Abstract
Background
Delta-like ligand 3 (DLL3), a member of the Notch pathway, has been identified as a potential therapeutic target as it is highly expressed in small cell lung cancer (SCLC), a subtype accounting for 15% of lung cancer cases.
Objective
A systematic literature review (SLR) was conducted to understand the prevalence and prognostic impact of DLL3 expression on survival of patients with SCLC and treatment response.
Patients and Methods
Systematic literature searches were conducted across multiple databases to capture studies of any SCLC population that evaluated DLL3 expression. Specific outcomes of interest included prevalence of DLL3 expression, method of expression analysis, and impact on outcome, including treatment response and survival (overall, progression-free, disease-free) according to varying levels of DLL3 expression/positivity. Standard risk of bias tools were used to evaluate study quality.
Results
Among the 30 included studies, the most common DLL3 testing method was immunohistochemistry (N = 26, 86.7%). For comparability, results focused on the 13 (22.3%) studies that used the Ventana DLL3 (SP347) immunohistochemistry assay. The prevalence of DLL3 positivity ranged from 80.0–93.5% for studies using a threshold of ≥ 1% of tumor cells (N = 4) and 58.3–91.1% for studies with a ≥ 25% threshold (N = 4). DLL3 expression was generally categorized as high using cutoffs of ≥ 50% (prevalence range: 45.8–79.5%; N = 6) or ≥ 75% (prevalence range: 47.3–75.6%; N = 5) of cells with positivity. Two studies used an H-score of ≥ 150 to define high DLL3 expression with prevalence ranging from 33.3–53.1%. No consistent associations were seen between DLL3 expression level and patient age, sex, smoking history, or disease stage. Two studies reported change in DLL3 expression category (high versus low) before and after chemotherapy. No statistically significant differences were reported between DLL3 expression groups and survival (overall, progression-free, or disease-free) or treatment response.
Conclusions
There is a high prevalence of DLL3 expression in SCLC. Further research and analytical methods may help to characterize different populations of patients with SCLC based on DLL3 expression. While no significant prognostic factor in the included studies was identified, additional cohort studies using standardized methodology, with longer follow-up, are needed to better characterize any potential differences in patient survival or response by DLL3 expression level in SCLC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11523-023-01008-x.
Key Points
| Despite the variability in definitions of DLL3 positivity and the tumor heterogeneity among the 30 identified studies, most patients with SCLC were found to be DLL3-positive. |
| This SLR demonstrates that DLL3 is expressed in most patients with SCLC and presents a potentially impactful therapeutic target. |
| Future investigations should explore the impact of DLL3 expression using large populations with validated tests for DLL3 expression to better understand the dynamics of DLL3 as a therapeutic target for SCLC. |
Introduction
Small cell lung cancer (SCLC) comprises approximately 15% of all lung cancers. The incidence rate of SCLC in the USA increased from 1975 (6.4 per 100,000) to 1990 (10.7 per 100,000 in 1988), but steadily decreased from 1990 to 2019 (4.5 per 100,000) [1]. This deadly disease is characterized by a high rate of metastasis, with 70% of patients having extensive-stage metastatic disease at diagnosis [2].
Despite expanded treatment options in the past few years, the 5-year survival rate of patients with SCLC is only 10% [3]. Molecular aberrations are common in SCLC, most frequently in TP53 (78.7–98.0%) and retinoblastoma protein 1 (44.7–91.0%), but also in PIK3CA, PTEN, MEK1, AKT, FGFR, and C-MET. First-line treatment options are determined by stage at diagnosis. Patients with limited-stage disease (tumor confined to one hemi-thorax and one radiation port with no malignant pleural or pericardial effusion) are treated with curative-intent etoposide and platinum agent, concurrently with radiation. The small proportion with resectable node-negative disease may undergo surgical resection. For the majority of patients that present with extensive-stage disease (disease not meeting criteria for limited stage), recommended treatment includes etoposide, a platinum agent with immunotherapy (atezolizumab/durvalumab). Despite overall response rates of 40–70%, the duration of response is short and most patients relapse, resulting in a median survival of only 7–12 months. Second-line treatment options are sparse. Single agent topotecan is widely approved; however, it is associated with significant toxicity, making it an unpopular choice. Lurbinectedin offers a second option after receiving accelerated US approval for this indication, with significant uptake in the USA and recently in other countries as well. Several other drugs such as irinotecan, paclitaxel, or combination regimens such as cyclophosphamide, adriamycin, and vincristine (CAV; all unapproved in this setting), as well as a platinum-based rechallenge are used. This reflects the limited efficacy and high toxicity seen when treating the second line patients with SCLC population reflecting a broad dissatisfaction with current options [3, 4].
Delta-like ligand 3 (DLL3) is an inhibitory Notch pathway ligand that has been implicated in the tumorigenesis of neuroendocrine tumors such as SCLC. DLL3 is highly upregulated in SCLC and aberrantly expressed on the surface of SCLC cells [5, 6]. As DLL3 has been found to be overexpressed in approximately 85% of SCLC tumors but only minimally in normal tissues, it may be a potential therapeutic target for SCLC [6, 7]. DLL3 expression can be measured using the proportion of cells with tumor positivity or a semi-quantitative H-score method [8]. One recent study of a large international cohort of patients with SCLC (N = 1073 patients from 19 countries) reported that 85% of patients had positive DLL3 expression (≥ 25% of tumor cells) and 68% had high levels of expression (≥ 75% of tumor cells) [6]. However, the prevalence of DLL3 expression in patients with SCLC has not yet been systematically examined.
The prognostic impact of DLL3 positivity and expression level on survival of patients with SCLC was described in a meta-analysis comprising six studies [7]. The authors reported that high DLL3 expression was a significant prognostic factor for overall survival (OS) among five studies conducted in Asian populations [summary hazard ratio (HR) = 1.37; 95% confidence interval (CI): 1.05, 1.69]; however, the results were no longer significant when the sixth study (conducted in the USA) was added to the analysis (summary HR = 1.13; 95% CI 0.61, 1.65). This meta-analysis did not account for heterogeneity in the prognostic impact of DLL3 by demographic or clinical factors such as tumor stage, DLL3 testing method, percent (%) of tumor cell positivity, and/or staining intensity and/or H-score (i.e., the percentage of positive cells by staining intensity), or smoking status. In addition, this meta-analysis was conducted in early 2020, and additional relevant studies have been published since that time [6, 9, 10]. Therefore, an updated and expanded systematic literature review (SLR) on the prevalence and prognostic impact of DLL3 expression on patient survival and treatment response in SCLC is warranted. The primary objectives of this SLR were to review the published scientific literature reporting the prevalence of DLL3 expression in patients with SCLC as well as the prognostic impact of DLL3 expression on patient survival and treatment response. Secondary objectives included evaluating DLL3 expression, prevalence, and prognostic impact by demographic and clinical factors such as age, sex, race/ethnicity, smoking status, DLL3 testing method, and percentage of tumor cell positivity and/or staining intensity and/or H-score.
Methods
This SLR was conducted and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11]. The protocol was registered a priori in the International Prospective Register of Systematic Reviews (PROSPERO identifier: CRD42022351119).
