Abstract
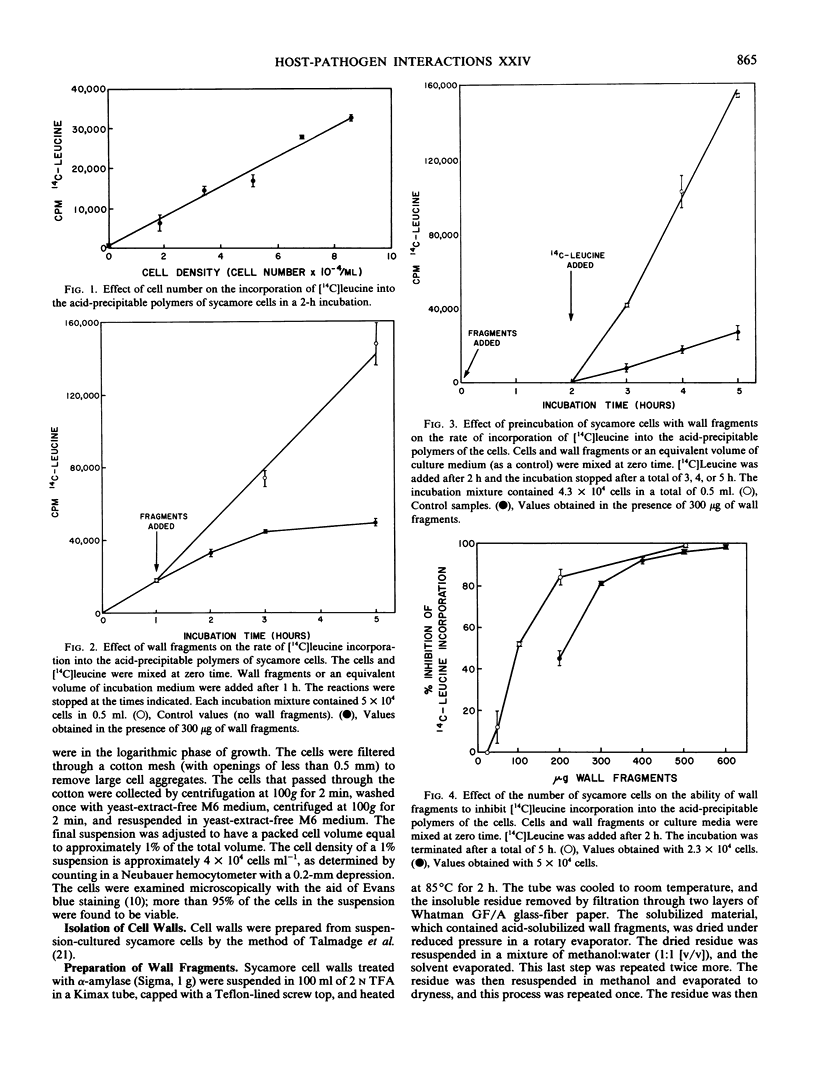
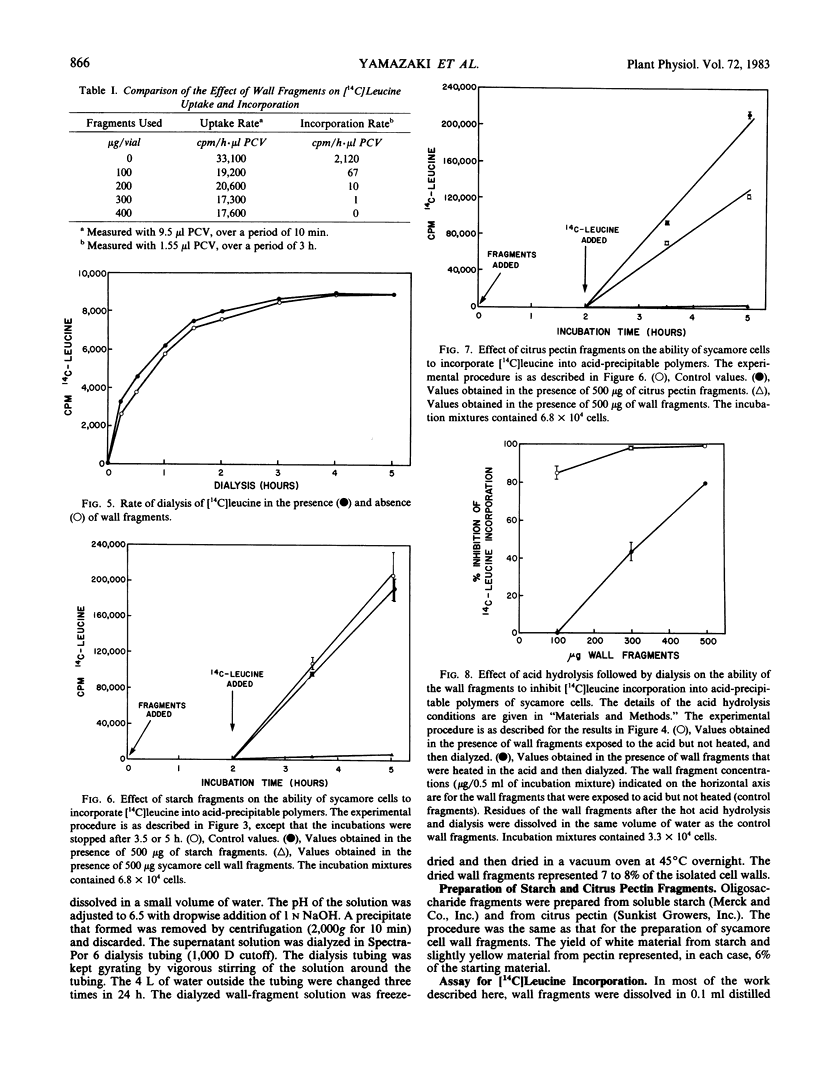
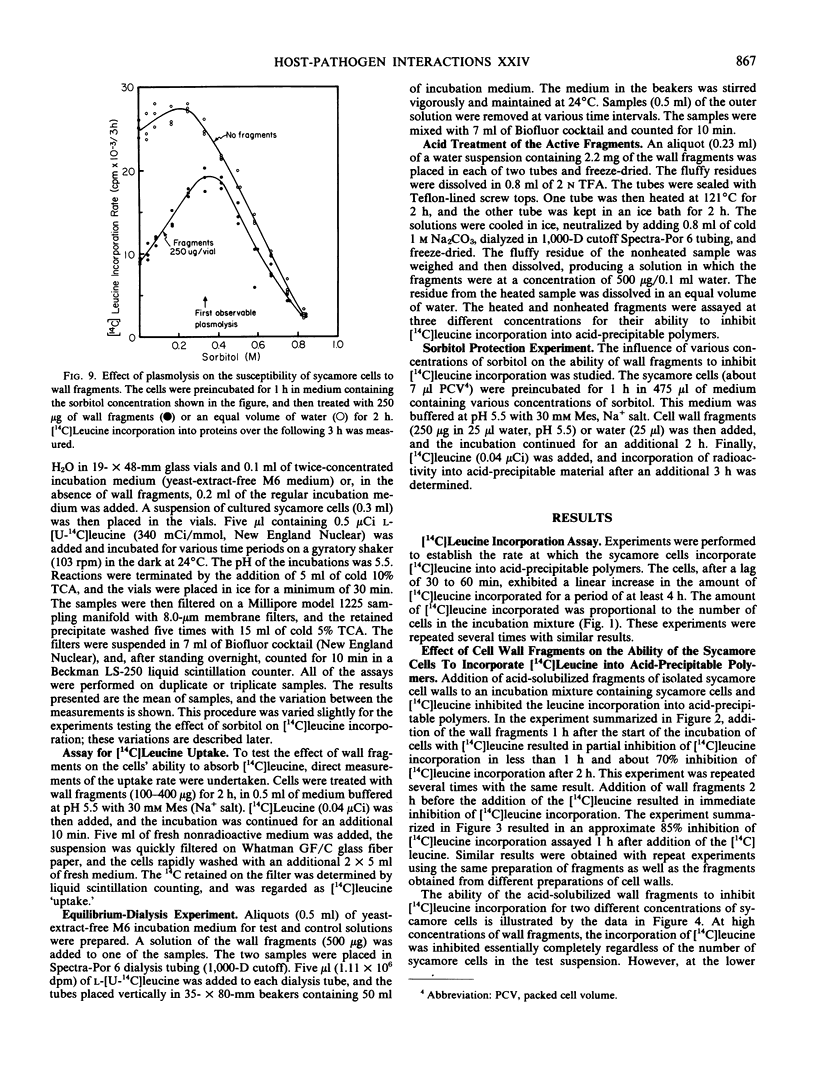
A bioassay to measure the incorporation of [14C]leucine into acid-precipitable polymers of suspension-cultured sycamore (Acer pseudoplatanus L.) cells is described. Using this assay, cell wall fragments solubilized from sycamore cell walls by partial acid hydrolysis are shown to contain components that inhibit the incorporation of [14C]leucine into the acid-precipitable polymers. This inhibition was not attributable to a suppression of [14C]leucine uptake. The effectiveness of the wall fragments in inhibiting [14C]leucine incorporation was substantially relieved by plasmolysis of the cells. Fragments released from starch and citrus pectin are shown not to possess such inhibitory activities.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hahn M. G., Darvill A. G., Albersheim P. Host-Pathogen Interactions : XIX. THE ENDOGENOUS ELICITOR, A FRAGMENT OF A PLANT CELL WALL POLYSACCHARIDE THAT ELICITS PHYTOALEXIN ACCUMULATION IN SOYBEANS. Plant Physiol. 