (N = 66) |
HRD negative (N = 52) |
HRD unknown (N = 81) |
BRCA wild type and HRD positive (N = 25) |
|---|---|---|---|---|---|---|---|---|
| Events at 6 months, n | 25 | 1 | 23 | 1 | 4 | 12 | 9 | 3 |
| 6-month PFS rate, % (95% CI) | 87.4 (83.0, 92.2) | 97.5 (92.8, 100.0) | 82.4 (76.2, 89.2) | 96.4 (89.8, 100.0) | 93.9 (88.4, 99.9) | 76.9 (66.3, 89.3) | 88.9 (82.3, 96.0) | 88.0 (76.1, 100.0) |
| Events at 12 months, n | 23 | 2 | 16 | 5 | 5 | 7 | 11 | 3 |
| 12-month PFS rate, % (95% CI) | 75.9 (70.2, 82.1) | 92.5 (84.7, 100.0) | 70.2 (62.8, 78.5) | 78.6 (64.8, 95.3) | 86.4 (78.5, 95.1) | 63.5 (51.6, 78.0) | 75.3 (66.5, 85.3) | 76.0 (61.0, 94.7) |
| Events at 18 months, n | 19 | 0 | 14 | 5 | 1 | 7 | 11 | 1 |
| 18-month PFS rate, % (95% CI) | 63.6 (56.7, 71.2) | 92.5 (84.7, 100.0) | 56.5 (48.0, 66.4) | 51.7 (33.6, 79.4) | 84.8 (76.5, 93.9) | 46.6 (34.0, 63.8) | 53.2 (41.2, 68.8) | 71.5 (55.7, 91.9) |
| Events at 24 months, n | 4 | 0 | 4 | 0 | 3 | 1 | 0 | 3 |
| 24-month PFS rate, % (95% CI) | 56.1 (47.4, 66.5) | 92.5 (84.7, 100.0) | 30.5 (14.9, 62.5) | 51.7 (33.6, 79.4) | 76.0 (64.6, 89.3) | 34.9 (18.3, 66.7) | 53.2 (41.2, 68.8) | 31.3 (11.0, 89.0) |
| Events at 30 months, n | 11 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| 30-month PFS rate, % (95% CI) | 51.8 (41.1, 65.3) | 84.1 (68.4, 100.0) | 30.5 (14.9, 62.5) | 51.7 (33.6, 79.4) | 70.1 (56.0, 87.9) | 34.9 (18.3, 66.7) | 53.2 (41.2, 68.8) | 31.3 (11.0, 89.0) |
Patients enrolled initiated niraparib maintenance therapy from 1 January 2019 to 31 December 2021. The database lock-time was on 31 December 2022. A significant proportion of patients were still taking the drug on 31 December 2022
CI confidence interval, HRD homologous recombination deficiency, PFS progression-free survival
Fig. 4.

TTD of stratified populations by using Kaplan–Meier method and the log-rank test. a TTD of patients with different postoperative status after primary cytoreductive surgery, b TTD of patients with different BRCA mutational status, and c TTD of patients with different HRD mutation status. HRD homologous recombination deficiency, TTD time to treatment discontinuation
Predictive Models of Prognostic PFS Based on LASSO Logistic Regression
Univariate analysis showed that factors associated with a prolonged PFS at 18 months for 93 patients included age (P = 0.050), BRCA1/2 mutation (P < 0.001), HRD-positive status (P < 0.001), R0 status after initial tumor reduction (P < 0.001), and the interval of niraparib MT ≤ 12 weeks after chemotherapy (P = 0.014) (Fig. 5). However, we did not include the HRD status in the LASSO regression analysis due to the issue that it was unknown in almost 40% of the patients. Yet we included the variables of BMI, chemotherapy outcome, and interaction of surgery and chemotherapy outcome for analysis, as they are of relevance to the PFS.
Fig. 5.
Forest plots for different subgroups in 93 patients (PFS) by univariate analysis. CI confidence interval, HRD homologous recombination deficiency, OR odds ratio, PFS progression-free survival, yr years
The variation characteristics of coefficient of variables by LASSO regression analysis are shown in Fig. 6. Each coefficient was included once the condition was met (Fig. 6a), and the 10-fold cross-validation method was applied to the iterative analysis, while a model with excellent performance but minimum number of variables was obtained when λ was 0.023 (Fig. 6b). Finally, the screened variables included age (< 65 years versus ≥ 65 years), BRCA, BMI, interval (≤ 12 weeks versus > 12 weeks) between completion of first-line chemotherapy and the start of niraparib MT and R0/R1 (Fig. 6b).
