Abstract
Introduction
Cryptosporidium, Cystoisospora, and Giardia duodenalis are gastrointestinal protozoa parasites that cause diarrhea in various animals. However, information regarding the detection and phylogenetic characterization of gastrointestinal protozoa parasites in cats is limited throughout South Korea. Therefore, this study aimed to determine the detection and identify subspecies of gastrointestinal protozoa parasites in cats from South Korea.
Methods
A total of 290 fecal samples were collected from stray, companion, and shelter cats in six provinces. Cryptosporidium, Cystoisospora, and G. duodenalis were identified by PCR. All positive samples were subtyped by PCR and sequencing of gp60, ITS-1, tpi, bg, and gdh.
Results
The overall detection of gastrointestinal protozoan parasitic infection was 17.93%. G. duodenalis was the most prevalent, with 7.93%, followed by Cystoisospora spp. (7.24%) and Cryptosporidium spp. (4.48%). In addition, C. felis (n=10), C. parvum (n=2), C. ryanae (n=1), Cystoisospora felis (n=14), Cystoisospora suis (n=5), Cystoisospora ohioensis (n=1), Cystoisospora spp. were identified in subspecies analysis of positive samples. C. felis showed a significant association with diarrhea (7.81%) and living condition (6.04%), and Cystoisospora felis in diarreha (9.38%) according to detection. Through phylogenetic analysis of the tpi, bg, and gdh genes from 23 G. duodenalispositive samples, it was confirmed that the samples of present study belonged to assemblage A, B, C, and D.
Discussion
South Korean cats have a high rate of gastrointestinal protozoan parasites infection with cat-specific Cryptosporidium and Cystoisospora, which are associated with living conditions and diarrhea symptoms. Moreover, zoonotic and other animal-specific subtype of protozoan parasites have been detected in cat feces.
Keywords: Cryptosporidium, Cystoisospora, Giardia duodenalis, gastrointestinal protozoa parasite, cat infection
Background
With the growth of the pet industry in South Korea, interest in animal health, including cats, has increased, and gastrointestinal parasitic infections have received significant attention for the general health of cats (Kwak and Seo, 2020; Moon et al, 2022). The three most prevalent gastrointestinal protozoa parasites in cats are Cryptosporidium, Cystoisospora, and Giardia duodenalis. Cryptosporidium is a protozoan parasite that infects the small intestine and can cause diarrhea, abdominal pain, and fever in cats (de Oliveira et al, 2021; Karimi et al, 2023). This parasite is excreted in the feces of infected cats and can contaminate food and water sources, potentially transmitting to other animals and humans (Current and Haynes, 1984; Feng et al, 2018). Cryptosporidium infections are a concern in immunocompromised individuals, causing severe and life-threatening digestive disease. In Cryptosporidium, Cryptosporidium felis cause a cat-specific infection, and Cryptosporidium parvum, a zoonoses infectious genus, is identified in cats (Zahedi et al, 2016; Feng et al, 2018). Cystoisospora is another protozoan parasite that infects the small intestine of cats and can cause diarrhea, vomiting, and dehydration (Lindsay et al, 1997; Dubey, 2018). While Cystoisospora infections are generally mild and self-limiting, they can be more severe in young or immunocompromised cats (Itoh et al, 2013; Dubey, 2018; Scorza et al, 2021). G. duodenalis is a flagellated protozoan parasite that infects the small intestine and can cause diarrhea, vomiting, and weight loss in cats (Epe et al, 2010; Feng and Xiao, 2011). G. duodenalis infections are particularly concerning as the parasite can persist in the environment and be difficult to eliminate (Janeczko and Griffin, 2010; Maciel and Sabogal-Paz, 2016).
The detection of gastrointestinal protozoa parasites infections in cats in South Korea is not well documented; however, previous studies have suggested that these infections were detected in several location of the country. For example, the identification of 0.6% of Cryptosporidium and 3.8% of Giardia infections have been reported in shelter cats in Jeju (Kwak and Seo, 2020) and 30.7% of Giardia infections in Daejeon (Lee et al, 2022). In addition, Cystoisospora infection has been confirmed microscopically using fecal samples from cats in Daegu (Lee et al, 2019), Suwon (Youn et al, 2012), and around major rivers (Ahn et al, 2019). However, since these previous studies were regionally limited, it is difficult to grasp the pattern of protozoa parasitic infection according to the species and district in domestic cats.
The current status of cats in South Korea is complex, with a large population of stray and feral cats living in urban and rural areas (Lee et al, 2010; Hwang et al, 2016). In addition, cats in abandoned animal shelters encounter various challenges, including overcrowding, lack of resources, and risk of disease transmission (Oh et al., 2021). These gastrointestinal protozoa parasites are a concern for the health of cats as well as have zoonotic potential, meaning they can be transmitted from cats to humans (Zahedi et al, 2016; Dixon, 2021). Therefore, understanding the detection and transmission of these parasites in cats is important for feline health as well as public health. Studying the detection of gastrointestinal protozoa parasites in cats in different environments, including strays, pets, and shelters, can provide important insights into the health status of cats in South Korea and inform efforts to improve their welfare. Furthermore, understanding the zoonotic potential of these parasites can inform public health efforts to reduce the risk of transmission to humans. Therefore, this study aimed to determine the detection and phylogenetic patterns of gastrointestinal protozoa parasites in South Korean cats.
Methods
Sample collection
Between January and December 2022, 290 fecal samples were collected from stray (n=149), companion (n=17), and shelter cats (n=124) in six provinces. The sample collection date, sex, and location were recorded using application documents. Dead companion and stray cat bodies were submitted to the Animal and Plant Quarantine Agency (APQA, South Korea) to determine the cause of death and digestive lesions and diarrhea were confirmed at this time, whereas in the case of shelter cats, fecal samples were secured with the cooperation of animal shelters in each city and province in 2022. All fecal samples were stored at 4°C until further experimentations.
DNA extraction
DNA extraction was performed according to a fecal sample-based adaptation of the Maxwell® RSC PureFood GMO Kit (REF AS1600; Promega Co., Madison, WI, USA) developed by Promega. Briefly, 200–500 mg of fecal samples were placed into a 1.5 mL microcentrifuge tube, 500 mL of CTAB Buffer was added with 35 uL of Proteinase K, and the tubes were heated at 70°C for 30 min and 95°C for 10 min after vortexing. Maxwell® RSC Cartridge preparation and loading on the Maxwell RSC Extraction System (Promega, USA) were performed as described by Promega. All samples were eluted in 100 uL of elution buffer. Extracted DNA was stored at -20°C until further applications.