Study Eligibility
The eligibility criteria were organized using the Population, Exposure, Comparator, Outcome, and Study Type (PECOS) format. Studies were required to include populations of patients with SCLC, with no geographic restrictions. The evaluated exposure was DLL3 protein expression. Comparators were not required for inclusion of studies evaluating DLL3 prevalence, as descriptive studies were eligible; prognostic studies were required to evaluate different levels of DLL3 expression/positivity. Outcomes included prevalence of DLL3 expression in SCLC or comparison of SCLC treatment response (complete response, partial response, overall response, disease control rate, or progressive disease) or survival (overall or progression-free survival) according to varying levels of DLL3 expression/positivity. Eligible study types included clinical trials, observational studies (prospective or retrospective cohort studies), and case series of ≥ 20 patients published in the English language in either peer-reviewed journals or as conference abstracts. Articles published up to the date the search strategy was executed with no lower bound on time period were eligible for inclusion.
Study Identification and Screening
Comprehensive literature searches were conducted in the PubMed, EMBASE, Web of Science, and Cochrane Library databases, as well as on ClinicalTrials.gov, on 3 August 2022 (described in Additional file 1). Articles identified in the searches were uploaded into a standardized software for conducting literature reviews (DistillerSR) [12] and deduplicated using both automated and hand-screening methods. Articles were first screened at the level of title and abstract by a single reviewer according to the PECOS criteria, with 10% of the articles screened by a second reviewer as a quality control (QC) measure. After exclusions were made at the title and abstract level, full-text articles were obtained and assessed for eligibility by two independent reviewers to determine agreement on all included articles. All disputes were resolved by a senior researcher. Bibliographies of the eligible literature (as well as key reviews) were also screened to identify additional references. If more than one article from the same study population was identified, data from the publication with the longest follow-up or most relevant population and/or outcomes were abstracted. For studies with overlapping data, data from the publication with a larger population size or more relevant population and/or outcomes were abstracted.
Data Abstraction
Data abstraction was conducted in DistillerSR on all full-text articles and conference abstracts meeting the PECOS eligibility criteria. Abstracted data elements included study characteristics, patient demographics, disease and treatment characteristics, DLL3 testing method, number of patients by DLL3 expression level, and outcomes related to response or survival by DLL3 expression level, along with any associated comparative effect measures and adjustment factors. Abstraction was performed by a single reviewer and all data elements underwent 100% QC by an independent reviewer. Discrepancies were resolved by a senior reviewer.
Risk of Bias Evaluation
Evaluation of risk of bias (RoB) in clinical trials was conducted using the Cochrane Collaboration’s ‘Risk of Bias’ tool [13]. Clinical trials were evaluated for selection, performance, detection, attrition, and reporting bias and scored as “low risk,” “some concerns,” or “high risk” of bias for each domain. Observational studies were assessed for risk of bias (RoB) using the Newcastle–Ottawa Scale (NOS) [14]. Observational studies were evaluated for selection, comparability, and outcome bias. Scores were transformed to measures of study quality (“good,” “fair,” or “poor”) using the Agency for Healthcare Research and Quality (AHRQ) standards (Additional file 2). The NOS for cohort studies was modified to evaluate the cross-sectional studies of DLL3 prevalence in SCLC by eliminating questions 2, 4, and 7, and the conversion to AHRQ standards is described in Additional file 2. RoB evaluations were conducted by a single reviewer and evaluated by an independent reviewer for QC.
Results
Study Identification
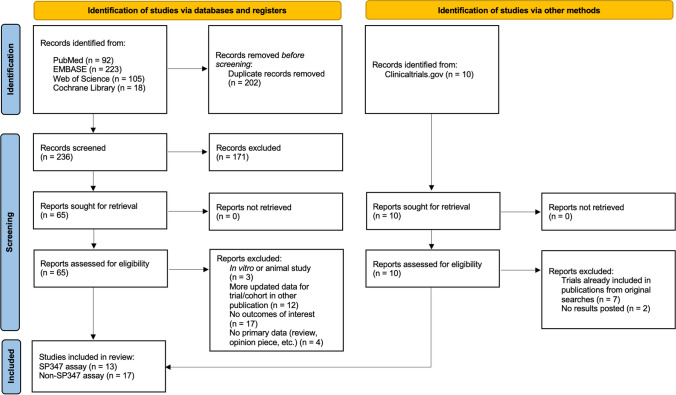
A PRISMA diagram for the identification of studies is shown in Fig. 1. After deduplication across databases, 236 studies were screened at the level of title and abstract. A total of 75 studies were evaluated at the full-text level, after the addition of 10 trials resulting from the search performed on ClinicalTrials.gov. A total of 45 studies were excluded at the full-text level; 17 did not include any outcomes of interest, 12 were linked to another publication with more updated data, 7 clinical trials were captured in the database searches, 4 had no primary data, 3 were in vitro or animal studies, and 2 clinical trials had no results available. Thus, a total of 30 studies were included for abstraction. A total of 13 [6, 10, 15–25] of these 30 studies utilized SP347 assays, which have been both validated and subsequently used in the clinical setting in multiple populations [24], to evaluate DLL3 expression and are the focus of this paper. Details about the remaining 17 papers are provided in Additional files 1–7 for purposes of comparability. Additional file 8 contains the supplementary text that corresponds with Additional files 1–7.
Fig. 1.
PRISMA study flow diagram
Study Characteristics
The characteristics of the 30 included studies are presented in Additional file 3. Of the 13 studies using SP347 assays, there were 5 retrospective cohorts [6, 10, 15, 17, 23], 4 cross-sectional studies [16, 21, 24, 25], 2 single-arm trials [20, 22], 1 randomized trial [18], and 1 prospective cohort study [19]. Most (N = 8) studies had less than 100 participants. Study locations were varied; two studies were conducted in the USA [23, 24], two were conducted in Germany [10, 16], two were conducted in Japan [17, 22], and three were conducted in multiple countries [6, 18, 20]. The remaining studies were conducted in Italy [15], Greece [19], Canada [21], and Sweden [25].
Risk of Bias
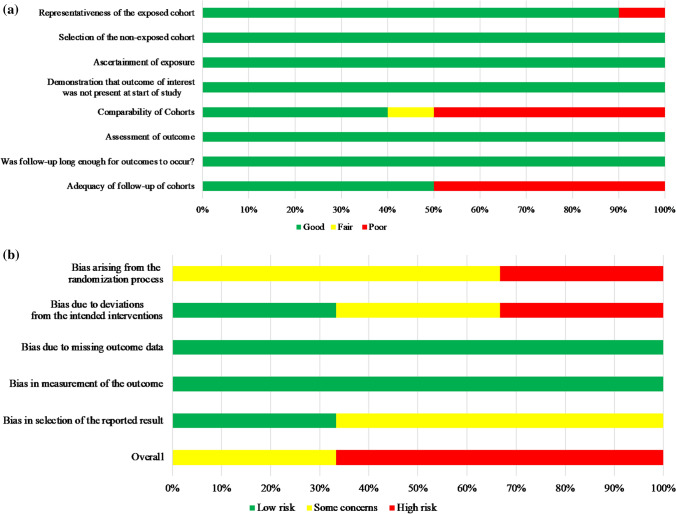
The NOS scores of the ten observational studies that used SP347 assays ranged from 3 to 9 with a mean of 6.1 and median of 6. When converted to the AHRQ standards, five studies were scored as good quality [6, 15, 19, 23, 25], three studies were scored as fair quality [16, 21, 24], and two studies were scored as poor quality [10, 17]. When evaluating bias by domain, risk was most apparent in the comparability of cohorts and adequacy of follow-up (Fig. 2A). In the Cochrane RoB scores of the three clinical trials that used SP347 assays, two studies [18, 22] were scored as “high risk” and one study [20] had “some concerns.” Risk was most apparent in the randomization process (Fig. 2B). Details about the remaining 17 papers are provided in Additional file 7.