Fig. 6.

Screening of variables based on LASSO regression. a The variation characteristics of the coefficient of variables. b The selection process of the optimum value of parameter l in the LASSO regression model using the cross-validation method
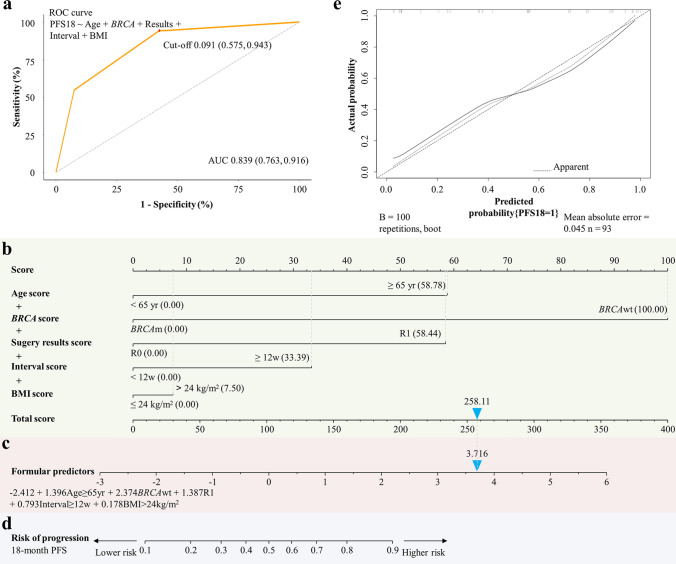
The 18-month PFS possibility formula was then created: − 2.412 + 1.396Age≥65yr + 2.374BRCAwt + 1.387R1 + 0.793Interval≥12w + 0.178BMI>24kg/m2. It was plotted as the AUC = 0.839 (0.763–0.916) with a cut-off value of 0.091, a sensitivity of 94.3% and an accuracy of 78.5% (Fig. 7a, c).
Fig. 7.
Predictive progressive probability of prognostic PFS for OC patients given niraparib. a ROC curve analysis. b Nomogram prediction model. Higher total points in b indicate a lower likelihood of patients reaching 18-month PFS when given niraparib (as shown in d). The correspondence between 0 and 1 in the predictor formula are designed as 0 was used to represent age < 65 years, BRCAm, R0, interval < 12 weeks, and BMI ≥ 24 kg/m2, whereas 1 was used to represent age ≥ 65 years, BRCAwt, R1, interval ≥ 12 weeks, and BMI > 24 kg/m2. c The value calculated from the formula higher than the cut-off value indicated lower possibility of patients reaching 18-month PFS when given niraparib. e Calibration curve with a 45° diagonal line in patients with a mean absolute error of 0.045. AUC area under the ROC curve, OC ovarian cancer, PFS progression-free survival, ROC receiver-operating characteristic
Nomogram as a Tool for Visualization of Prognostic 18-month PFS
Based on the LASSO regression analysis, a nomogram was developed as a prediction tool. Each predictor included in the nomogram is represented on one row, and a corresponding number of points assigned to different magnitudes of the predictor. The cumulative point axis is presented at the end of the nomogram (Fig. 7b). Higher total points indicate a lower likelihood of patients reaching 18-month PFS when given niraparib (Fig. 7b, d), while the value calculated from the formula higher than the cut-off value indicated lower possibility of patients reaching 18-month PFS when given niraparib (Fig. 7c). The calibration curve showed a good coincidence with a 45° diagonal line in the patients with a mean absolute error of 0.045 (Fig. 7e).
Incidence of TEAEs and PFS in Patients Treated with Different Niraparib Doses
Most patients received the ISD strategy as the initial dose of niraparib (300 mg, n = 2; 200 mg, n = 184; 100 mg, n = 13, which is lower than the standard dose). There were 26 patients who were adjusted to a long-term stable dose of 100 mg due to TEAEs, from a 200 mg starting dose. The 18-month PFS was 69.2% in patients adjusted from a 200 to 100 mg dose (26 patients), and greater than 49.2% in patients started on 100 mg (13 patients). The percentage of patients who experienced grade ≥ 3/4 TEAEs was not lower in patients started on 100 mg (69.2%) niraparib compared with patients on 200 mg (53.2%). These data suggested that the niraparib ISD strategy administration (200 mg) and dose adjustment (from 200 to 100 mg) were more reasonable (Table 4).