PCR amplification and molecular identification
Molecular identification of Cryptosporidium, Cystoisospora, and G. duodenalis was performed by extracting DNA with specific target genes from fecal samples using a thermocycler (Takara, Shiga, Japan) ( Table 1 ). All samples were screened using 18S rRNA gene for Cryptosporidium and G. duodenalis, and ITS-1 gene for Cystoisospora as described by previous reports (Power et al, 2011; Pallant et al, 2015; Shafiei et al, 2016). The PCR were performed for the gp60 gene for C. felis (Koseoglu et al, 2022), and tpi, gdh and bg for G. duodenalis (Sulaiman et al, 2003; Lalle et al, 2005; Caccio et al, 2008) to identify their subtypes. A negative template control sample (RNase-free water) was included in each PCR to confirm contamination with the PCR reaction mixture. PCR-positive amplification products were sequenced using forward and reverse primers (Macrogen Inc., Seoul, South Korea). The nucleotide sequences obtained in the present study were aligned and analyzed with reference sequences from GenBank using BioEdit version 7.2.5. The Cryptosporidium and Cystoisospora and the assemblages and sub-assemblages of G. duodenalis were initially identified from the GenBank database (http://blast.ncbi.nlm.nih.gov). The obtained sequences were deposited in GenBank under the accession numbers OQ598555-OQ598563 for gp60 of C. felis, OQ473126, OQ473172-OQ473185, OQ534549 and OQ534551-OQ534555 for ITS-1 of Cystoisospora, OQ442978-OQ442994 for tpi of G. duodenalis, OQ442958-OQ442969 for b-giardin of G. duodenalis, and OQ442970-OQ442977 for gdh of G. duodenalis.
Table 1.
Primer sequences and PCR conditions used for the molecular identification and characterization of Cryptosporidium, Cystoisospora, and Giardia duodenalis.
| Target species | Gene | Primer nucleotide sequences (5'-3') | Size (bp) | Cycling conditions | Ref. |
|---|---|---|---|---|---|
| Cryptosporidium | 18S rRNA | F: AGTGACAAGAAATAACAATACAGG | 295 | 2m/96°C, 40 cycles (30s/94°C, 30s/60°C, 60s/72°C), 7m/72°C | Power et al, 2011 |
| R: CCTGCTTTAAGCACTCTAATTTTC | |||||
| Cryptosporidium felis | gp60 | F1: TTTCCGTTATTGTTGCAGTTGCA | 1,200 | PCR1 and PCR2 : 4m/95°C, 35 cycles (30s/95°C, 30s/55°C, 90s/72°C), 7m/72°C | Koseoglu et al, 2022 |
| R1: ATCGGAATCCCACCATCGAAC | |||||
| F2: GGGCGTTCTGAAGGATGTAA | 900 | ||||
| R2: CGGTGGTCTCCTCAGTCTTC | |||||
| Cystoisospora | ITS-1 | F1: CCGTTGCTCCTACCGATTGAGTG | PCR1 and PCR2 : 60s/94°C, 40 cycles (10s/98°C, 15s/62°C, 60s/68°C), 5m/68°C | Shafiei et al, 2016 | |
| R1: GCATTTCGCTGCGTCCTTCATCG | |||||
| F2: GATCATTCACACGTGGCCCTTG | 450 | ||||
| R2: GACGACGTCCAAATCCACAGAGC | |||||
| Giardia duodenalis | 18S rRNA | F1: CATCCGGTCGATCCTGCC | PCR1 : 15m/95°C, 35 cycles (30s/95°C, 30s/65°C, 60s/72°C), 7m/72°C PCR2 : 15m/95°C, 35 cycles (30s/95°C, 30s/55°C, 60s/72°C), 7m/72°C | Pallant et al, 2015 | |
| R1: GTCGAACCCTGATTCTCCG | |||||
| F2: GACGCTCTCCCCAAGGAC | 170 | ||||
| R2: CTGCGTCACGCTGCTCG | |||||
| tpi | AL3543: AAATIATGCCTGCTCGTCG | 605 | PCR1 and PCR2 : 5m/94°C, 35 cycles (45s/94°C, 45s/50°C, 60s/72°C), 10m/72°C | Sulaiman et al, 2003 | |
| AL3546: CAAACCTTITCCGCAAACC | |||||
| AL3544: CCCTTCATCGGIGGTAACTT | 532 | ||||
| AL3545: GTGGCCACCACICCCGTGCC | |||||
| gdh | Gdh1: TTCCGTRTYCAGTACAACTC | 754 | PCR1 and PCR2 : 60s/94°C, 40 cycles (10s/98°C, 15s/62°C, 60s/68°C), 5m/68°C | Caccio et al, 2008 | |
| Gdh2: ACCTCGTTCTGRGTGGCGCA | |||||
| Gdh3: ATGACYGAGCTYCAGAGGCACGT | 530 | ||||
| Gdh4: GTGGCGCARGGCATGATGCA | |||||
| bg | G7: AAGCCCGACGACCTCACCCGCAGTGC) | 753 | PCR1 : 15m/95°C, 35 cycles (30s/95°C, 30s/65°C, 60s/72°C), 7m/72°C PCR2 : 15m/95°C, 35 cycles (30s/95°C, 30s/55°C, 60s/72°C), 7m/72°C | Lalle et al, 2005 | |
| G759: GAGGCCGCCCTGGATCTTCGAGACGAC | |||||
| GiarF: GAACGAACGAGATCGAGGTCCG | 511 | ||||
| GiarR: CTCGACGAGCTTCGTGTT |
Phylogenetic analysis
Phylogenetic analysis was performed with MEGA X (www.megasoftware.net) using the maximum likelihood method. Phylogenetic tree stability was assessed using a bootstrap value of 1,000 replicates. For multilocus genotyping of G. duodenalis, the DNA sequences of tpi, bg, and gdh loci were concatenated in MEGA X to form the MLG, and the reference sequences were selected according to previous studies (Feng and Xiao, 2011; Wang et al, 2019; Wu et al, 2022).
Statistical analysis
All results are expressed as the percentage of isolates. The frequency of detection of gastrointestinal protozoa parasite isolates from fecal samples was statistically compared using the chi-square test or Fisher’s exact test with a 95% confidence interval, followed by Holm’s post-hoc test. The P-value was calculated, and statistical significance was set at P< 0.05.
Results
Detection of Cryptosporidium, Cystoisospora, and Giardia duodenalis in fecal samples of cats
The detection of each parasite infection according to region, season, sex, fecal state, and living conditions is shown in Table 2 . The detection of Cryptosporidium spp. was 4.48% (13/290; CI 2.24–4.98), with 3.45% of C. felis (10/290; CI 1.52–4.03), 0.69% of C. parvum (2/290; CI 0.23–0.88) and 0.34% of C. ryanae (1/290; CI 0.06–0.49). C. felis showed a statistically significant difference between fecal states and living conditions (P< 0.05). However, no statistically significant differences were observed in the infection of Cryptosporidium by region, season, and gender.
Table 2.