Fig. 2.
Summary of risk of bias scores in A observational studies and B clinical trials. A Risk of bias in observational studies (Newcastle–Ottawa Scale) using SP347 assay (N = 10). B Risk of bias in clinical trials (Cochrane Risk of Bias Tool) using SP347 assay (N = 3)
Patient and Treatment Characteristics
Patient and treatment characteristics varied across the studies using the SP347 assay (Additional file 3). The distribution of sex was reported in ten studies, with males ranging from 43% [25] to 84.3% [19]. One study reported race/ethnicity, with 82% of the study population being white [18]. Three studies reported smoking status [6, 15, 17], of which most patients were current/former smokers (range: 85.2% [17] to 100% [15]). Eastern Cooperative Oncology Group (ECOG) performance status was reported in six studies, with the majority of patients having an ECOG status of 1 or 0 in most studies [6, 18, 20, 22, 25]. Six studies reported tumor stage at diagnosis as limited or extensive [6, 15, 19] and/or by tumor, node, metastasis (TNM) stage [15, 17, 18, 20]. Patients with extensive disease at diagnosis ranged from 0% in an Italian cohort of 32 patients with SCLC from 2007–2019 [15] to 63% in a large (N = 1073) multicountry retrospective cohort (2008–2017) [6]; patients with TNM stage III–IV at diagnosis ranged from 11.6% in a retrospective Japanese cohort of 95 patients with SCLC (2003–2013) [17] to 84% in the phase III MERU trial (2017–2019) [18].
DLL3 positivity was determined by the percentage of tumor cell positivity in 13 studies [6, 10, 15–25] and/or H-score in two studies [10, 15]. The threshold for tumor cell positivity ranged from 1–25% and was often classified as “high” or “low” with cut-offs at 50% or 75% of positive tumor cells. The H-score threshold for “high” DLL3 expression was consistent in both studies at 150. All 13 studies reported DLL3 prevalence as an outcome. Other endpoints assessed in the studies included stratification of DLL3 expression by demographic or clinical factors (N = 5), OS (N = 8), progression-free survival (PFS) or disease-free survival (DFS) (N = 5), and treatment response (N = 3).
Prevalence of DLL3 Expression in SCLC
Positive Versus Negative DLL3 Expression
All 13 studies using the SP347 assay evaluated the prevalence of DLL3 expression (Table 1). Additional file 4 describes the prevalence of DLL3 expression (N = 15) among non-SP347 assay studies. DLL3 expression was categorized as positive or negative in nine studies using the SP347 assay [6, 10, 16, 17, 20–22, 24, 25] and ten non-SP347 studies [26–35] (Additional file 4). One cross-sectional US study reported 76.4% of patients with SCLC were DLL3-positive, defined as reactivity > 0% [24]. Among four studies using a threshold of ≥ 1% to define DLL3 positivity [10, 17, 21, 25], patients that were DLL3-positive ranged from 80.0% among chemo-relapsed German patients (time period not specified) [10] to 93.5% in patients who had completed at least one cycle of chemotherapy between 2008 and 2015 in Sweden [25]. Of the four studies that used a 25% threshold [6, 16, 20, 22], the prevalence of DLL3 positivity ranged from 58.3% in a cohort of German chemo-naïve surgically resected samples from 1996–2012 [16] to 91.1% in an international clinical trial of 339 adult patients with advanced stage SCLC from 2016 to 2017 [20].
Table 1.
Prevalence of DLL3 expression in the tissue of patients with SCLC using SP347 assay (N = 13)
| Author, year | study design (cohort name or NCT number, if applicable); population inclusion details | Geographic location (year) | N patients | DLL3 positivity threshold (proportion of positive cells), N (%) | DLL3 expression (definition), N (%) | H-score threshold, N (%) | ||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | High | Low | |||||
| Ali [15], 2021 | Retrospective cohort; chemo- and radiation-naïve surgical specimens | Italy (2007–2019) | 32 | NR | NR | ≥ 50%: 24 (75) | < 50%: 8 (25) |
High (≥ 150): 15 (53.1) Low (< 150): 17 (46.9) |
| Brcic [16], 2019 | Cross-sectional; chemo-naïve surgical specimens | Germany (1996–2012) | 24 | ≥ 25%: 14 (58.3) | < 25%: 10 (41.7) |
≥ 75%: 5 (20.8) ≥ 50%: 11 (45.8) |
< 50%: 13 (54.2) | NR |
| Furuta [17], 2019 | Retrospective cohort; surgical resections of primary tumors | Japan (2003–2013) | 93a | ≥ 1%: 77 (82.8) | 0%: 16 (17.2) | ≥ 75%: 44 (47.3) | < 75%: 49 (52.7) | NR |
| Huang [24], 2019b | Cross-sectional; primary and metastatic SCLC samples | USA (NR) | 1362 | Reactivity (> 0%): 1040 (76.4) | Non-reactive (0%): 322 (23.6) | NR | NR | NR |
| Johnson [18], 2021 | Randomized controlled trial (MERU; NCT03033511); patients with ES–SCLC that did not progress after 1L platinum-based chemotherapy | Multicountry (2017–2019) | Intervention: 365b | NR | NR | ≥ 75%: 217 (59.5) | < 75%: 148 (40.5) | NR |
| Placebo: 360b | NR | NR | ≥ 75%: 240 (66.7) | < 75%: 120 (33.3) | NR | |||
| Kuempers [10], 2021 | Retrospective cohort; paired chemo-naïve and recurrent SCLC post-chemotherapy | Germany (NR) | Chemo-naïve: 30c | ≥ 1%: 26 (86.7) | 0%: 4 (13.3) | ≥ 50%: 16 (53.3) | 1–49%: 10 (33.3) |
High (≥ 150): 10 (33.3%) Low (< 150): 16 (53.3%) Negative (0): 4 (13.3%) |
| Chemo-relapsed: 30c | ≥ 1%: 24 (80.0) | 0%: 6 (20.0) | ≥ 50%: 19 (63.3) | 1–49%: 5 (16.7) |
High (≥ 150): 16 (53.3) Low (< 150): 8 (26.7) Negative (0): 6 (20.0) |
|||
| Messaritakis, 2019[19] | Prospective cohort; SCLC samples from pre-chemotherapy, after a single cycle, or at progression |
Greece (NR) |
20d | NR | NR | ≥ 50%: 14 (70.0) | < 50%: 6 (30.0) | NR |
| Morgensztern [20], 2019 | Single-arm clinical trial (TRINITY; NCT02674568); relapsed/ refractory SCLC | Multicountry (2016–2017) | 315e | ≥ 25%: 287 (91.1) | < 25%: 28 (8.9) | ≥ 75%: 238 (75.6) | < 75%: 77 (24.4) | NR |
| Odashiro [21], 2020 | Cross-sectional; SCLC samples | Canada (NR) | 39 | ≥ 1%: 36 (92.3) | < 1%: 3 (7.7) |
> 75%: 25 (64.1) 50–74%: 2 (5.1) |
1–49%: 9 (23.1) | NR |
| Rojo [6], 2020 | Retrospective cohort; SCLC samples | Multi-country (2008–2017) | 1050f | ≥ 25%: 895 (85.2) | 0–24%: 155 (14.8) | High positive (≥ 75%): 719 (68.5) | Non-high positive (25–74%): 176 (16.8) | NR |