Table 4.
Relationship between the incidence of TEAEs and PFS in patients treated with different doses of niraparib
| Patients | Starting dose | Dose after 3 months | TEAEs, n (%) | Grade 3/4 TEAEs, n (%) | Number of events, n | PFS rate at 18 months, % | |
|---|---|---|---|---|---|---|---|
| Total | N = 199 | – | – | 180 (90.5) | 113 (56.8) | 67 | 63.6 |
| ISD | N = 186 | 300/200 mg | 300/200/100 mg | 167 (89.8) | 104 (55.9) | 61 | 64.7 |
| N = 2 | 300 mg | 300 mg | 2 (100.0) | 0 (0.0) | 1 | 50.0 | |
| N = 158 | 200 mg | 200 mg | 139 (88.0) | 84 (53.2) | 52 | 64.3 | |
| N = 26 | 200 mg | 100 mg | 26 (100.0) | 20 (76.9) | 8 | 69.2 | |
| Non-ISD | N = 13 | 100 mg | 100 mg | 13 (100.0) | 9 (69.2) | 6 | 49.2 |
PFS progression-free survival, TEAE treatment-emergent adverse events, ISD individualized starting dose
Discussion
Several Chinese phase 3 clinical trials have confirmed that niraparib MT for recurrent OC produced significantly prolonged PFS times compared with placebo [18, 20]. However, compared with randomized clinical trials, real-world studies are less restrictive and have broader enrollment criteria, with the patients more relevant to clinical practice. Until the end of 2021, niraparib was not covered by Chinese medical insurance. From 2021, niraparib as second-line MT was covered by Chinese medical insurance, and then first-line MT was covered by 2022. Therefore, niraparib has not been regularly administered until healthcare reimbursement, resulting in our multicenter data patient cohort being not as large as expected. At the time of the data cut-off, 131 patients remained free of disease progression, which explains why our data maturity was 36.2% (95% CI 23.4–36.5%) and the duration of follow-up differed considerably compared with other studies resulting in a not-reached median PFS (95% CI 23.8–NE). However, in the present study, niraparib as MT for OC patients within 12 weeks of completion of first-line chemotherapy regimens produced overall PFS rates of 87.4%, 75.9%, 63.6%, 56.1%, and 51.8% at 6, 12, 18, 24, and 30 months, respectively, after initiation of treatment for the entire cohort. A similar PFS rate at 18 months of 62% for niraparib plus bevacizumab MT has been published recently [26].
LASSO regression analysis revealed that the factors influencing prolonged PFS times included age, germline BRCA mutation status, and cytoreductive surgery outcomes. HRD-positive patients and BRCA mutation cases had superior PFS rates, respectively, compared with HRD-negative and BRCAwt patients, which confirms that BRCA status and HRD positivity are factors influencing the effectiveness of niraparib therapy in patients with advanced OC [27]. It is noteworthy that niraparib in contrast to other PARP inhibitors (e.g. olaparib) was approved in the EU and USA for first-line MT of OC, regardless of patient HRD status [23].
Also, the cytoreductive surgery outcome status has been described as being a major factor for PFS times when niraparib MT was used for advanced OC patients [28]. With regard to age, previous trials found that it was not a limiting factor for PFS times of OC patients receiving niraparib MT [29, 30], but a real-world study on olaparib MT for OC patients noted that the clinical benefit regarding median PFS times was different in older, compared with younger, patients. Selection in RCTs prefers older patients with a good performance status without other major health issues, but in real-life unselected frail older patients represent a significantly larger proportion than in the cohorts enrolled in randomized controlled trials [31].