The detection of Cryptosporidium, Cystoisospora and Giardia duodenalis in the stray, companion and shelter cats of South Korea.
| No. of isolates (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cryptosporidium | Cystoisospora | Giardia duodenalis | Total | |||||||
| C. felis | C. parvum | C. ryanae | C. felis | C. suis | C. ohioensis | others | ||||
| Region | Gyeonggi (n=112) | 5 (4.46) | – | – | 3 (2.68) | 1 (0.89) | – | – | 11 (9.82) | 17 (15.18) |
| Gangwon (n=11) | – | – | – | – | – | – | – | 2 (18.18) | 2 (18.18) | |
| Chungcheong (n=43) | 2 (4.65) | – | – | 3 (6.98) | – | – | – | 3 (6.98) | 8 (18.60) | |
| Gyeongsang (n=56) | 3 (5.36) | – | – | 2 (3.57) | 1 (1.79) | – | – | 4 (7.14) | 10 (17.86) | |
| Jeolla (n=38) | – | 2 (5.26) | 1 (2.63) | 3 (7.89) | – | – | 1 (2.63) | 1 (2.63) | 7 (18.42) | |
| Jeju (n=30) | – | – | – | 3 (10.00) | 3 (10.00) | 1 (3.33) | – | 2 (6.67) | 8 (26.67) | |
| Season | Spring (n=94) | 4 (4.26) | 1 (1.06) | – | 1 (1.06) | – | 1 (1.06) | – | 3 (3.19) | 10 (10.64) |
| Summer (n=53) | – | – | 1 (1.89) | 3 (5.66) | 4 (7.55) | – | – | 4 (7.55) | 10 (18.87) | |
| Autumn (n=52) | 2 (3.85) | – | – | 5 (9.62) | 1 (1.92) | – | – | 6 (11.54) | 13 (25.00) | |
| Winter (n=91) | 4 (4.40) | 1 (1.10) | – | 5 (5.50) | – | – | 1 (1.10) | 10 (10.99) | 19 (20.88) | |
| Gender | male (n=124) | 7 (5.65) | 1 (0.81) | – | 4 (3.23) | 2 (1.61) | – | – | 12 (9.68) | 24 (19.35) |
| female (n=120) | 3 (2.50) | 1 (0.83) | 1 (0.83) | 7 (5.83) | 1 (0.83) | 1 (0.83) | – | 6 (5.00) | 19 (15.83) | |
| unknown (n=46) | – | – | – | 3 (6.52) | 2 (4.35) | – | 1 (2.17) | 5 (10.87) | 9 (19.57) | |
| Fecal states | Diarrhea (n=64) | 5 (7.81)* | – | – | 6 (9.38)* | 2 (3.13) | – | – | 3 (4.69) | 15 (23.44) |
| Noramal (n=226) | 5 (2.21) | 2 (0.88) | 1 (0.44) | 8 (3.54) | 3 (1.33) | 1 (0.44) | 1 (0.44) | 20 (8.85) | 37 (16.37) | |
| Living condition | Stray (n=149) | 9 (6.04)* | 1 (0.67) | – | 7 (4.70) | 2 (1.34) | – | – | 13 (8.72) | 29 (19.46) |
| Companion (n=17) | – | 1 (5.88) | – | – | – | – | – | 2 (11.76) | 3 (17.65) | |
| Shelter (n=124) | 1 (0.81) | – | 1 (0.81) | 7 (5.65) | 3 (2.42) | 1 (0.81) | 1 (0.81) | 8 (6.45) | 20 (16.13) | |
| Total | Total (n=290) | 10 (3.45) | 2 (0.69) | 1 (0.34) | 14 (4.83) | 5 (1.72) | 1 (0.34) | 1 (0.34) | 23 (7.93) | 52 (17.93) |
| 13 (4.48) | 21 (7.24) | |||||||||
*significant difference (P < 0.05) in parasite prevalence within comparison criteria.
The detection of Cystoisospora spp. was 7.24% (21/290; 95% CI 4.16–7.50), including 4.83% of Cystoisospora felis (14/290; 95% CI 2.77–5.00), 1.72% of Cystoisospora suis (5/290; 95% CI 0.79–1.98), 0.34% of Cystoisospora ohioensis (1/290; 95% CI 0.06–0.49) and 0.34% of Cystoisospora spp. (1/290; 95% CI 0.07–0.49). The detection of Cystoisospora infection was not statistically related to region, season, gender, or living conditions. Cystoisospora felis infection was affected by fecal state (P< 0.05), as diarrhea (6/64, 9.39%) was higher than normal (8/226, 3.54%) feces.
The detection of G. duodenalis was 7.93% (23/290; 95% CI 4.06–8.72). G. duodenalis was detected in all regions, seasons, gender, fecal states, and living conditions, although no statistical differences were observed in the detection of G. duodenalis infection.
Collectively, the results revealed gastrointestinal protozoa parasite infection was confirmed in 52 out of 290 (17.93%; 95% CI 10.24–18.65) fecal samples of cats. Among them, multiple protozoa parasites were detected in five fecal samples: three were co-infected with Cystoisospora felis and G. duodenalis, one with C. felis and G. duodenalis, and one with Cystoisospora felis and C. parvum (data not shown).
Cryptosporidium felis genotype in fecal samples of cats
The isolates of C. felis identified using the 18S rRNA gene were sequenced using the gp60 gene for the subtype of C. felis. Based on sequence analysis of the gp60 gene, nine of the 10 isolates were successfully sequenced, while one isolate was non-typable. Four of the sequenced isolates had high homology (98.93 to 99.46%) with the GenBank sequence accession number MH240847. Two isolates had 99.43 to 99.62% homology with MW351825, and the other two isolates had 98.17 to 98.73% homology with MH240865. One isolate showed 97.71% homology to MH240868. Phylogenetic analysis of C. felis using gp60 revealed that all nine isolates in the present study clustered with C. felis subtype XIXa ( Figure 1 ).
Figure 1.
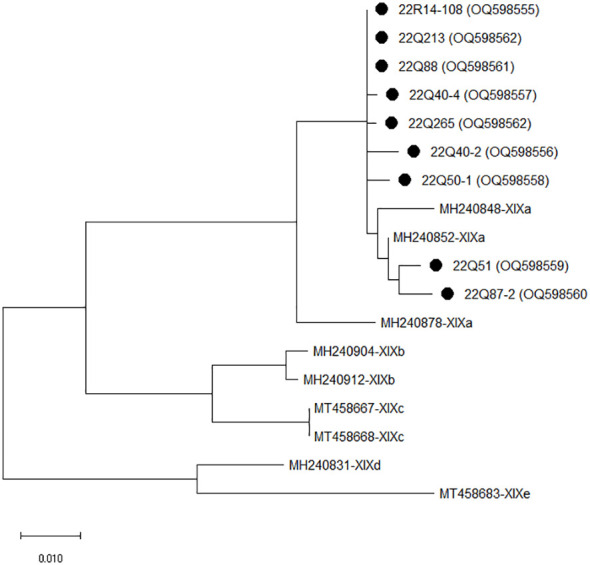
The phylogenetic analysis of Cryptosporidium felis based on the gp60 gene. The phylogenetic tree was constructed using the maximum likelihood method with 1,000 replicates of bootstrap value based on the nucleotide sequence of the gp60 gene of C. felis isolated from stray (Q) and shelter (R) cats. The nucleotide sequences isolated in this study were compared with nucleotide sequences of C. felis retrieved from GenBank. Evolutionary analyses were conducted in MEGA X.