| Tendler [25], 2020 | Cross-sectional; patients with SCLC completing ≥ 1 cycle of platinum-doublet chemotherapy | Sweden (2008–2015) | 46 | ≥ 1%: 43 (93.5) | 0%: 3 (6.5) |
Undefined: 38 (82.6) > 75%: 19 (41.3) 51–75%: 9 (19.6) 26-50%: 9 (19.6) |
Undefined: 8 (17.4) 1–25%: 6 (13.0) | NR |
| Udagawa [22], 2019 | Single-arm clinical trial (NCT03086239); advanced recurrent SCLC that progressed after ≥ 2 chemotherapy regimens | Japan (2017–2018) | 28g | ≥ 25%: 24 (82.3) | < 25%: 4 (13.8) | ≥ 75%: 18 (64.3) | < 75%: 10 (35.7) | NR |
| Xie [23], 2019 | Retrospective cohort; SCLC resection samples | USA (1995–2017) | 44 | NR | NR | ≥ 50%: 35 (79.5) | < 50%: 9 (20.5) | NR |
1L first-line, ES-SCLC extensive-stage small cell lung cancer, NR not reported
aFurata et al. 2019 included 95 patients, but DLL3 expression was evaluated in 93 patients
bJohnson et al. 2021 included 748 patients, but DLL3 expression was evaluated in 725 patients
cKuempers et al. 2021 included 42 patients, but DLL3 expression was evaluated in a subcohort of 30-paired chemo-naive and chemo-relapsed patients
dMessaritakis et al. 2019 included 108 patients, but DLL3 expression was evaluated in 20 patients
eMorgensztern et al. 2019 included 339 patients, but DLL3 expression was evaluated in 315 patients
fRojo et al. 2020 included 1073 patients, but DLL3 expression was evaluated in 1050 patients
gUdagawa et al. 2019 included 29 patients, but DLL3 expression was evaluated in 28 patients
High Versus Low DLL3 Expression
DLL3 expression was broadly categorized as “high” or “low” in 12 SP347 studies [6, 10, 15–23, 25] and 11 non-SP347 studies [27, 29–38] (Additional file 4). High expression was defined as positivity in ≥ 50% of tumor cells in six studies [10, 15, 16, 19, 21, 23] and ranged from 45.8% of patients in a cohort of German chemo-naïve surgically resected samples from 1996–2012 [16] to 79.5% of patients in a retrospective cohort of 44 resected patients with SCLC at the Mayo Clinic Rochester from 1995 to 2017 [23]. A threshold of 75% was reported in eight studies [6, 16–18, 20–22, 25]; the prevalence of high DLL3 expression ranged from 20.8% in a cohort of German chemo-naïve surgically resected samples from 1996–2012 [16] to 75.6% in an international clinical trial of 339 adult patients with advanced stage SCLC from 2016–2017 [20]. In three of these studies, the 75% cut-off was described without being used as a threshold to define “high” DLL3 expression [16, 21, 25]. A cohort of patients from the Swedish Lung Cancer Registry who had completed at least one cycle of chemotherapy between 2008 and 2015 reported 82.6% of patients with “high” DLL3 expression without definition of the threshold [25].
H-Score Categorization of DLL3 Expression
H-score was used to categorize DLL3 expression as “high” or “low” in two SP347 studies (and one non-SP347 study [39]) using a threshold score of 150 [10, 15]. Prevalence of patients with high H-score (≥ 150) ranged from 33.3% in a study of chemo-naïve German patients (time period not specified) [10] to 53.1% in an Italian cohort of resected patients with SCLC from 2007 to 2019 [15].
Factors Significantly Associated with DLL3 Expression
Among studies using the SP347 assay, five studies investigated demographic and clinical factors in relation to DLL3 expression [6, 10, 17, 23, 25]; Additional file 5 reports DLL3 expression by demographic and clinical factors (N = 15) among non-SP347 assay studies. Table 2 shows DLL3 expression by age, sex, smoking history, and disease stage. No studies stratified DLL3 expression by race or ethnicity. Univariate analyses were used to examine the differences between clinical and demographic factors in all studies, aside from the multivariate analyses conducted in Rojo et al. 2020 [6].
Table 2.
Stratification of DLL3 expression in SCLC by demographic and clinical factors, SP347 assay studies (N = 5)
| Author, year | DLL3 expression (definition) | N patients | Age | Sex | Smoking status | Stage |
|---|---|---|---|---|---|---|
| Furuta [17], 2019 | High (≥ 75%) | 44 |
< 65: 38.6% ≥ 65: 61.4% |
Male: 79.5% Female: 20.5% |
Pack-years ≥ 20: 84.1% < 20: 9.1% Unknown: 6.8% |
TNM stage I–II: 79.5% III–IV: 20.5% |
| Low (< 75%) | 49 |
< 65: 26.5% ≥ 65:73.5% |
Male: 75.5% Female: 24.5% |
Pack-years ≥ 20: 77.6% < 20: 14.3% Unknown: 8.2% |
TNM stage I–II: 95.9% III–IV: 4.1% |
|
| p value (univariate; high versus low) | 0.268 | 0.805 | 0.526 | 0.022 | ||
| Kuempers [10], 2021 | High (≥ 50%) | 16 chemo-naïve | Median (IQR): 57.0 (65.0, 88.0) |
Male: 78.6% Female: 21.4% |
NR |
TNM stage < IV: 14.3% IV: 28.6% Missing: 57.1% |
| Low (< 50%) | 14 chemo-naïve | Median (IQR): 67.0 (61.0, 75.0) |
Male: 56.2% Female: 43.8% |
NR |
TNM stage < IV: 12.5% IV: 37.5% Missing: 50.0% |
|
| p value (univariate; high versus low in chemo-naïve) | 0.024 | 0.26 | NR | 1.0 | ||
| High (≥ 50%) | 19 chemo-relapsed | NR | NR | NR | NR | |
| Low (< 50%) | 11 chemo-relapsed | NR | NR | NR | NR | |
| p value (univariate; high versus low in chemo-relapsed) | > 0.05 | > 0.05 | NR | > 0.05 | ||
| Rojo [6], 2020 | Positive (≥ 25%) | 895 |
< 65: 44.5% ≥ 65: 55.2% Missing: 0.3% |
Male: 65.1% Female: 34.9% |
NR |
Limited: 31.1% Extensive: 64.2% Missing: 4.7% |
| Negative (< 24%) | 155 |
< 65: 40.0% ≥ 65: 60.0% Missing: 0% |
Male: 60.6% Female: 39.4% |
NR |
Limited: 36.8% Extensive: 59.4% Missing: 3.9% |
|
| p value (multivariate, positive versusnegative) | NR (p > 0.05 on univariate analyses) | NR (p > 0.05 on univariate analyses) | NR | 0.1995; OR 0.767 (95% CI 0.511, 1.151) | ||
| High positive (≥ 75%) | 719 | NR | NR | NR | NR | |
| p value (multivariate, high positive versus negative + non-high positive) | NR (p > 0.05 on univariate analyses) | NR (p > 0.05 on univariate analyses) | NR | 0.0835; OR 0.752 (95% CI 0.545, 1.039) | ||
| Non-high positive (25–74%) | 176 | NR | NR | NR | NR | |
| p value (multivariate, high positive versus non-high positive) | NR (p > 0.05 on univariate analyses) | 0.0580; OR 1.450 (95% CI: 0.987, 2.129) | NR | 0.1061; OR 1.357 (95% CI: 0.937, 1.965) | ||
| Tendler [25], 2020 | High (undefined) | 38 |
< 70: 34.2% ≥ 70: 65.6% |