The initial dose given to most patients in the present study was 200 mg/day, which produced the best clinical results. After 3 months from the initiation of treatment, some patients chose to discontinue or take a reduced dose due to TEAEs or personal choice. However, an average 200 mg dose led to somewhat higher PFS rates at 18 months with even lower rates of grade 3/4 TEAEs, indicating that a standard dose of 200 mg was efficient and safe, while switching to 100 mg might be necessary, mainly for cases with distinct TEAE susceptibilities, without essentially lessening the long term efficacy of treatment as reported in the PRIME study [20]. According to a meta-analysis of recent trials, patients taking niraparib were at a higher risk of developing nausea, fatigue, anemia, platelet count decreases, vomiting, neutrophil count decreases, headache, constipation, and insomnia of any grade, as well as at a higher risk for grade 3 or 4 fatigue [32], all findings that are in good agreement with those of the present study. The percentages of dose reduction (23.6%) and treatment discontinuation (5.5%) in our study were lower than the 70.9% and 12.0% previously observed [19], and 59.9% and 4% [18] reported in other studies, but similar to the 16.7% and 5.6% in the Chinese real-world study of Ni and colleagues that used only 200 mg doses [33]. This may be due to the low number of patients taking 300 mg in the present study.
Some limitations of the present study should be considered. First, its retrospective nonexperimental design may have increased selection bias. Second, the follow-up times were not long enough to establish long-term survival outcomes. Third, the absence of BRCA mutation and especially HRD status may have affected the interpretation of the findings to some extent and led to the exclusion of HRD status in determining a PFS correlation. Finally, due to the small sample size, we were not able to divide the dataset into the training dataset and validation dataset to test the robustness of the prediction model we have developed. As a result, the present predictive model requires a larger database and further confirmation in prospective research before its clinical application.
Conclusions
The significant effectiveness and manageable safety of niraparib in the registration trials have been confirmed in this real-world study despite the more complex treatment and status of the patients in clinal practice. For Chinese OC patients, it is important to use an ISD strategy of niraparib. The clinical predictive model established in this database needs more data to become more mature and unequivocally verified.
Declarations
Funding
This study was supported by the National Natural Science Foundation of China (82072078), the Primary Research and Development Plan of Jiangsu Province (SBE2020741118), the Scientific Research Project of Jiangsu Health Commission (ZDA2020012), the National Key Research and Development Program (2022YFC2403400), the Anhui Provincial Key Research and Development Program (2022e07020013), the 2020 USTC Affiliated Hospital Introduction Project to Medical Leading Technology (2020LXJS 05), and the Beijing Science and Technology Innovation Fund (KC2021-JX-0186-143). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
Jun Chen and Jingcheng Xu are employees as well as shareholders of Zai Lab (Shanghai) Co., Ltd, Shanghai, China. The remaining authors declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics Approval
The study was performed following the guiding principles of the Declaration of Helsinki and the International Conference on Harmonization of Good Clinical Practice and appropriate legal guidelines for non-interventional and observational studies and approved by the Ethics Committee of Zhongda Hospital, School of Medicine, Southeast University on 9 September 2022 (no.: 2022040092). The study was registered with ClinicalTrials.gov (Registration number: NCT05734911).
Consent to Participate
As the study was retrospective and data collection anonymized, the informed consent of patients was not required.
Consent for Publication
Not applicable.
Data Availability
The data generated in this study are available upon reasonable request from the corresponding author.
Code Availability
Not applicable.
Author Contributions
Minmin Z: formal analysis, investigation, writing—original draft, writing—review and editing, visualization. SQ: formal analysis, writing—original draft, writing—review and editing, visualization. XW, PM, ZJ, TZ, XX, Yanling Z, BZ, DY, Yang Z, WS, AH, Min Z, WH, Yingli Z, ZS, MJ and ML: investigation, writing—review and editing, visualization. JC and JX: formal analysis, writing—review and editing, visualization. BC: conceptualization, formal analysis, resources, writing—review and editing, visualization, supervision, project administration. Ying Z: conceptualization, investigation, resources, writing—original draft, writing—review and editing, visualization, supervision, project administration, funding acquisition. YS: conceptualization, formal analysis, resources, writing—original draft, writing—review and editing, visualization, supervision, project administration, funding acquisition. All authors approved the final submitted version of the manuscript.
Footnotes
Minmin Zhao and Shanhu Qiu contributed equally to the study and should be regarded as co-first authors.
Contributor Information
Bingwei Chen, Email: drchenbw@126.com.
Ying Zhou, Email: caddiezy@ustc.edu.cn.
Yang Shen, Email: shenyang@seu.edu.cn.