Phylogenetic analysis of Cystoisospora in fecal samples of cats
Sequencing and phylogenetic analyses of the amplification products of ITS-1 in Cystoisospora are shown in Figure 2 . The comparative analysis of 14 Cystoisospora felis with the GenBank sequence revealed 99.68 to 100% homology with KP411388. Five Cystoisospora suis and one Cystoisospora ohioensis isolates showed 90.13% and 90.61% homology with OM870399 and GU292307, respectively. One isolate identified as Cystoisospora spp. showed 99.67% homology with MN556343 isolated from tigers and 86.51% with the KP411388 of Cystoisospora felis.
Figure 2.
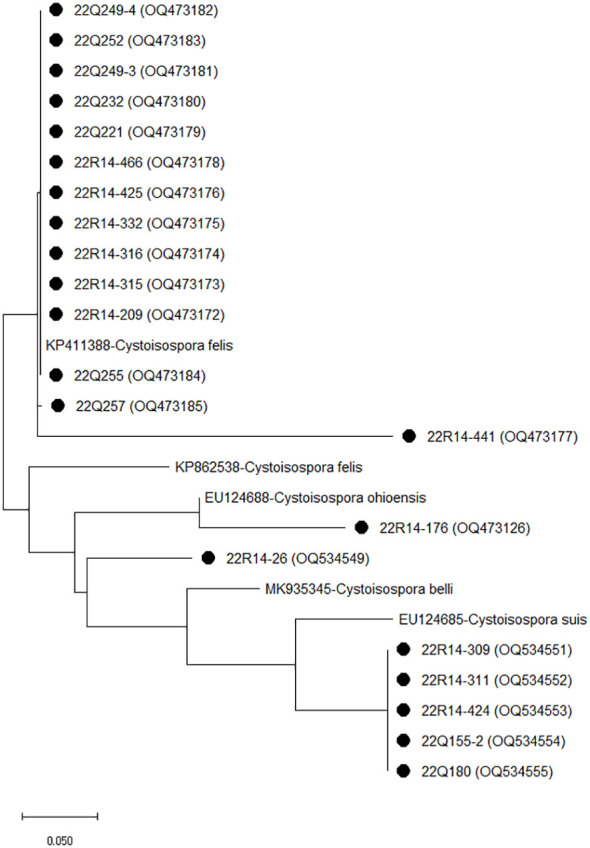
The phylogenetic analysis of Cystoisospora based on the ITS-1 gene. The phylogenetic tree was constructed using the maximum likelihood method with 1,000 replicates of bootstrap value based on the nucleotide sequence of the ITS-1 gene of Cystoisospora isolated from stray (Q) and shelter (R) cats. The nucleotide sequences isolated in this study were compared with nucleotide sequences of Cystoisospora retrieved from GenBank. Evolutionary analyses were conducted in MEGA X.
Giardia duodenalis assemblages and genotypes in fecal samples of cats
Sequence analysis of the tpi, bg, and gdh loci revealed an assemblage of G. duodenalis ( Table 3 ). By sequencing analysis, assemblage A4 (n=4), A5 (n=2), B (n=7), and C (n=4) were identified at the tpi locus, and assemblage A (n=2), B5 (n=6), C (n=1), and D (n=3) at the bg locus, and assemblage A (n=1), A2 (n=1), B (n=5), and D (n=1) at the gdh locus. Among the 23 G. duodenalis-positive fecal samples, five were amplified at all tpi, bg, and gdh loci, and concatenated nucleotide sequences were used for MLG ( Figure 3 ). The isolates from stray cats were classified as AI-2 and B18. In companions, one isolate of G. duodenalis was classified as assemblage AI-1, two isolates from shelter cats were classified as assemblage B, and the other as assemblage D.
Table 3.
Multilocus genotypes of tpi, bg, and gdh genes for Giardia duodenalis positive isolates.
| Living condition | Prevalence | Giardia duodenalis | |||
|---|---|---|---|---|---|
| tpi | bg | gdh | MLG | ||
| Stray | 13/149 (8.72 %) | A5 | A | A2 | AI-2 |
| B | B5 | B | B18 | ||
| B | B5 | ||||
| A4 | |||||
| A4 | |||||
| A5 | |||||
| B | |||||
| C | |||||
| B5 | B | ||||
| C | |||||
| D | |||||
| D | |||||
| B | |||||
| Companion | 2/17 (11.76 %) | A4 | A | A | AI-2 |
| A4 | |||||
| Shelter | 8/124 (6.45 %) | B | B5 | B | B |
| C | D | D | D | ||
| B | |||||
| B | |||||
| B | B5 | ||||
| C | |||||
| C | |||||
| B5 | B | ||||
Figure 3.
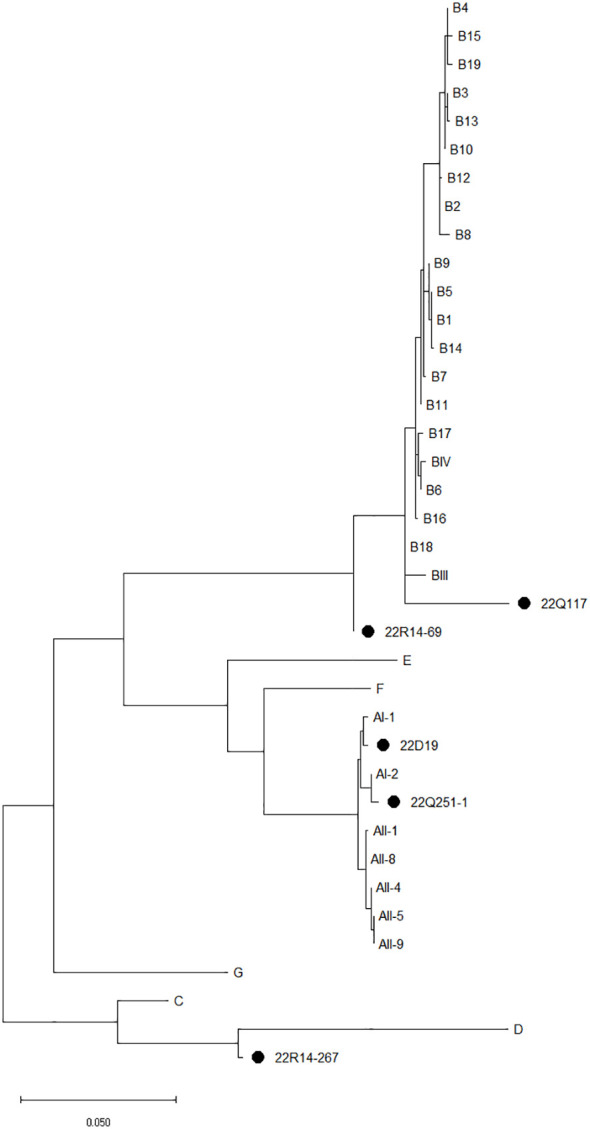
The phylogenetic analysis of Giardia duodenalis based on the concatenated multilocus genotypes MLG using tpi, bg, and gdh genes. The phylogenetic tree was constructed using the maximum likelihood method with 1,000 replicates of bootstrap value based on the nucleotide sequence of tpi, bg, and gdh genes, and MLG of G. duodenalis isolated from stray (Q), companion (D), and shelter (R) cats. The nucleotide sequences isolated in this study were compared with those of G. duodenalis retrieved from GenBank. Reference sequences for MLG were selected according to previous studies (Feng and Xiao, 2011; Wang et al, 2019; Wu et al, 2022). Evolutionary analyses were conducted in MEGA X.