Male: 47.4% Female: 52.6% |
NR |
Limited disease: 44.7% Extensive disease: 55.3% |
| Low (undefined) | 8 |
< 70: 50.0% ≥ 70: 50.0% |
Male: 25.0% Female: 75.0% |
NR |
Limited disease: 25.0% Extensive disease: 75.0% |
|
| p value (univariate; high versus low) | < 0.05 | > 0.05 | NR | > 0.05 | ||
| Xie [23], 2019 | High (≥ 50%) | 35 | Median (range): 69.5 (53.2–81.8) |
Male: 34.3% Female: 65.7% |
NR |
TNM stage I: 46.4% II: 10.7% III/IV: 42.9% |
| Low (< 50%) | 9 | Median (range): 71.7 (41.9–88.1) |
Male: 77.8% Female: 22.2% |
NR |
TNM stage I: 28.6% II: 14.3% III/IV: 57.1% |
|
| p value (univariate; high versus low) | 0.49 | 0.03 | NR | 0.73 | ||
Bold values indicate statistical significance (p < 0.05)
NR not reported
Four studies did not demonstrate any significant difference in age distribution by DLL3 expression in univariate analyses [6, 17, 23, 25]. The fifth study (a multicenter German cohort) reported a statistically significant lower median age among patients with high DLL3 expression (≥ 50% positivity in tumor cells) compared with those with low expression (univariate p = 0.024), but only among chemo-naïve patients [10]. Similarly, only one [23] of the five studies [6, 10, 17, 25] with information on sex distribution by DLL3 expression found a significant difference: the Mayo Clinic cohort of patients with SCLC (1995–2017) reported significantly fewer males among the high DLL3 expression group (≥ 50% of tumor cell positivity) than the low expression group (univariate p = 0.03), although case numbers were small (N = 9 patients in the low expression group) [23]. History of smoking was only evaluated in a retrospective cohort study of surgically resected primary patients in Japan from 2003–2013; no statistically significant difference was reported for pack-years of smoking by DLL3 expression (p = 0.526) [17].
Only one study reported DLL3 expression by stage of disease [17]. A retrospective cohort study of 95 patients who had undergone complete surgical resection of a primary lung tumor in Japan from 2003–2013 reported that the proportion of patients with higher disease stage (TNM stage III–IV) was statistically significantly higher among patients with DLL3‐high expression (≥ 75% tumor cell positivity) than those with low expression in univariate analysis (p = 0.022) [17].
ECOG status was not statistically significantly associated with DLL3 expression in either univariate analysis from the Japanese cohort study [17] or multivariate analyses in a large multicountry cohort of patients with SCLC [6]. A retrospective cohort study conducted in Germany (time period not specified) reported that DLL3 expression may be influenced by therapy. Paired samples from patients with SCLC (N = 30), prechemotherapy and post-relapse, showed that approximately 43% of patient samples were discordant to their prechemotherapy sample after relapse. Of these discordant pairs, 69.2% shifted from the DLL3-low (H-score < 150) to DLL3-high (H-score ≥ 150) after relapse, while 30.8% shifted from DLL3-high to DLL3-low after relapse. Specimens of patients at diagnosis and at relapse were also compared in a multicountry retrospective cohort study. This study reported a 12% discordance at relapse, with 23% discordance among samples with high DLL3 (≥ 75% of tumor cells) at diagnosis and 35% discordance among samples with non-high DLL3 (25–74% of tumor cells) at diagnosis [6].
Overall Survival
Among studies using the SP347 assay, OS was assessed by DLL3 expression status in eight studies (Table 3). No significant associations were reported between OS and DLL3 expression level in univariate analyses in the six studies with this data [6, 10, 17, 18, 22, 25] or in multivariate analysis of the Mayo Clinic Rochester cohort of patients with SCLC (1995–2017), after adjustment for age, tumor size, and stage (HR: 1.0; 95% CI 0.98, 1.01) [23]. The TRINITY trial only reported median OS for DLL3-high (≥ 75% of tumor cells) or DLL3-positive (≥ 25%) patients without comparison to low level patients [20]. Two studies evaluated the impact of DLL3 expression on OS stratified by disease stage but did not identify any significant prognostic differences [17, 25].
Table 3.
SCLC treatment response and survival by DLL3 expression level, SP347 assay studies (N = 8)
| Author, year | Geographic location (dates) | DLL3 expression (definition) | N patients | Response | Overall survival | Progression-free survival or disease-free survival |
|---|---|---|---|---|---|---|
| Median or % (95% CI) | Median (95% CI) or % by milestone | Median (95% CI) or % by milestone | ||||
| Furuta [17], 2019 | Japan (2003–2013) | High (≥ 75%) | 44 | NR |
Overall: 24.4 months (16.8–32.7) Stage I/II: 25.8 months (18.1–39.4) Stage III/IV: 10.8 months (1.1–61.1) |
NR |
| Low (< 75%) | 49 | NR |
Overall: 33.3 months (23.5–N/A) Stage I/II: 40.2 months (24.1–N/A) Stage III/IV: 11.2 months (3.1–19.3) |
NR | ||
| p value (univariate; high versus low) | NR |
Overall: p = 0.16 Stage I/II: p = 0.182 Stage III/IV: p = 0.641 |
NR | |||
| Johnson [18], 2021 | Multicountry (2017–2019) | High (≥ 75%) |
Intervention: 217 |
ORR: 10.0% (6.2–15.3) CBR: 72.0% (65.5–78.7) CR: 0.0% PR: 10.0% |
8.5 months (7.3–10.2) | PFS: 4.0 months (3.2–4.1) |
| Placebo: 240 |
ORR: 5.0% (2.6–9.0) CBR: 30.0% (24.3–37.0) CR: 0.0% PR: 5.0% |
9.8 months (8.4–10.9) | PFS: 1.4 months (1.4–1.5) | |||
| Low (< 75%) |
Intervention: 148 |
NR | 9.0 months (8.1–10.1) | PFS: 2.8 months (2.6–4.0) | ||
| Placebo: 120 | NR | 11.3 months (8.3–13.0) | PFS: 1.5 months (1.4–1.7) | |||
| p value (univariate, low versus high) | NR |
Intervention: p > 0.05 Placebo: p > 0.05 |
NR | |||
| Kuempers [10], 2021 | Germany (NR) | High (≥ 50%) | Chemo-naïve: 14 | NR | NR | NR |
| Chemo-relapsed: 19 | NR | NR | NR | |||
| Low (< 50% | Chemo-naïve: 16 | NR | NR | NR | ||
| Chemo-relapsed: 11 | NR | NR | NR | |||
| p value (univariate, high versus low) | NR |
Chemo-naïve: p = 0.42 Chemo-relapsed: 0.57 |
NR | |||
| Morgensztern [20], 2019 | Multicountry (2016–2017) | High (≥ 75%) | 238 |
ORR: 14.3% (10.1–19.4) DCR: 73.5% (67.4–79.0) DOR: 3.7 months (2.9–4.2) |
5.7 months (4.9–6.7) | PFS: 3.8 months (3.2–4.1) |
