Abstract
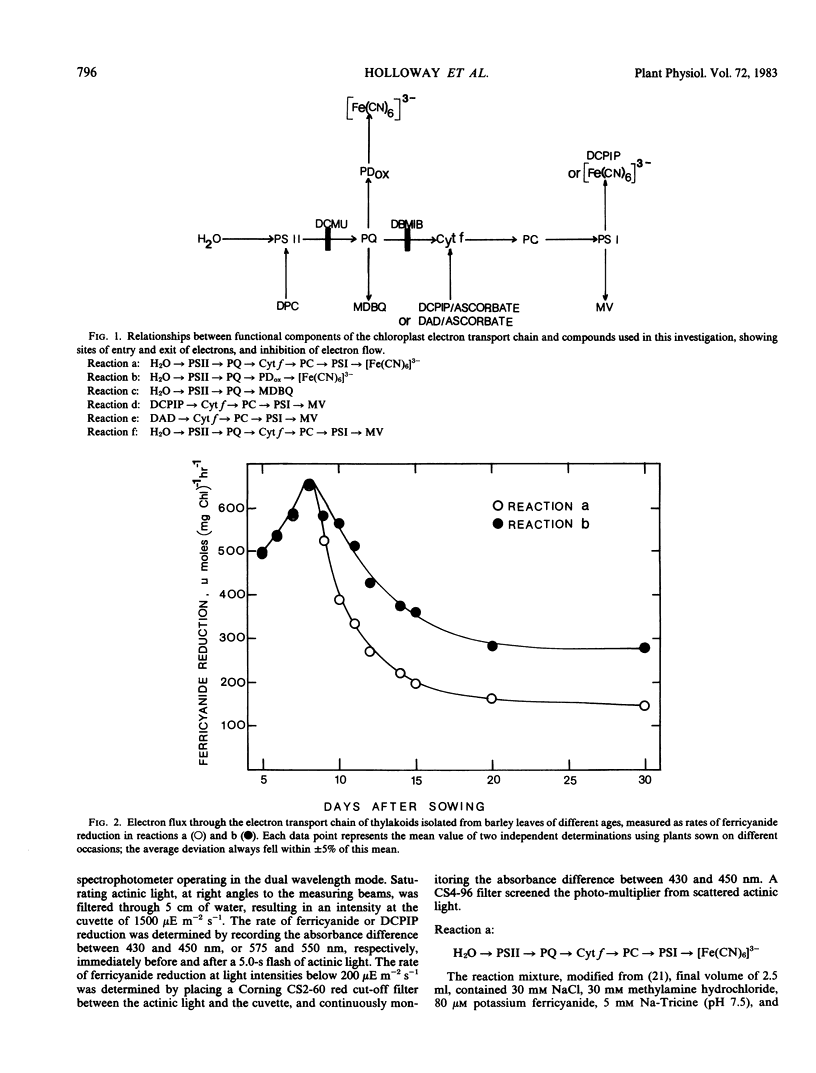
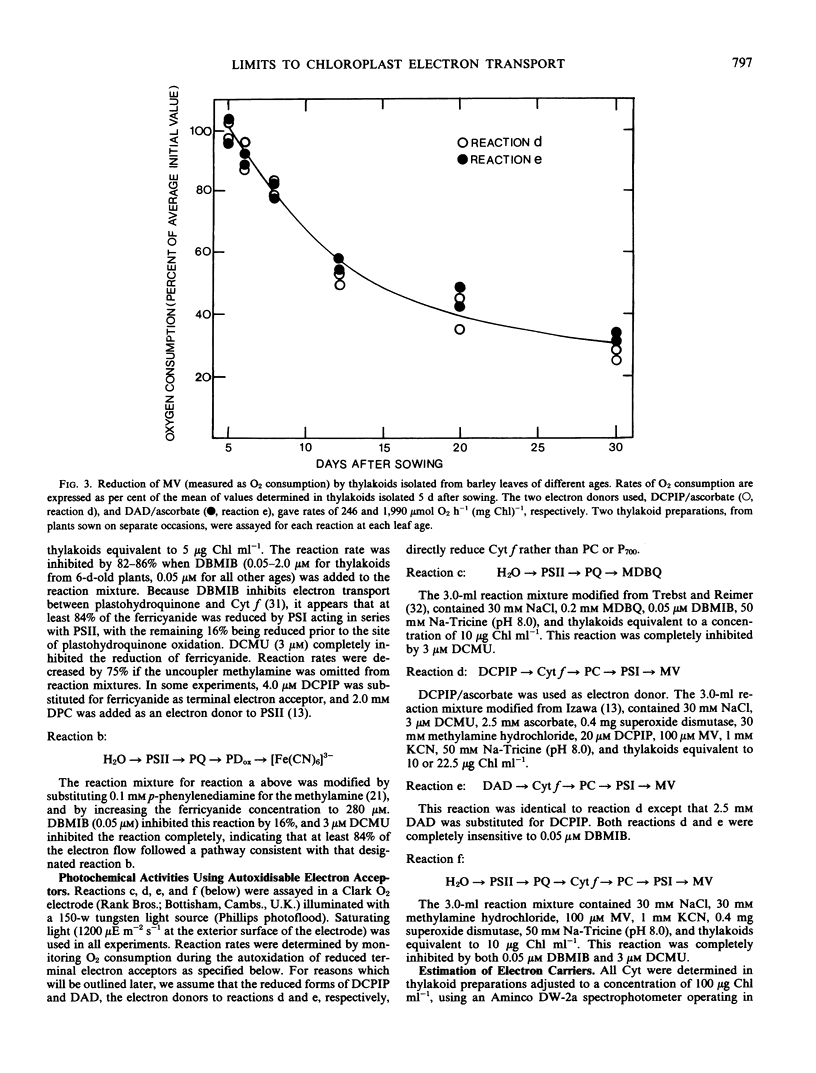
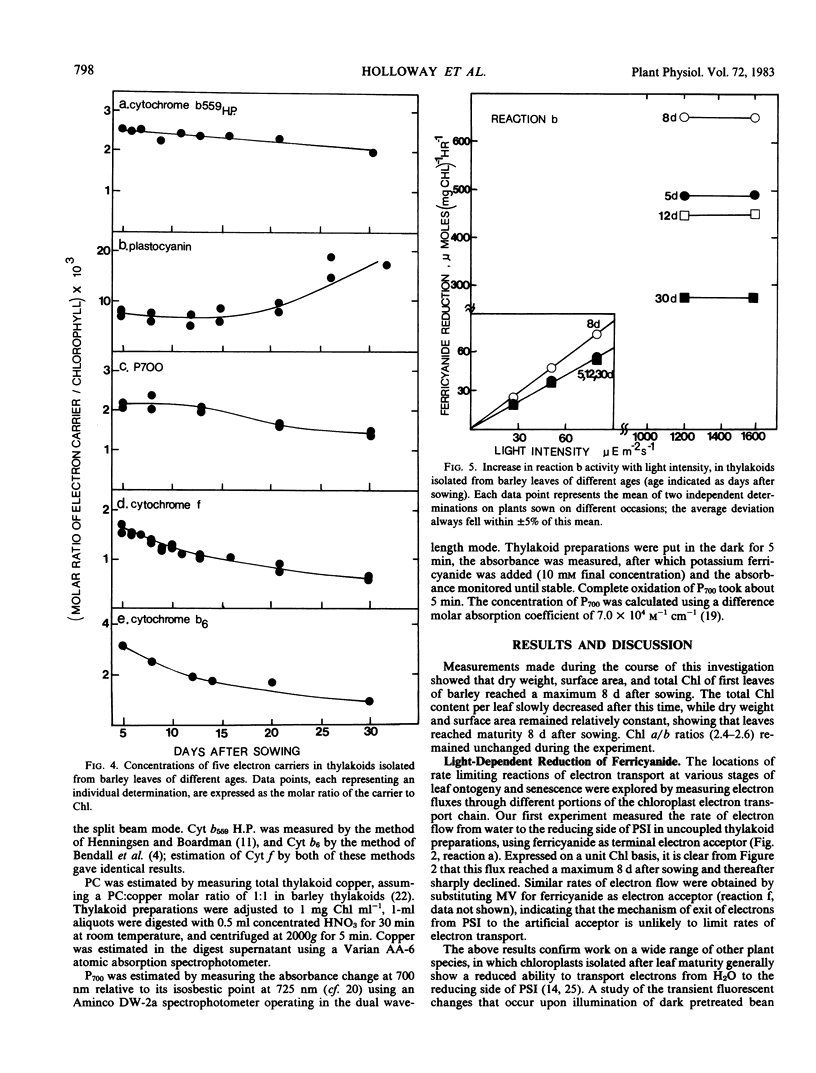
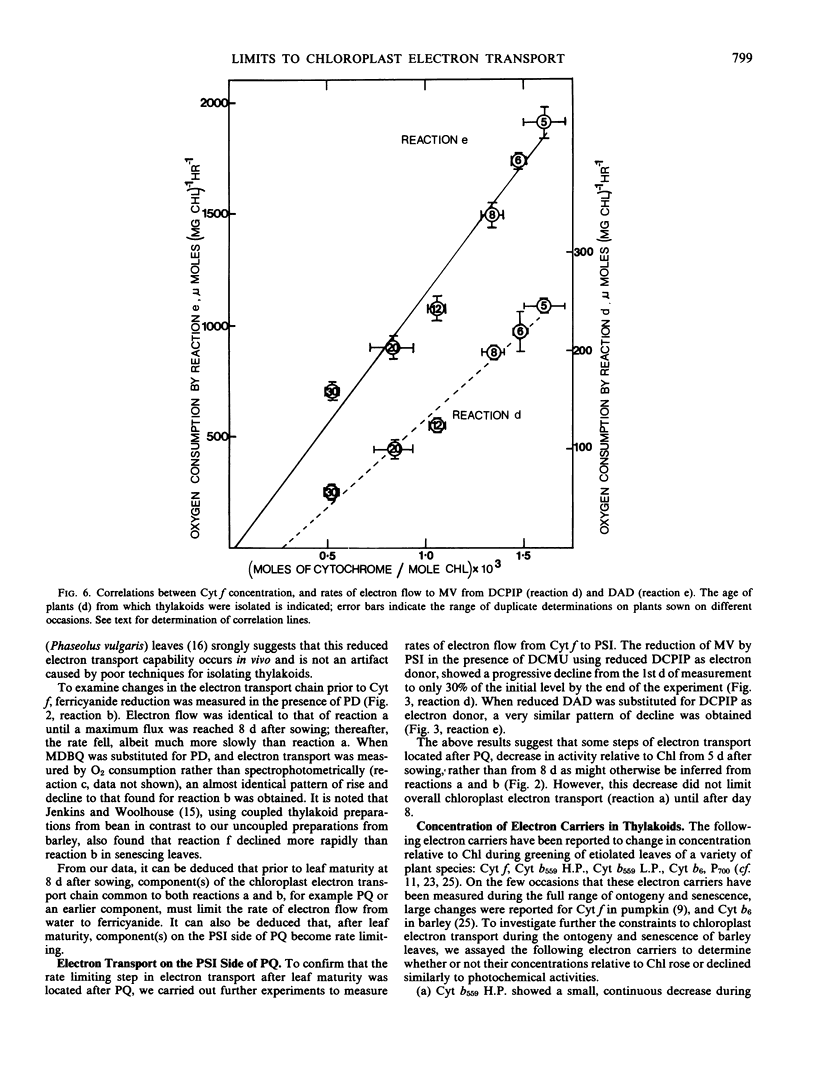
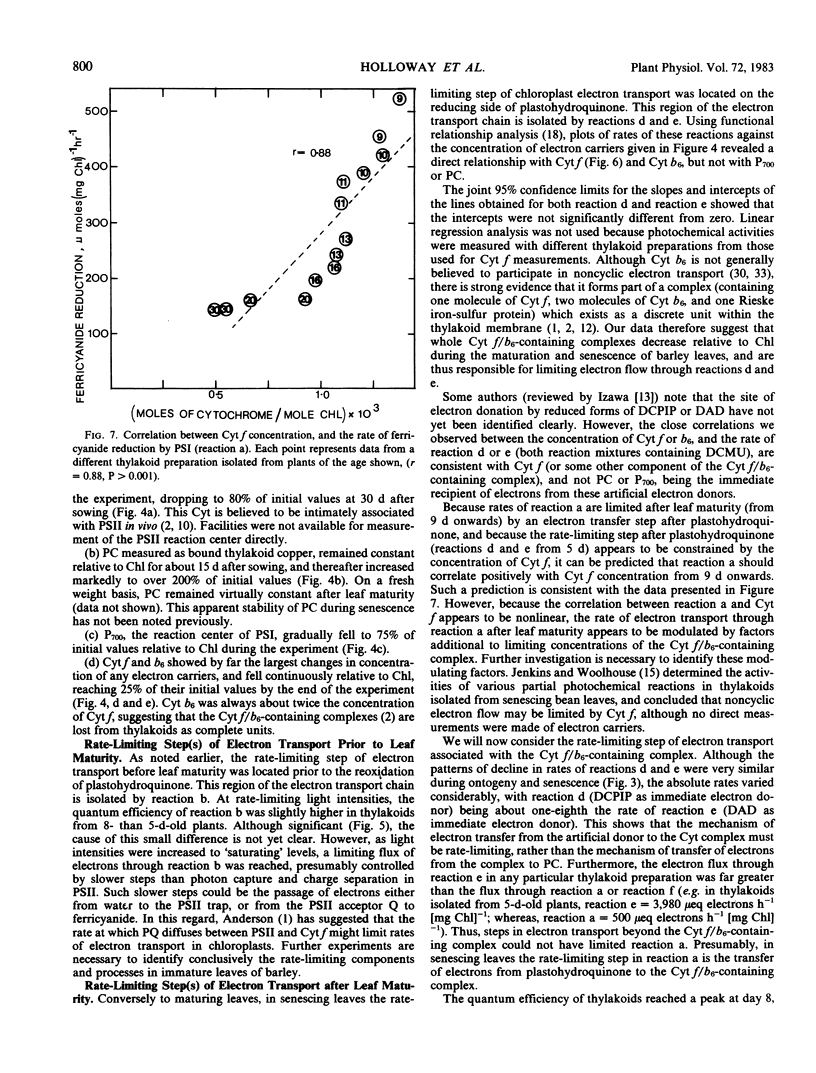
Partial photochemical activities and concentrations of electron carriers were measured relative to chlorophyll in barley (Hordeum vulgare L.) thylakoids, isolated from primary leaves during ontogeny and senescence. Thylakoids from mature leaves generated somewhat higher quantum efficiencies than thylakoids from premature or senescing leaves; this phenomenon did not appear to be caused by any deficiency of water-splitting enzyme. Under conditions of saturating light, the noncyclic electron flux from water to the reducing side of photosystem I increased during leaf ontogeny, peaked at maturity, and declined during senescence. However, electron fluxes appeared to be limited at different steps before and after leaf maturity. Before leaf maturity, the rate-limiting step was located prior to the reoxidation of plastohydroquinone. After leaf maturity, the decline in noncyclic electron flux correlated with a decrease in the concentration of cytochromes f