Discussion
In the present study, molecular analysis was conducted to identify the detection of infection and the species, and subtypes of Cryptosporidium, Cystoisospora, and Giardia duodenalis of cats in South Korea. Moreover, differences according to the provinces of South Korea, seasons, gender, diarrheal symptoms, and living conditions were analyzed.
With the growing pet industry, the increasing number of cats raised by people, and the large population of stray and abandoned cats in South Korea, concerns about zoonotic parasite infection in cats have been highlighted (Cho et al, 2018). Cryptosporidium, Cystoisospora, and Giarida are the most important gastrointestinal protozoa parasites that infect humans, livestock, and wild animals, which have zoonotic and zooanthroponotic characteristics, and can be transmitted between humans and animals, causing enteric disorders such as diarrhea (Appelbee et al, 2005; Dubey, 2018). Stray cats have an increased risk of transmitting pathogens through contact with humans or infected excrement while roaming the streets (Kwak and Seo, 2020; Mendoza and Otranto, 2023). In shelters, cats raised by humans were included, but kittens and injured cats were also admitted (Cho et al, 2020). At this time, there is a possibility of cross-infection by pathogens in co-breeding environments. These three gastrointestinal protozoa parasites show asymptomatic or weak clinical symptoms in adults; however, they cause acute diarrhea in kitten and young-age cat and can lead to death in severe cases (Tzannes et al., 2008; Epe et al., 2010; Certad et al, 2017). Therefore, considering the risk of infection of humans and young-age cats, it is important to analyze the infection status and characteristics of these protozoa parasites
Cryptosporidium has a wide host range, including humans and mammals, and C. felis is a cat-specific species that cause diarrhea in cats (Appelbee et al, 2005; Certad et al, 2017). In this study, C. felis infection was the most frequent at 3.45% of the total 4.48% Cryptosporidium infection. This is consistent with the global Cryptosporidium infection rate of 6.0% on average and 3– 9% in Asian countries for cats (Meng et al., 2021; Taghipour et al, 2021). This was higher than the infection rate of C. felis recently reported in Jeju, South Korea at 0.6% (Kwak and Seo, 2020), China at 2.3 to 5.02% (Li et al., 2019; Li et al, 2019) and Turkey at 0.0% (Onder et al, 2021). However, the infection rate of C. felis is lower than that in Denmark at 6.7% (Rojas-Lopez et al, 2020) and Brazil at 5.4% (de Oliveira et al, 2021). The detection of Cystoisospora had an infection rate of 7.24% overall, with a relatively high infection rate of Cystoisospora felis at 4.83%. This was higher than the recently reported Cystoisospora felis infection rates of 0.53–0.73% in cats in South Korea (Ahn et al, 2019; Lee et al, 2019). Eight cases (8,7%) of Cystoisospora infection were previously confirmed by microscopic evaluation in hospitalized and stray cats in South Korea (Youn et al, 2012), but the sampling area was limited to certain areas. Cystoisospora felis shows cat-specific infectious properties and has a 12.8% (0.5–76.0%) infection rate in cats worldwide (Lindsay et al, 1997). The present study showed a higher rate of Cryptosporidium and Cystoisospora infections than previous studies in South Korea, but this varies depending on the region where the survey and sample were obtained. A gp60 subtyping has been used to analyze the genetic characteristics of C. felis (Rojas-Lopez et al, 2020). In the present study, all isolated C. felis strains belonged to the XIXa subtype family. The seven C. felis in this study showed a high homology rate of 97.71–99.46% with previous studies isolated from humans in the UK (Jiang et al, 2020). The other two isolates from stray cats showed a 99.43–99.62% homology with those isolated from cats in China (Li et al., 2021). In addition, all 14 Cystoisospora felis isolates in this study showed 99.68–100% homology with those from cats in the United States (Dubey et al., 2015). As exchanges between countries and civilian travel have become more active, the possibility of protozoa parasitic infection through contact with infected or contaminated humans, animals, water, food, and the environment in other countries has continuously increased (Certad et al, 2017; Kostopoulou et al, 2017). Since the limited information of C. felis and Cystoisospora felis reported in South Korea, it is unclear whether the C. felis and Cystoisospora felis in this study were introduced from other countries or originally present in South Korea; thus, continuous observation and further research are required.
In this study, one case each of C. parvum infection was detected in stray and companion cats. Cats can be infected with zoonotic C. parvum, which can be transmitted to humans and mammals through fecal-oral transmission (Appelbee et al, 2005; Certad et al, 2017; Mendoza and Otranto, 2023). Recently, several cases of C. parvum infections in cats have been reported (Li et al., 2019; Tangtrongsup et al, 2020; Taghipour et al, 2021), and the possibility of C. parvum infection in cats is expected as well as a carrier that spreads to other animal species in South Korea. The present study showed that one, five, and one cases of C. ryanae, Cystoisospora suis, and Cystoisospora ohioensis, respectively, were detected in cats. C. ryanae causes cattle-specific infections (Zahedi et al, 2016). Cystoisospora suis and Cystoisospora ohioensis are associated with infections in pigs and dog (Lindsay et al., 1997). Moreover, the one Cystoisospora spp. isolate showed high homology (99.67%) with MN556343 isolated from tigers in China, which is associated with Cystoisospora suis and Cystoisospora belli (Chiu et al, 2021). The route of inflow or infection of other animal-specific Cryptosporidium and Cystoisospora subspecies in cats remains unclear. Information on Cystoisospora suis and Cystoisospora ohioensis infections in cats is lacking, but one case of cat infection with C. ryanae has been reported (Yang et al., 2015). However, some animals, including mice, act as paratenic or reservoir hosts (Dubey, 2018). A previous study showed that Cystoisospora ohioensis can remain infectious in mice and be transmitted to other animals (Dubey and Mehlhorn, 1978). Cats are the top predators of small animals, suggesting the possibility of parasite transmission (Mendoza and Otranto, 2023). In a previous study, Cryptosporidium muris, which causes rodent-specific infections, was detected in cats that probably ate infected rodents (FitzGerald et al., 2011; Yang et al., 2015). Therefore, although it is uncertain whether the cat is a primary or paratenic host for C. ryanae, Cystoisospora suis, and Cystoisospora ohioensis, it is possible that other animal-specific parasites may be detected in cats by contact with the animal feces, contaminated water near livestock farms, or ingested prey infected with C. ryanae and Cystoisospora spp as reservoir host. This study showed that rare cases of C. ryanae, Cystoisospora suis and Cystoisospora ohioensis were detected in cats, but further research is needed to determine whether cats act as reservoir host without infection or primary/paratenic host with clinical symptoms.