References
- 1.Wang Z, Guo E, Yang B, Xiao R, Lu F, You L, et al. Trends and age-period-cohort effects on mortality of the three major gynecologic cancers in China from 1990 to 2019: cervical, ovarian and uterine cancer. Gynecol Oncol. 2021;163(2):358–363. doi: 10.1016/j.ygyno.2021.08.029. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Wang Z, Zhang Z, Wang H, Peng J, Hong L. Burden of ovarian cancer in China from 1990 to 2030: a systematic analysis and comparison with the global level. Front Public Health. 2023;11:1136596. doi: 10.3389/fpubh.2023.1136596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanker LC, Loibl S, Burchardi N, Pfisterer J, Meier W, Pujade-Lauraine E, et al. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann Oncol. 2012;23(10):2605–2612. doi: 10.1093/annonc/mds203. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Y, Zhao J, Zhang L, Tian S, Yang T, Wang L, et al. Evaluation of the efficacy and safety of PARP inhibitors in advanced-stage epithelial ovarian cancer. Front Oncol. 2020;10:954. doi: 10.3389/fonc.2020.00954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mirza MR, Coleman RL, González-Martín A, Moore KN, Colombo N, Ray-Coquard I, et al. The forefront of ovarian cancer therapy: update on PARP inhibitors. Ann Oncol. 2020;31(9):1148–1159. doi: 10.1016/j.annonc.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Zheng H, Gao Y, Guo H, Li L, Li Q, Cui H, et al. Real-world experience of olaparib treatment in patients with ovarian cancer: a Chinese multicenter study. Mol Cancer Ther. 2021;20(9):1735–1742. doi: 10.1158/1535-7163.MCT-20-1064. [DOI] [PubMed] [Google Scholar]
- 8.Moore K, Colombo N, Scambia G, Kim B-G, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 9.DiSilvestro P, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Efficacy of maintenance olaparib for patients with newly diagnosed advanced ovarian cancer with a BRCA mutation: subgroup analysis findings from the solo1 trial. J Clin Oncol. 2020;38(30):3528–3537. doi: 10.1200/JCO.20.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harter P, Mouret-Reynier MA, Pignata S, Cropet C, González-Martín A, Bogner G, et al. Efficacy of maintenance olaparib plus bevacizumab according to clinical risk in patients with newly diagnosed, advanced ovarian cancer in the phase III PAOLA-1/ENGOT-ov25 trial. Gynecol Oncol. 2022;164(2):254–264. doi: 10.1016/j.ygyno.2021.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Eriksson I, Wettermark B, Bergfeldt K. Real-world use and outcomes of olaparib: a population-based cohort study. Target Oncol. 2018;13(6):725–733. doi: 10.1007/s11523-018-0604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paik J. Olaparib: a review as first-line maintenance therapy in advanced ovarian cancer. Target Oncol. 2021;16(6):847–856. doi: 10.1007/s11523-021-00842-1. [DOI] [PubMed] [Google Scholar]
- 13.Jones P, Altamura S, Boueres J, Ferrigno F, Fonsi M, Giomini C, et al. Discovery of 2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide (MK-4827): a novel oral poly(ADP-ribose)polymerase (PARP) inhibitor efficacious in BRCA-1 and -2 mutant tumors. J Med Chem. 2009;52(22):7170–7185. doi: 10.1021/jm901188v. [DOI] [PubMed] [Google Scholar]
- 14.European Medicines Agency. European Medicines Agency decision EMA/CHMP/574018/2017; 2017.
- 15.Food and Drug Administration. FDA approves maintenance treatment for recurrent epithelial ovarian, fallopian tube or primary peritoneal cancers; 2017.
- 16.National Medical Products Administration. Niraparib has been approved by NMPA with conditions; 2019.