and b6. This correlation, together with a consideration of mechanisms of entry and exit of electrons in 3-(3,4-dichlorophenyl)-1,1-dimethylurea-treated thylakoids, suggests that the cytochrome f/b6-containing complex, and not plastocyanin or P700, is the site of entry of electrons from the reduced forms of 2,6-dichlorophenolindophenol and diaminodurene. It is therefore proposed that in senescing leaves the cytochrome f/b6-containing complex limited electron transport by constraining the rate of reduction of cytochrome f by plastohydroquinone.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUINSMA J. A comment on the spectrophotometric determination of chlorophyll. Biochim Biophys Acta. 1961 Sep 30;52:576–578. doi: 10.1016/0006-3002(61)90418-8. [DOI] [PubMed] [Google Scholar]
- Heber U., Kirk M. R., Boardman N. K. Photoreactions of Cytochrome b-559 and cyclic electron flow in photosystem II of intact chloroplasts. Biochim Biophys Acta. 1979 May 9;546(2):292–306. doi: 10.1016/0005-2728(79)90047-1. [DOI] [PubMed] [Google Scholar]
- Henningsen K. W., Boardman N. K. Development of Photochemical Activity and the Appearance of the High Potential Form of Cytochrome b-559 in Greening Barley Seedlings. Plant Physiol. 1973 Jun;51(6):1117–1126. doi: 10.1104/pp.51.6.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E., Hauska G. A cytochrome f/b6 complex of five polypeptides with plastoquinol-plastocyanin-oxidoreductase activity from spinach chloroplasts. Eur J Biochem. 1981 Jul;117(3):591–595. doi: 10.1111/j.1432-1033.1981.tb06379.x. [DOI] [PubMed] [Google Scholar]
- Melis A., Brown J. S. Stoichiometry of system I and system II reaction centers and of plastoquinone in different photosynthetic membranes. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4712–4716. doi: 10.1073/pnas.77.8.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen N. C., Smillie R. M. The effect of p-phenylenediamine and dibromothymoquinone on the postosynthetic electron transport and proton pump activities of isolated barley chloroplats. Arch Biochem Biophys. 1978 Feb;186(1):52–59. doi: 10.1016/0003-9861(78)90462-9. [DOI] [PubMed] [Google Scholar]
- Plesnicar M., Bendall D. S. The photochemical activities and electron carriers of developing barley leaves. Biochem J. 1973 Nov;136(3):803–812. doi: 10.1042/bj1360803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesnicar M., Bendall D. S. The plastocyanin content of chloroplasts from some higher plants estimated by a sensitive enzymatic assay. Biochim Biophys Acta. 1970 Aug 4;216(1):192–199. doi: 10.1016/0005-2728(70)90170-2. [DOI] [PubMed] [Google Scholar]
- Smillie R. M., Nott R. Assay of chilling injury in wild and domestic tomatoes based on photosystem activity of the chilled leaves. Plant Physiol. 1979 May;63(5):796–801. doi: 10.1104/pp.63.5.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie R. M. Photosynthetic & respiratory activities of growing pea leaves. Plant Physiol. 1962 Nov;37(6):716–721. doi: 10.1104/pp.37.6.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebst A. Measurement of Hill reactions and photoreduction. Methods Enzymol. 1972;24:146–165. doi: 10.1016/0076-6879(72)24065-4. [DOI] [PubMed] [Google Scholar]
- Wood P. M., Bendall D. S. The reduction of plastocyanin by plastoquinol-1 in the presence of chloroplasts. A dark electron transfer reaction involving components between the two photosystems. Eur J Biochem. 1976 Jan 15;61(2):337–344. doi: 10.1111/j.1432-1033.1976.tb10027.x. [DOI] [PubMed] [Google Scholar]