G. duodenalis occurred most frequently among the three parasites, at 7.93%, although there were no significant differences according to region, season, gender, diarrhea, or living conditions. The information on G. duodenalis infection in cats is limited, and it has been reported only in certain regions of South Korea: with 3.8% in Jeju (Kwak and Seo, 2020) and 30.7% in Daejeon (Li et al, 2019). The G. duodenalis infection rate in this study was higher than 2.3% in the United States (Bouzid et al, 2015), 7% in Denmark (Enemark et al, 2020), 1.4–3.6% in China (Li et al, 2019; Li et al, 2019), and 1.5% in Iran (Karimi et al., 2023). However, it was lower than Brazil at 9.0% (de Oliveira et al, 2021), Australia at 10.1% (Yang et al, 2015), and Turkey at 8.0% (Onder et al, 2021). Giardia is the common enteric parasite that causes digestive problems, and infections are frequently confirmed in cats in developed countries (Feng and Xiao, 2011; Pallant et al, 2015). In this study, G. duodenalis was the most frequently detected gastrointestinal protozoa parasite, indicating that G. duodenalis infections are prevalent in South Korea. Assemblage analysis of G. duodenalis using three loci showed infections in assemblages A, B, C, and D. Assemblages AI-2, B18, and D were identified by MLG analysis. G. duodenalis has a wide host range compared to many other mammalian species. Each assemblage shows a specific host, and cats have been reported to have zoonotic infections of assemblages A and B, dog-specific assemblages C and D, and cat-specific assemblage F (Appelbee et al, 2005; Feng and Xiao, 2011; Capewell et al, 2021). In a recent study, assemblage F was identified in Jeju, South Korea (Kwak and Seo, 2020). In contrast, assemblages A and B, which are zoonotic subtypes in cats, have been reported in other countries (Li et al., 2019; Li et al., 2019; Enemark et al, 2020; Onder et al, 2021; Procesi et al, 2022). Giardia is host-adapted in most species and has a high infection rate (Appelbee et al, 2005; Feng and Xiao, 2011; Wang et al, 2019);. In addition, cats raised by humans have the potential to transmit Giardia through contact with people and sharing their environment (Ballweber et al, 2010; Yang et al., 2015; Dixon, 2021). Thus the infections of G. duodenalis assemblages A and B in this study are likely to be transmitted to humans. The detection of assemblages C and D (Tangtrongsup et al, 2020) and assemblage D (Palmer et al, 2008; Jaros et al, 2011) in cats has been reported in previous studies. Other animal infections with dog-specific assemblages C and D remain unclear. However, the rare detection in cats, including in this study, indicates the possibility of infection or carriers. Overall, G. duodenalis infection in cats is prevalent in South Korea, and it is suggested that cats were infected with the zoonotic type by sharing living environments with humans or other animals.
In this study, C. felis and Cystoisospora felis were significantly associated with diarrhea. Although it does not cause clinical symptoms in paratenic hosts, it has been reported in previous studies on digestive diseases in cats following infection with Cryptosporidium (Lindsay and Zajac, 2004; Rambozzi et al, 2007), C. felis (Beser et al, 2015) and Cystoisospora felis (Dubey, 2018). However, previous studies have shown no association between the pathological symptoms in the infection of C. felis and Cystoisospora felis (Ballweber et al, 2010; Lucio-Forster et al, 2010; Dubey et al, 2015; Ito et al, 2017; Li et al, 2019; Meng et al, 2021). Therefore, although an association between C. felis and Cystoisospora felis infection in cats and diarrhea symptoms was identified in this study, continuous observation with more samples is required. According to the results of the present study, significant differences in infection by living conditions were observed for C. felis. Moreover, despite no significant differences, Cryptosporidium and Cystoisospora infections in companion cats were not identified as subspecies infections other than C. parvum. The housing environment of companion animals contributes to the prevalence of parasitic infection (Appelbee et al, 2005). Previous studies have reported a higher rate of C. felis infection in stray and shelter cats than in companion cats, although companions also show parasitic infection (Itoh et al, 2013; Li et al, 2019; Li et al, 2021; Taghipour et al, 2021; Karimi et al, 2023). The present results showed that the detection of protozoa parasites varies depending on the habitat of cats and that the low rate of companion cat infection with Cryptosporidium and Cystoisospora is consistent with the breeding style of companion cats with less outside access in South Korea (Kim et al, 2018). However, the number of fecal samples of companions is very limited compared to the number of samples from stray and shelter cats, and further research through more fecal samples from companion cats is required to identify accurate infection rates. In contrast, the overall detection of G. duodenalis in this study was identified in all categories of regions, seasons, gender, fecal state, and living conditions. In previous studies, although the infection rate of G. duodenalis was significantly higher in females in Daejeon (Lee et al, 2022), there were no significant differences in infection rates by region, season, gender, diarrhea, age, or living conditions in South Korea (Kwak and Seo, 2020), China (Li et al, 2019; Li et al, 2019), Iran (Karimi et al, 2023), Greece (Kostopoulou et al, 2017), and Italy (Procesi et al, 2022). G. duodenalis usually exhibits asymptomatic infections (Lucio-Forster et al, 2010; Certad et al, 2017; Lee et al, 2019). Despite the relatively high detection rate of G. duodenalis in cats, this study did not show an association between the infection rate and analytical condition (region, season, gender, fecal states, and living condition), which is consistent with the results of previous studies. The detection rate of G. duodenalis was relatively high in companion cats as well as overall infection, and the possibility that it can have clinical symptoms of co-infection with other pathogens as well as G. duodenalis itself was suggested in previous studies (Lee et al, 2016; Tangtrongsup et al, 2020). However, it is clear that the number of fecal samples from companion cats secured in this study is small, continuous diagnosis and research of cats will be required.
Conclusion
The results of the present study showed the detection according to region, gender, diarrhea symptoms, and living conditions of Cryptosporidium, Cystoisospora, and G. duodenalis, which are gastrointestinal protozoa parasites from all provinces of South Korea. Cat-specific C. felis and Cystoisospora felis were identified most frequently and were associated with living conditions and diarrhea symptoms caused by infection. Moreover, C. parvum and G. duodenalis assemblages A and B, which are zoonotic subspecies, were detected, suggesting that transmission between humans and cats is possible through environmental sharing. Unexpectedly, other species-specific C. ryanae, Cystoisospora ohioensis, and G. duodenalis assemblages C and D were detected, and further research on cat infections caused by these subspecies is required.