- 17.Monk BJ, González-Martin A, Buckley L, Matulonis UA, Rimel BJ, Wu X, et al. Safety and management of niraparib monotherapy in ovarian cancer clinical trials. Int J Gynecol Cancer. 2023;33:971–981. doi: 10.1136/ijgc-2022-004079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu XH, Zhu JQ, Yin RT, Yang JX, Liu JH, Wang J, et al. Niraparib maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer using an individualized starting dose (NORA): a randomized, double-blind, placebo-controlled phase III trial. Ann Oncol. 2021;32(4):512–521. doi: 10.1016/j.annonc.2020.12.018. [DOI] [PubMed] [Google Scholar]
- 19.González-Martín A, Pothuri B, Vergote I, DePont CR, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381(25):2391–2402. doi: 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- 20.Li N, Zhu J, Yin R, Wang J, Pan L, Kong B, et al. Efficacy and safety of niraparib as maintenance treatment in patients with newly diagnosed advanced ovarian cancer using an individualized starting dose (PRIME Study): a randomized, double-blind, placebo-controlled, phase 3 trial (LBA 5) Gynecol Oncol. 2022;166:S50–S51. doi: 10.1016/S0090-8258(22)01298-7. [DOI] [Google Scholar]
- 21.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 22.Moore KN, Secord AA, Geller MA, Miller DS, Cloven N, Fleming GF, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20(5):636–648. doi: 10.1016/S1470-2045(19)30029-4. [DOI] [PubMed] [Google Scholar]
- 23.Lee A. Niraparib: a review in first-line maintenance therapy in advanced ovarian cancer. Target Oncol. 2021;16(6):839–845. doi: 10.1007/s11523-021-00841-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caleyachetty R, Barber TM, Mohammed NI, Cappuccio FP, Hardy R, Mathur R, et al. Ethnicity-specific BMI cutoffs for obesity based on type 2 diabetes risk in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2021;9(7):419–426. doi: 10.1016/S2213-8587(21)00088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Wu Y, Liu P, Zhang X. Developing a validated nomogram for predicting ovarian metastasis in endometrial cancer patients: a retrospective research. Arch Gynecol Obstet. 2022;305(3):719–729. doi: 10.1007/s00404-021-06214-4. [DOI] [PubMed] [Google Scholar]
- 26.Hardesty MM, Krivak TC, Wright GS, Hamilton E, Fleming EL, Belotte J, et al. OVARIO phase II trial of combination niraparib plus bevacizumab maintenance therapy in advanced ovarian cancer following first-line platinum-based chemotherapy with bevacizumab. Gynecol Oncol. 2022;166(2):219–229. doi: 10.1016/j.ygyno.2022.05.020. [DOI] [PubMed] [Google Scholar]
- 27.Akay M, Funingana I-G, Patel G, Mustapha R, Gjafa E, Ng T, et al. An in-depth review of niraparib in ovarian cancer: mechanism of action, clinical efficacy and future directions. Oncol Ther. 2021;9(2):347–364. doi: 10.1007/s40487-021-00167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Cearbhaill RE, Pérez-Fidalgo JA, Monk BJ, Tusquets I, McCormick C, Fuentes J, et al. Efficacy of niraparib by time of surgery and postoperative residual disease status: a post hoc analysis of patients in the PRIMA/ENGOT-OV26/GOG-3012 study. Gynecol Oncol. 2022;166(1):36–43. doi: 10.1016/j.ygyno.2022.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valabrega G, Pothuri B, Oaknin A, Graybill W, Sánchez AB, McCormick C, et al. 819P Efficacy and safety of niraparib in older patients (pts) with advanced ovarian cancer (OC): results from the PRIMA/ENGOT-OV26/GOG-3012 trial. Ann Oncol. 2020;31:S619. doi: 10.1016/j.annonc.2020.08.958. [DOI] [Google Scholar]
- 30.Fabbro M, Moore KN, Dørum A, Tinker AV, Mahner S, Bover I, et al. Efficacy and safety of niraparib as maintenance treatment in older patients (≥ 70 years) with recurrent ovarian cancer: results from the ENGOT-OV16/NOVA trial. Gynecol Oncol. 2019;152(3):560–567. doi: 10.1016/j.ygyno.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Liposits G, Wulff CN, Otland A, Fokdal LU. Olaparib treatment in older patients with ovarian cancer: need for ‘real-world’ data beyond clinical trials. Ecancermedicalscience. 2020;14:1104. doi: 10.3332/ecancer.2020.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pagkali A, Mamais I, Michalinos A, Agouridis AP. Safety profile of niraparib as maintenance therapy for ovarian cancer: a systematic review and meta-analysis. Curr Oncol. 2022;29(1):321–336. doi: 10.3390/curroncol29010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nie J, Wu H, Sun L, Ding Y, Luan Y, Wu J. Cost-effectiveness of fuzuloparib compared to routine surveillance, niraparib and olaparib for maintenance treatment of patients with germline BRCA1/2 mutation and platinum-sensitive recurrent ovarian carcinoma in China. Front Pharmacol. 2022;13:987337. doi: 10.3389/fphar.2022.987337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in this study are available upon reasonable request from the corresponding author.