Abstract
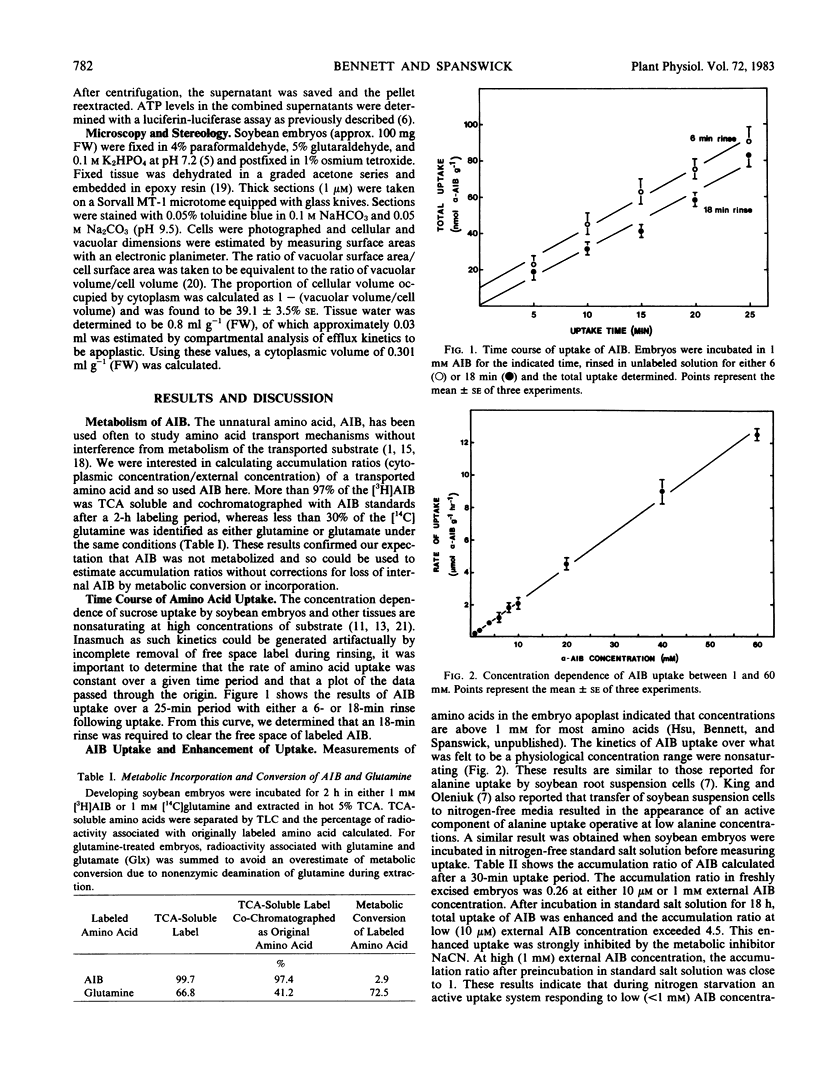
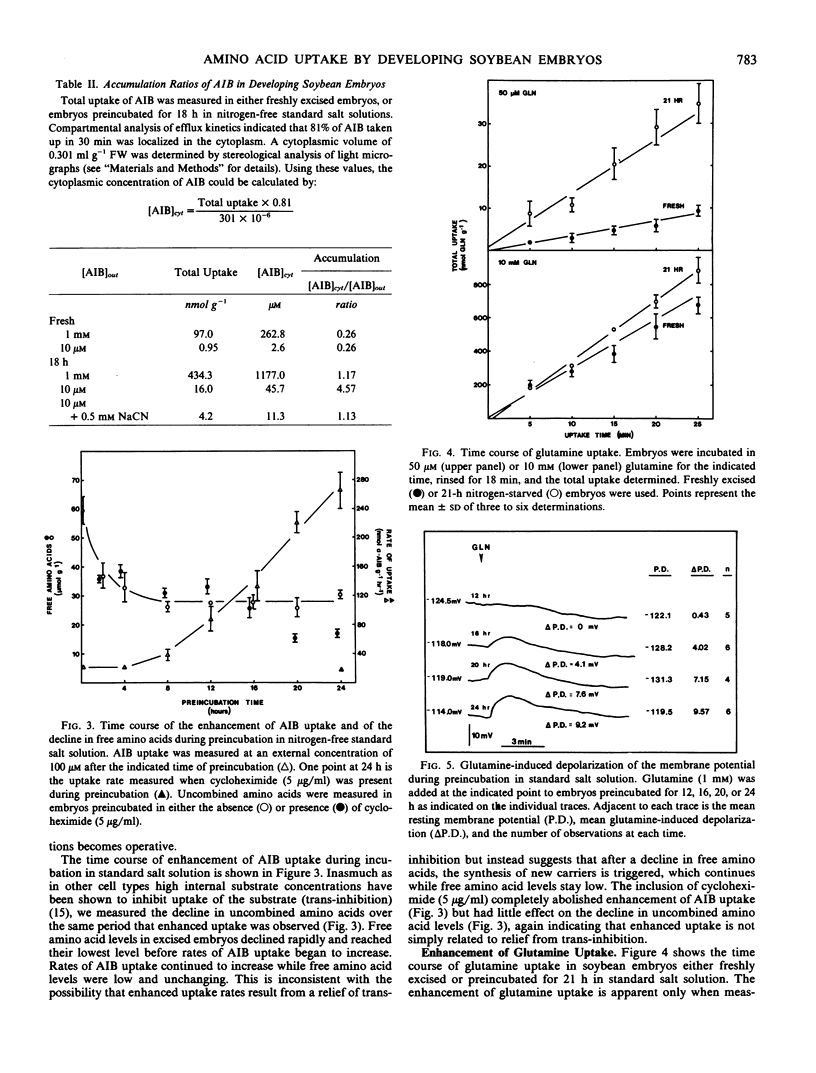
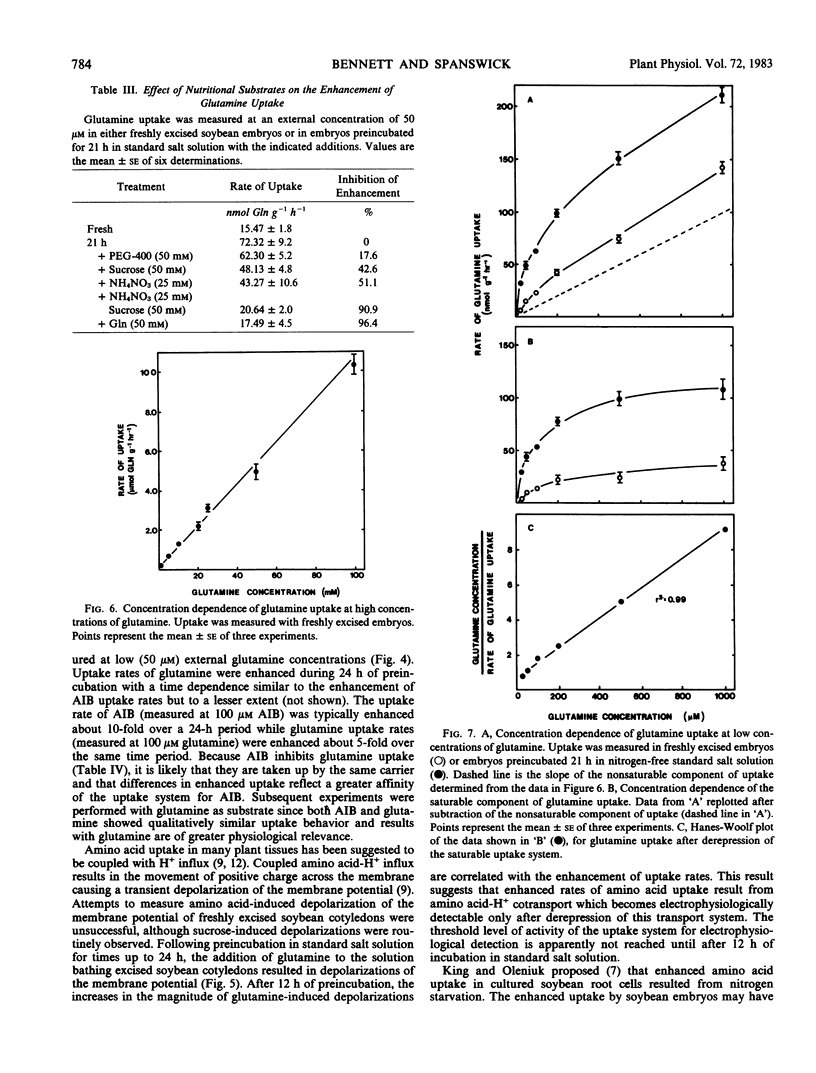
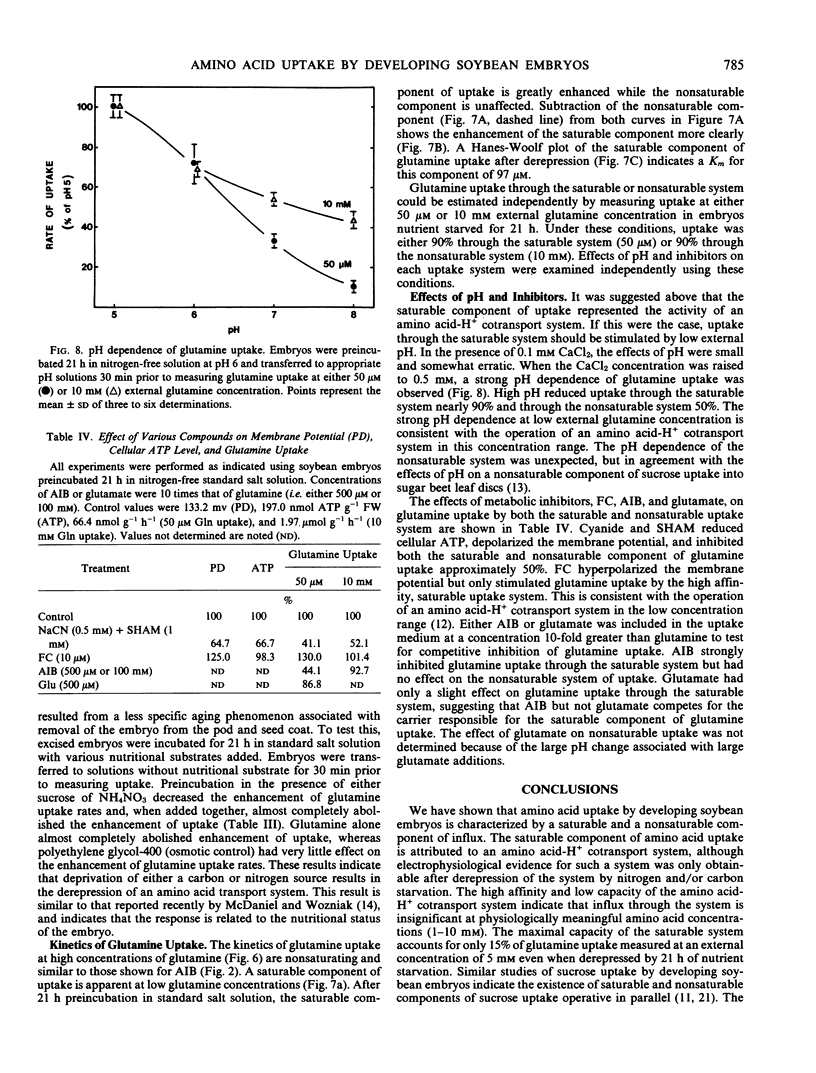
The uptake of the unnatural amino acid α-aminoisobutyric acid (AIB) and glutamine by developing soybean (Glycine max Merr. cv Chippewa 64) embryos was investigated. In freshly excised embryos, the accumulation ratio (cytoplasmic concentration/external concentration) of AIB did not exceed 1.0. After an 18-hour preincubation in nitrogen-free medium the accumulation ratio of AIB exceeded 4.5 at an external AIB concentration of 10 micromolar. This indicates the derepression of an active amino acid uptake mechanism operative at low external amino acid concentration. The presence of sucrose, NH4NO3, or glutamine during a 21-hour preincubation prior to measuring glutamine uptake inhibited the enhancement of uptake by 43%, 51%, and 96%, respectively. The time course of the decline in free amino acids and the time course of enhancement of amino acid uptake was not consistent with enhanced