Data availability statement
Data presented in this study are available upon request from the corresponding author. Representative DNA sequences from the present study were deposited in the GenBank database under the accession numbers OQ598555-OQ598563 for gp60 of C. felis, OQ473126, OQ473172-OQ473185, OQ534549, and OQ534551-OQ534555 for ITS-1 of Cystoisospora, OQ442978-OQ442994 for tpi of G. duodenalis, OQ442958-OQ442969 for b-giardin of G. duodenalis, and OQ442970-OQ442977 for gdh of G. duodenalis.
Ethics statement
The animal studies were approved by Animal and Plant Quarantine Agency. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
CY: Data curation, Investigation, Methodology, Software, Writing – original draft, Formal Analysis. B-YM: Conceptualization, Writing – review & editing. KL: Conceptualization, Writing – review & editing. SK: Investigation, Writing – review & editing. B-KK: Funding acquisition, Writing – review & editing. M-HH: Formal Analysis, Writing – review & editing, Data curation.
Acknowledgments
We would like to thank the owners of the affected cats for their cooperation.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the program (B-1543069-2023-24-01) of the Animal and Plant Quarantine Agency (APQA) and the Ministry of Agriculture, Food, and Rural Affairs (MARFA).
Abbreviations
PCR, polymerase chain reaction; 18S rRNA,18S ribosomal RNA; gp60,60-kDa glycoprotein; ITS-1, internal transcribed spacer 1; tpi, triosephosphate isomerase; bg, b-giardin; gdh, glutamate dehydrogenase; MLG, multilocus genotyping; CI, confidence interval.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Ahn K. S., Ahn A. J., Park S. I., Sohn W. M., Shim J. H., Shin S. S. (2019). Excretion of Toxoplasma gondii oocysts from Feral Cats in Korea. Korean J. Parasitol. 57, 665–670. doi: 10.3347/kjp.2019.57.6.665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbee A. J., Thompson R. C., Olson M. E. (2005). Giardia and Cryptosporidium in mammalian wildlife–current status and future needs. Trends Parasitol. 21, 370–376. doi: 10.1016/j.pt.2005.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballweber L. R., Xiao L., Bowman D. D., Kahn G., Cama V. A. (2010). Giardiasis in dogs and cats: update on epidemiology and public health significance. Trends Parasitol. 26, 180–189. doi: 10.1016/j.pt.2010.02.005 [DOI] [PubMed] [Google Scholar]
- Beser J., Toresson L., Eitrem R., Troell K., Winiecka-Krusnell J., Lebbad M. (2015). Possible zoonotic transmission of Cryptosporidium felis in a household. Infect. Ecol. Epidemiol. 5, 28463. doi: 10.3402/iee.v5.28463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzid M., Halai K., Jeffreys D., Hunter P. R. (2015). The prevalence of Giardia infection in dogs and cats, a systematic review and meta-analysis of prevalence studies from stool samples. Vet. Parasitol. 207, 181–202. doi: 10.1016/j.vetpar.2014.12.011 [DOI] [PubMed] [Google Scholar]
- Caccio S. M., Beck R., Lalle M., Marinculic A., Pozio E. (2008). Multilocus genotyping of Giardia duodenalis reveals striking differences between assemblages A and B. Int. J. Parasitol. 38, 1523–1531. doi: 10.1016/j.ijpara.2008.04.008 [DOI] [PubMed] [Google Scholar]
- Capewell P., Krumrie S., Katzer F., Alexander C. L., Weir W. (2021). Molecular epidemiology of Giardia infections in the genomic era. Trends Parasitol. 37, 142–153. doi: 10.1016/j.pt.2020.09.013 [DOI] [PubMed] [Google Scholar]
- Certad G., Viscogliosi E., Chabé M., Cacciò S. M. (2017). Pathogenic mechanisms of cryptosporidium and giardia. Trends Parasitol. 33, 561–576. doi: 10.1016/j.pt.2017.02.006 [DOI] [PubMed] [Google Scholar]
- Chiu H. C., Fan K., Sun X., Lin K., Chen T., Yang F., et al. (2021). Detection and molecular characterisation of intestinal parasites in the South China tiger Panthera tigris amoyensis (Hilzheimer). Folia Parasitol. 68, 1–5. doi: 10.14411/fp.2021.029 [DOI] [PubMed] [Google Scholar]
- Cho J., Seo G., Kim H., Kim W., Ji I. (2018). The estimation of current and future market size of pet related industries. Korean J. Agric. Manag Polic. 45, 611–629. doi: 10.30805/KJAMP.2018.45.3.611 [DOI] [Google Scholar]
- Cho Y., Kim K., Kim M. S., Lee I. (2020). Application of a high-quality, high-volume trap–neuter–return model of community cats in Seoul, Korea. PeerJ. 8, e8711. doi: 10.7717/peerj.8711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Current W. L., Haynes T. B. (1984). Complete development of Cryptosporidium in cell culture. Science. 224, 603–605. doi: 10.1126/science.6710159 [DOI] [PubMed] [Google Scholar]
- de Oliveira A. G. L., Sudré A. P., Bergamo do Bomfim T. C., Santos H. L. C. (2021). Molecular characterization of Cryptosporidium spp. in dogs and cats in the city of Rio de Janeiro, Brazil, reveals potentially zoonotic species and genotype. PloS One 16, e0255087. doi: 10.1371/journal.pone.0255087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon B. R. (2021). Giardia duodenalis in humans and animals–transmission and disease. Res. Vet. Sci. 135, 283–289. doi: 10.1016/j.rvsc.2020.09.034 [DOI] [PubMed] [Google Scholar]
- Dubey J. P. (2018). A review of Cystoisospora felis and C. rivolta-induced coccidiosis in cats. Vet. Parasitol. 263, 34–48. doi: 10.1016/j.vetpar.2018.09.016 [DOI] [PubMed] [Google Scholar]
- Dubey J. P., Houk A. E., Verma S. K., Calero-Bernal R., Humphreys J. G., Lindsay D. S. (2015). Experimental transmission of Cystoisospora felis-like coccidium from bobcat (Lynx rufus) to the domestic cat (Felis catus). Vet. Parasitol. 211, 35–39. doi: 10.1016/j.vetpar.2015.03.027 [DOI] [PubMed] [Google Scholar]
- Dubey J. P., Mehlhorn H. (1978). Extraintestinal stages of Isospora ohioensis from dogs in mice. J. Parasitol. 64, 689–695. doi: 10.2307/3279961 [DOI] [PubMed] [Google Scholar]
- Enemark H. L., Starostka T. P., Larsen B., Takeuchi-Storm N., Thamsborg S. M. (2020). Giardia and Cryptosporidium infections in Danish cats: risk factors and zoonotic potential. Parasitol. Res. 119, 2275–2286. doi: 10.1007/s00436-020-06715-2 [DOI] [PubMed] [Google Scholar]
- Epe C., Rehkter G., Schnieder T., Lorentzen L., Kreienbrock L. (2010). Giardia in symptomatic dogs and cats in Europe—results of a European study. Vet. Parasitol. 173, 32–38. doi: 10.1016/j.vetpar.2010.06.015 [DOI] [PubMed] [Google Scholar]