| Positive (≥ 25%) | 287 |
ORR: 13.2% (9.5, 17.7) DCR: 71.8% (66.2–76.9) DOR: 3.7 months (2.9 4.2) |
5.8 months (5.1–6.7) | PFS: 3.8 months (3.2–4.0) | ||
| HR (95% CI), p value | NR | NR | NR | |||
| Rojo [6], 2020 | Multicountry (2008–2017) | High positive (≥ 75%) | 719 | NR | 9.5 months | NR |
| Non-high positive (25–74%) | 176 | NR | 9.5 months | NR | ||
| Positive (≥ 25%) | 895 | NR | 9.5 months | NR | ||
| Negative (0–24%): | 155 | NR | 9.5 months | NR | ||
| p value (univariate, positive versus negative) | NR | p > 0.05 | NR | |||
| Tendler [25], 2020 | Sweden (2008–2015) | High (undefined) | 38 | NR | 11.5 months (9.2–13.7) | PFS: 7.4 months (6.1–8.7) |
| Low (undefined) | 8 | NR | 10.6 months (8.9–12.4) | PFS: 5.6 months (2.5–8.7) | ||
| p value (univariate, low versus high) | NR | p > 0.05 | p > 0.05 | |||
| Udagawa [22], 2019 | Japan (2017–2018) | High (≥ 75%) | 18 |
ORR: 16.7% DCR: 55.6% DOR: 3.0 months (2.9-4.1) |
7.4 months (4.1–11.9) | PFS: 2.9 months (1.2–3.6) |
| Low (< 75%) | 10 |
ORR: 0.0% DCR: 60.0% DOR: NR |
5.1 months (1.8–7.8) | PFS: 2.0 months (0.7–2.7) | ||
| p value (univariate, low versus high) | NR | p = 0.346 | PFS: p = 0.082 (investigator); 0.157 (central review) | |||
| Xie [23], 2019 | USA (1995–2017) | High (≥ 50%) | 35 | NR | 5-year survival: 33.0% | DFS: NR |
| Low (< 50%) | 9 | NR | 5-year survival: 0.0% | DFS: NR | ||
| HR (95% CI), p value [low versus high; multivariate (OS) or univariate (DFS)] | NR | 1.0 month (0.985–1.008), p = 0.49 | DFS: p = 0.27 | |||
CBR clinical benefit rate, CI confidence interval, CR complete response, DCR disease control rate, DFS disease-free survival, DOR duration of objective response, HR hazard ratio, NR not reported, ORR overall response rate, PR partial response
Progression-Free Survival or Disease-Free Survival
The relationship between DLL3 expression in SCLC and PFS or DFS was assessed in five studies (Table 3). Two studies reported no statistically significant impact of DLL3 expression on PFS in univariate analyses [22, 25], while two additional studies reported PFS by DLL3 expression but did not conduct statistical analyses for the difference [18, 20]. PFS trended higher among DLL3-high patients than DLL3-low patients in three of these studies [18, 22, 25], although the association was either not statistically significant, or the statistical significance was unknown. The impact of DLL3 expression on DFS was assessed in the Mayo Clinic Rochester cohort of patients with SCLC (1995–2017); univariate analysis did not demonstrate a statistically significant association (p = 0.27) [23].
Treatment Response
Treatment response was only reported among DLL3-high (DLL3-high: ≥ 75%) patients in two studies; objective response rate (ORR) ranged from 5.0% in the placebo arm of the MERU trial [18] to 14.3% in the TRINITY trial [20] (Table 3). The Phase I trial of Rova-T in Japanese patients with advanced recurrent SCLC reported treatment response for both DLL3-high (≥ 75% of tumor cells) and DLL3-low (< 75% of tumor cells) groups; while the ORR was higher in the DLL3-high group (16.7% versus 0.0%), no tests for statistical significance were conducted [22]. Additional file 6 reports survival and treatment response by DLL3 expression levels (N = 11) among non-SP347 assay studies.
Discussion
This SLR identified and evaluated studies of the prevalence and the prognostic impact of DLL3 expression among patients with SCLC worldwide. The review focused on studies that utilized the Ventana SP347 immunohistochemistry (IHC) assay to measure DLL3 expression to allow for comparable results across studies; however, other methods were used in additional eligible studies (i.e., ab103102, Stemcentryx, bs-7860R, E3J5R). Notable heterogeneity was further observed in study location, population characteristics (age, sex, clinical stage), time periods, sample type, pathologist interpretation, inclusion criteria, and treatment history, which may have contributed towards inconsistent observations of prevalence across studies. Despite the variability in definitions of DLL3 positivity and the tumor heterogeneity among the 30 identified studies, most patients with SCLC were found to be DLL3-positive across studies (58.3% [16] to 93.5% [25]). Higher levels of DLL3 prevalence generally corresponded with lower thresholds of “high” DLL3 expression reported.
The largest available international cohort of non-selected patients with SCLC at various stages of disease and lines of therapy evaluated the prevalence of DLL3 expression by IHC using DLL3 antibody (clone SP347, Ventana, Tucson, AZ). This study reported that 85% (N = 895/1050) of patients had positive (≥ 25% of tumor cells) DLL3 expression and 68% (N = 719/1050) had high (≥ 75% of tumor cells) DLL3 expression [6]. A high selective expression of DLL3 in SCLC tumors makes it an attractive option to guide therapeutic targets, as DLL3 is not present in normal adult tissues, including the vasculature and skin tissue [27]. A biomarker expressed in the majority of patients with SCLC is advantageous in comparison to other therapeutic targets that are minimally expressed in SCLC, such as PD-L1, which has been reported to be positive (≥ 1% of tumor cells) in < 20% of patients [40].
Among the included studies, DLL3 expression was not consistently associated with any demographic or clinical characteristics such as age, sex, smoking history, ECOG performance status, or disease stage. However, these associations were examined in a limited number of studies; methods and cut-off values used to detect DLL3 expression in each study were inconsistent; and analyses were largely univariate with small sample sizes. While none of the studies in the current review assessed differences in DLL3 expression by race/ethnicity, the previously published meta-analysis reported a statistically significant decreased prognosis for DLL3-high patients from Asian countries after excluding the single US study [7]. Further investigation is warranted to evaluate whether this potential therapeutic target has any clear defining demographic or clinical correlates among patients with SCLC.