uptake resulting from relief of transinhibition, but suggested instead the derepression of synthesis of new carriers. The time course of enhancement of amino acid uptake was paralleled by an increase in glutamine-induced depolarization of the membrane potential. The kinetics of glutamine uptake indicated the presence of a saturable and a nonsaturable component of uptake. The saturable component of uptake is attributed to a mechanism of amino acid-H+ cotransport which is derepressed by nitrogen and/or carbon starvation. At physiological concentrations of amino acids, uptake through the saturable system in freshly excised embryos is negligible. Thus, uptake through the nonsaturable system is of primary importance in the nitrogen nutrition of developing soybean embryos.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen S. R. A comparison of the rate equations, kinetic parameters, and activation energies for the initial uptake of L-lysine, L-valine, gamma-aminobutyric acid, and alpha-aminoisobutyric acid by mouse brain slices. J Membr Biol. 1975 Jun 3;22(1):53–72. doi: 10.1007/BF01868163. [DOI] [PubMed] [Google Scholar]
- Keifer D. W., Spanswick R. M. Correlation of Adenosine Triphosphate Levels in Chara corallina with the Activity of the Electrogenic Pump. Plant Physiol. 1979 Aug;64(2):165–168. doi: 10.1104/pp.64.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide T. B., Etherton B. Electrical evidence for different mechanisms of uptake for basic, neutral, and acidic amino acids in oat coleoptiles. Plant Physiol. 1980 Jun;65(6):1085–1089. doi: 10.1104/pp.65.6.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide T. B. Interamino Acid Inhibition of Transport in Higher Plants : EVIDENCE FOR TWO TRANSPORT CHANNELS WITH ASCERTAINABLE AFFINITIES FOR AMINO ACIDS. Plant Physiol. 1981 Dec;68(6):1327–1333. doi: 10.1104/pp.68.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtner F. T., Spanswick R. M. Electrogenic sucrose transport in developing soybean cotyledons. Plant Physiol. 1981 Apr;67(4):869–874. doi: 10.1104/pp.67.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtner F. T., Spanswick R. M. Sucrose uptake by developing soybean cotyledons. Plant Physiol. 1981 Sep;68(3):693–698. doi: 10.1104/pp.68.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard J. W., Lucas W. J. A Reanalysis of the Two-Component Phloem Loading System in Beta vulgaris. Plant Physiol. 1982 Mar;69(3):734–739. doi: 10.1104/pp.69.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Thorne J. H. Characterization of the active sucrose transport system of immature soybean embryos. Plant Physiol. 1982 Oct;70(4):953–958. doi: 10.1104/pp.70.4.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne J. H., Koller H. R. Influence of assimilate demand on photosynthesis, diffusive resistances, translocation, and carbohydrate levels of soybean leaves. Plant Physiol. 1974 Aug;54(2):201–207. doi: 10.1104/pp.54.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse R. E., Saftner R. A. Reduction in Sink-Mobilizing Ability following Periods of High Carbon Flux. Plant Physiol. 1982 Jan;69(1):226–228. doi: 10.1104/pp.69.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]