1981 Nov;68(5):1161–1169. doi: 10.1104/pp.68.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMPORT D. T. CELL SUSPENSION CULTURES OF HIGHER PLANTS: ISOLATION AND GROWTH ENERGETICS. Exp Cell Res. 1964 Jan;33:195–206. doi: 10.1016/s0014-4827(64)81026-0. [DOI] [PubMed] [Google Scholar]
- Lee S. C., West C. A. Polygalacturonase from Rhizopus stolonifer, an Elicitor of Casbene Synthetase Activity in Castor Bean (Ricinus communis L.) Seedlings. Plant Physiol. 1981 Apr;67(4):633–639. doi: 10.1104/pp.67.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters B. M., Cribbs D. H., Stelzig D. A. Agglutination of plant protoplasts by fungal cell wall glucans. Science. 1978 Jul 28;201(4353):364–365. doi: 10.1126/science.201.4353.364. [DOI] [PubMed] [Google Scholar]
- Ryan C. A., Bishop P., Pearce G. A sycamore cell wall polysaccharide and a chemically related tomato leaf polysaccharide possess similar proteinase inhibitor-inducing activities. Plant Physiol. 1981 Sep;68(3):616–618. doi: 10.1104/pp.68.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge K. W., Keegstra K., Bauer W. D., Albersheim P. The Structure of Plant Cell Walls: I. The Macromolecular Components of the Walls of Suspension-cultured Sycamore Cells with a Detailed Analysis of the Pectic Polysaccharides. Plant Physiol. 1973 Jan;51(1):158–173. doi: 10.1104/pp.51.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatley M. H., Hunter N., Cantrell M. A., Hendrick C., Keegstra K., Sequeira L. Lipopolysaccharide Composition of the Wilt Pathogen, Pseudomonas solanacearum: CORRELATION WITH THE HYPERSENSITIVE RESPONSE IN TOBACCO. Plant Physiol. 1980 Mar;65(3):557–559. doi: 10.1104/pp.65.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm J. M. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 1972 Jul;47(4):189–194. doi: 10.3109/10520297209116483. [DOI] [PubMed] [Google Scholar]