The prognostic impact of DLL3 expression was investigated in a limited number of studies. Among the studies using the Ventana SP347 assay, no significant differences were reported for survival (OS, PFS, or DFS) or treatment response between DLL3-high and low groups. The previously published meta-analysis of the prognostic impact of DLL3 included six studies (all of which were included in the current review) and reported a statistically significant decreased prognosis for DLL3-high patients in Asian studies, after excluding the study conducted in the USA [7]. While methods for evaluating DLL3 and defining “high” expression varied across the included studies in the meta-analysis, results suggest that the use of DLL3-targeted drugs may improve survival and response in patients with SCLC with high expression. The phase I trial of Rova-T, an antibody–drug conjugate directed against DLL3, in recurrent SCLC did not test for statistical differences in response or survival between DLL3-high (≥ 50%) and low (< 50%) groups, but the ORR (35% versus 0%), OS (median 5.8 versus 2.7 months), and PFS (median 4.3 versus 2.2 months) trended higher in DLL3-high patients [33]. The phase 2 trial of Rova-T in relapsed/refractory patients with SCLC (which included patients with DLL3-expressing tumors only) demonstrated similar ORR among patients who were DLL3-positive (≥ 25%; ORR 13.2%) and DLL3-high (≥ 75%; ORR 14.3%), suggesting that DLL3 positivity (as opposed to high expression) was sufficient for benefits of response to Rova-T [20]. However, Phase III trials for Rova-T (MERU and TAHOE) did not demonstrate increased survival for DLL3-high patients compared with placebo [18] or topotecan [9] therapy and were terminated early. Additional studies of DLL3 targeting agents may provide more insight as to the potential benefit of DLL3 expression on patient survival or treatment response.
No standardized threshold values have been established or recommended by treatment guidelines to determine DLL3 positivity or “high” levels of expression. Among the included studies, thresholds of ≥ 1 or ≥ 25% of positive tumor cells were commonly used to define the DLL3-positive group, while ≥ 50% or ≥ 75% were frequently used to define “high” DLL3 expression. However, other threshold values were used in some studies (≥ 13.5%, ≥ 60%), whereas others did not report the threshold value used to define “high” expression. Without a standardized definition of DLL3 positivity or “high” expression, the prevalence of DLL3 expression cannot be summarized quantitatively across studies. Future studies of DLL3 expression in SCLC would benefit from harmonized definitions for DLL3 positivity and “high” expression or reporting results using multiple thresholds to allow comparability between studies.
While the current review focused on studies using the Ventana SP347 IHC assay, a variety of testing methods were reported in the literature, including other IHC assays and reverse transcription polymerase chain reaction (RT–PCR). Within each of these methods, there are further variances, including the use of antibodies and biopsy sampling, which may influence detectability. Brcic et al. [16] compared four different DLL3 antibodies (Ventana SP347, Novus NBP2–24669, Thermo Fisher PA5–26336, and Abcam ab103102) for their reliability in detecting DLL3 expression in high-grade neuroendocrine tumors of the lung. There was poor overall positive and negative agreement and kappa values when Ventana SP347 was compared with the other three antibodies. The authors concluded that only the Ventana SP347 assay could be reliably used to determine DLL3 expression. Recommendations for treatment decisions based on biomarker expression generally require the use of a validated test. This SLR highlights a need to clearly define a guideline for DLL3 evaluation to assess the impact of expression on clinical variables and prognosis across studies.
Strengths of this SLR include the use of a systematic methodology following PRISMA guidelines, evaluation of study quality, and a thorough evaluation of relevant survival and treatment response outcomes. A broad and comprehensive search strategy was incorporated across multiple databases to include all potentially relevant articles. Quality control measures were implemented at several steps to ensure accuracy as well as transparency and reproducibility of the review. Our restriction to English language publications may have resulted in missing key articles published in other languages; for this reason, we also examined bibliographies of relevant reviews. Most of the included literature was retrospective in design and may be biased if the patients that were tested for DLL3 were inherently different than those who were not. Due to the heterogeneity between studies in methods, included populations, and outcome definitions, we were unable to quantitatively summarize the prevalence of DLL3 expression in SCLC using meta-analytical techniques. The addition of more studies using consistent methods and definitions for evaluating DLL3 may allow for a meta-analysis in the future.
Conclusions
This SLR demonstrates that DLL3 is expressed in most patients with SCLC and presents a potentially impactful therapeutic target. To date, DLL3 expression has not been shown to be consistently associated with sociodemographic or clinical factors or have a significant impact on treatment response or patient survival. Future investigations should explore the impact of DLL3 expression using large populations with validated tests for DLL3 expression to better understand the dynamics of DLL3 as a therapeutic target for SCLC.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
This work was funded by Amgen Inc.
Conflict of interest
LCB, NH, NM, and JPF are employees of EpidStrategies, a division of ToxStrategies, LLC, which received funding for the conduct of this study. XP, AB, DJ, C-HJ, and EE are employees and shareholders of Amgen Inc. GB was an employee and shareholder of Amgen Inc. at the time of the manuscript creation.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Author contributions
XP, LCB, AB, and JPF contributed to study conceptualization and design. LCB, NH, and NM conducted the literature review and prepared the first draft of the manuscript. All authors reviewed, revised, and provided critical feedback on the manuscript. All authors provided final approval of the manuscript.
Code availability
Not applicable.
References
- 1.National Cancer Institute Surveillance, Epidemiology, and End Results Program. In: SEER*Explorer: An interactive website for SEER cancer statistics [Internet]. Surveillance Research Program, National Cancer Institute. 2023. https://seer.cancer.gov/statistics-network/explorer/.
- 2.Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nat Rev Dis Primers. 2021;7:3. doi: 10.1038/s41572-020-00235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang S, Zimmermann S, Parikh K, et al. Current diagnosis and management of small-cell lung cancer. Mayo Clin Proc. 2019:1599–622. [DOI] [PubMed]
- 4.Dingemans AC, Früh M, Ardizzoni A, et al. Small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up☆. Ann Oncol. 2021;32:839–853. doi: 10.1016/j.annonc.2021.03.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owen DH, Giffin MJ, Bailis JM, et al. DLL3: an emerging target in small cell lung cancer. J Hematol Oncol. 2019;12:1–8. doi: 10.1186/s13045-019-0745-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rojo F, Corassa M, Mavroudis D, et al. International real-world study of DLL3 expression in patients with small cell lung cancer. Lung Cancer. 2020;147:237–243. doi: 10.1016/j.lungcan.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 7.Chen B, Li H, Liu C, et al. Potential prognostic value of delta-like protein 3 in small cell lung cancer: a meta-analysis. World J Surg Oncol. 2020;18:1–9. doi: 10.1186/s12957-020-02004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aeffner F, Zarella MD, Buchbinder N, et al. Introduction to digital image analysis in whole-slide imaging: a white paper from the digital pathology association. J Pathol Inform. 2019;10:9. doi: 10.4103/jpi.jpi_82_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackhall F, Jao K, Greillier L, et al. Efficacy and safety of rovalpituzumab tesirine compared with topotecan as second-line therapy in DLL3-high SCLC: results from the phase 3 TAHOE study. J Thorac Oncol. 2021;16:1547–1558. doi: 10.1016/j.jtho.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Kuempers C, Jagomast T, Krupar R, et al. Delta-like protein 3 expression in paired chemonaive and chemorelapsed small cell lung cancer samples. Front Med. 2021;8:734901. [DOI] [PMC free article] [PubMed]
- 11.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 12.DistillerSR. DistillerSR: literature review software. 2022.
- 13.Sterne JA, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366. [DOI] [PubMed]
- 14.Wells GA, Shea B, O’Connell D, et al. Newcastle–Ottawa Quality Assessment Scale. 2019.
- 15.Alì G, Di Stefano I, Poma AM, et al. Prevalence of delta-like protein 3 in a consecutive series of surgically resected lung neuroendocrine neoplasms. Front Oncol. 2021;11:729765. [DOI] [PMC free article] [PubMed]
- 16.Brcic L, Kuchler C, Eidenhammer S, et al. Comparison of four DLL3 antibodies performance in high grade neuroendocrine lung tumor samples and cell cultures. Diagn Pathol. 2019;14:47. doi: 10.1186/s13000-019-0827-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furuta M, Sakakibara-Konishi J, Kikuchi H, et al. Analysis of DLL3 and ASCL1 in surgically resected small cell lung cancer (HOT1702) Oncologist. 2019;24:e1172–e1179. doi: 10.1634/theoncologist.2018-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson ML, Zvirbule Z, Laktionov K, et al. Rovalpituzumab tesirine as a maintenance therapy after first-line platinum-based chemotherapy in patients with extensive-stage–SCLC: results from the phase 3 MERU study. J Thorac Oncol. 2021;16:1570–1581. doi: 10.1016/j.jtho.2021.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Messaritakis I, Nikolaou M, Koinis F, et al. Characterization of DLL3-positive circulating tumor cells (CTCs) in patients with small cell lung cancer (SCLC) and evaluation of their clinical relevance during front-line treatment. Lung Cancer. 2019;135:33–9. [DOI] [PubMed]
- 20.Morgensztern D, Besse B, Greillier L, et al. Efficacy and safety of rovalpituzumab tesirine in third-line and beyond patients with DLL3-expressing, relapsed/refractory small-cell lung cancer: results from the phase II TrINITY study. Clin Cancer Res. 2019;25:6958–6966. doi: 10.1158/1078-0432.CCR-19-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Odashiro P, Tomarchio G, Gagné A, et al. DLL3 expression in small cell lung carcinomas (SCLC) Mod Pathol. 2020;33:1809–1810. [Google Scholar]
- 22.Udagawa H, Akamatsu H, Tanaka K, et al. Phase I safety and pharmacokinetics study of rovalpituzumab tesirine in Japanese patients with advanced, recurrent small cell lung cancer. Lung Cancer. 2019;135:145–150. doi: 10.1016/j.lungcan.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 23.Xie H, Boland JM, Maleszewski JJ, et al. Expression of delta-like protein 3 is reproducibly present in a subset of small cell lung carcinomas and pulmonary carcinoid tumors. Lung Cancer. 2019;135:73–79. doi: 10.1016/j.lungcan.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 24.Huang RSP, Holmes BF, Powell C, et al. Delta-like protein 3 prevalence in small cell lung cancer and DLL3 (SP347) assay characteristics. Arch Pathol Lab Med. 2019;143:1373–1377. doi: 10.5858/arpa.2018-0497-OA. [DOI] [PubMed] [Google Scholar]
- 25.Tendler S, Kanter L, Lewensohn R, et al. The prognostic implications of Notch1, Hes1, Ascl1, and DLL3 protein expression in SCLC patients receiving platinum-based chemotherapy. PLoS ONE. 2020;15(10):e0240973. [DOI] [PMC free article] [PubMed]
- 26.An E, Hong SH, An HJ, et al. Identifying treatment options for SCLC patients with multiplexed clinical proteomic testing. J Clin Oncol. 2018;36(15_suppl):8574.
- 27.Calvo E, Spira A, Miguel M, et al. Safety, pharmacokinetics, and efficacy of budigalimab with rovalpituzumab tesirine in patients with small cell lung cancer. Cancer Treat Res Commun. 2021;28:100405. doi: 10.1016/j.ctarc.2021.100405. [DOI] [PubMed] [Google Scholar]
- 28.Fu X, Liu Z, Xiang L, et al. PD-L1 predicts poor prognosis in surgically resected limited stage small-cell lung cancer. Cancer Manag Res. 2020;12:10939–10948. doi: 10.2147/CMAR.S260599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldman JW, Barve M, Patel JD, et al. Effects of rovalpituzumab tesirine on ventricular repolarization in patients with small-cell lung cancer. Clin Transl Sci. 2021;14:664–670. doi: 10.1111/cts.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W, Ye L, Huang Y, et al. Characteristics of Notch signaling pathway and its correlation with immune microenvironment in SCLC. Lung Cancer. 2022;167:25–33. doi: 10.1016/j.lungcan.2022.03.019. [DOI] [PubMed] [Google Scholar]
- 31.Malhotra J, Nikolinakos P, Leal T, et al. A phase 1–2 Study of rovalpituzumab tesirine in combination with nivolumab plus or minus ipilimumab in patients with previously treated extensive-stage SCLC. J Thorac Oncol. 2021;16:1559–1569. doi: 10.1016/j.jtho.2021.02.022. [DOI] [PubMed] [Google Scholar]
- 32.Regzedmaa O, Li Y, Li Y, et al. Prevalence of DLL3, CTLA-4 and mstn expression in patients with small cell lung cancer. Onco Targets Ther. 2019;12:10043–10055. doi: 10.2147/OTT.S216362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudin CM, Pietanza MC, Bauer TM, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2017;18:42–51. doi: 10.1016/S1470-2045(16)30565-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saito M, Saito K, Shiraishi K, et al. Identification of candidate responders for anti-PD-L1/PD-1 immunotherapy, Rova-T therapy, or EZH2 inhibitory therapy in small-cell lung cancer. Mol ClinOncol. 2018;8:310–314. doi: 10.3892/mco.2017.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka K, Isse K, Fujihira T, et al. Prevalence of delta-like protein 3 expression in patients with small cell lung cancer. Lung Cancer. 2018;115:116–120. doi: 10.1016/j.lungcan.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 36.Huang J, Cao D, Sha J, et al. DLL3 is regulated by LIN28B and miR-518d-5p and regulates cell proliferation, migration and chemotherapy response in advanced small cell lung cancer. Biochem Biophys Res Commun. 2019;514:853–860. doi: 10.1016/j.bbrc.2019.04.130. [DOI] [PubMed] [Google Scholar]
- 37.Lim S, Hong M, Kim SP, Chung SH. P2.12–18 prevalence of DLL3 expression and its prognostic role in extensive stage small cell lung cancer. J Thorac Oncol. 2019;14:S820. doi: 10.1016/j.jtho.2019.08.1763. [DOI] [Google Scholar]
- 38.Prieto T, Baldavira CM, Saber A, et al. P47.11 DLL-3 and ASCL-1 expression emerge as promising therapeutic targets in high-grade neuroendocrine lung tumors: a preliminary study. J Thorac Oncol. 2021;16:S496–S497. doi: 10.1016/j.jtho.2021.01.867. [DOI] [Google Scholar]
- 39.Yan LX, Liu YH, Li Z, et al. Prognostic value of delta-like protein 3 combined with thyroid transcription factor-1 in small-cell lung cancer. Oncol Lett. 2019;18:2254–2261. doi: 10.3892/ol.2019.10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iams WT, Porter J, Horn L. Immunotherapeutic approaches for small-cell lung cancer. Nat Rev Clin Oncol. 2020;17:300–312. doi: 10.1038/s41571-019-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.