- Feng Y., UM R., Xiao L. (2018). Genetic diversity and population structure of Cryptosporidium. Trends Parasitol. 34, 997–1011. doi: 10.1016/j.pt.2018.07.009 [DOI] [PubMed] [Google Scholar]
- Feng Y., Xiao L. (2011). Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 24, 110–140. doi: 10.1128/CMR.00033-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald L., Bennett M., Ng J., Nicholls P., James F., Elliot A., et al. (2011). Morphological and molecular characterisation of a mixed Cryptosporidium muris/Cryptosporidium felis infection in a cat. Vet. Parasitol. 175, 160–164. doi: 10.1016/j.vetpar.2010.10.003 [DOI] [PubMed] [Google Scholar]
- Hwang J., Gottdenker N., MS M., Lee H., Chun M. S. (2016). Evaluation of biochemical and haematological parameters and prevalence of selected pathogens in feral cats from urban and rural habitats in South Korea. J. Feline Med. Surg. 18, 443–451. doi: 10.1177/1098612X15587572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Itoh N., Iijima Y., Kimura Y. (2017). Molecular prevalence of Cryptosporidium species among household cats and pet shop kittens in Japan. J. Feline Med. Surg. Open Rep. 3, 2055116917730719. doi: 10.1177/2055116917730719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Ito Y., Kato A., Kanai K., Chikazawa S., Hori Y., et al. (2013). Prevalence of intestinal parasites in pet shop kittens in Japan. J. Feline Med. Surg. 15, 908–910. doi: 10.1177/1098612X13487362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeczko S., Griffin B. (2010). Giardia infection in cats. Compend Contin Educ. Vet. 32, E1. [PubMed] [Google Scholar]
- Jaros D., Zygner W., Jaros S., Wedrychowicz H. (2011). Detection of Giardia intestinalis assemblages A, B and D in domestic cats from Warsaw, Poland. Pol. J. Microbiol. 60, 259–263. doi: 10.33073/pjm-2011-036 [DOI] [PubMed] [Google Scholar]
- Jiang W., Roellig D. M., Lebbad M., Beser J., Troell K., Guo Y., et al. (2020). Subtype distribution of zoonotic pathogen Cryptosporidium felis in humans and animals in several countries. Emerg. Microbes Infect. 9, 2446–2454. doi: 10.1080/22221751.2020.1840312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi P., Shafaghi-Sisi S., Meamar A. R., Razmjou E. (2023). Molecular identification of Cryptosporidium, Giardia, and Blastocystis from stray and household cats and cat owners in Tehran, Iran. Sci. Rep. 13, 1554. doi: 10.1038/s41598-023-28768-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Kim H., Pfeiffer D., Brodbelt D. (2018). Epidemiological study of feline idiopathic cystitis in Seoul, South Korea. J. Feline Med. Surg. 20, 913–921. doi: 10.1177/1098612X17734067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koseoglu A. E., Can H., Karakavuk M., Güvendi M., Değirmenci Döşkaya A., Manyatsi P. B., et al. (2022). Molecular prevalence and subtyping of Cryptosporidium spp. in fecal samples collected from stray cats in İzmir, Turkey. BMC Vet. Res. 18, 89. doi: 10.1186/s12917-022-03190-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostopoulou D., Claerebout E., Arvanitis D., Ligda P., Voutzourakis N., Casaert S., et al. (2017). Abundance, zoonotic potential and risk factors of intestinal parasitism amongst dog and cat populations: the scenario of Crete, Greece. Parasit Vectors. 10, 43. doi: 10.1186/s13071-017-1989-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak D., Seo M. G. (2020). Genetic analysis of zoonotic gastrointestinal protozoa and Microsporidia in shelter cats in South Korea. Pathogens. 9, 894. doi: 10.3390/pathogens9110894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalle M., Pozio E., Capelli G., Bruschi F., Crotti D., Cacciò S. M. (2005). Genetic heterogeneity at the β-giardin locus among human and animal isolates of Giardia duodenalis and identification of potentially zoonotic subgenotypes. Int. J. Parasitol. 35, 207–213. doi: 10.1016/j.ijpara.2004.10.022 [DOI] [PubMed] [Google Scholar]
- Lee S. E., JY K., YA K., SH C., HJ A., HM W., et al. (2010). Prevalence of Toxoplasma gondii infection in stray and household cats in regions of Seoul, Korea. Korean J. Parasitol. 48, 267–270. doi: 10.3347/kjp.2010.48.3.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.-k., Lee H.-j., Song J.-h., Song K.-h. (2022). Prevalence of giardiasis of stray cats in the Daejeon city. Korean J. Vet. Serv. 45, 249–252. doi: 10.7853/kjvs.2022.45.4.249 [DOI] [Google Scholar]
- Lee S. H., Ock Y., Choi D., Kwak D. (2019). Gastrointestinal parasite infection in cats in Daegu, Republic of Korea, and efficacy of treatment using topical emodepside/praziquantel formulation. Korean J. Parasitol. 57, 243–248. doi: 10.3347/kjp.2019.57.3.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., VanBik D., HY K., Cho A., JW K., JW B., et al. (2016). Prevalence and molecular characterisation of Giardia duodenalis in calves with diarrhoea. Vet. Rec. 178, 633–. doi: 10.1136/vr.103534 [DOI] [PubMed] [Google Scholar]
- Li J., Dan X., Zhu K., Li N., Guo Y., Zheng Z., et al. (2019). Genetic characterization of Cryptosporidium spp. and Giardia duodenalis in dogs and cats in Guangdong, China. Parasit Vectors. 12, 571. doi: 10.1186/s13071-019-3822-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Liu X., Gu Y., Liu J., Luo J. (2019). Prevalence of Cryptosporidium, Giardia, Blastocystis, and trichomonads in domestic cats in East China. J. Vet. Med. Sci. 81, 890–896. doi: 10.1292/jvms.19-0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Yang F., Liang R., Guo S., Guo Y., Li N., et al. (2021). Subtype characterization and zoonotic potential of Cryptosporidium felis in cats in Guangdong and Shanghai, China. Pathogens. 10, 89. doi: 10.3390/pathogens10020089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay D. S., Dubey J. P., Blagburn B. L. (1997). Biology of Isospora spp. from humans, nonhuman primates, and domestic animals. Clin. Microbiol. Rev. 10, 19–34. doi: 10.1128/CMR.10.1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay D. S., Zajac A. (2004). Cryptosporidium infections in cats and dogs. Compendium on continuing education for the practising veterinarian-North American edition- 26, 864–876. [Google Scholar]
- Lucio-Forster A., Griffiths J. K., Cama V. A., Xiao L., Bowman D. D. (2010). Minimal zoonotic risk of cryptosporidiosis from pet dogs and cats. Trends Parasitol. 26, 174–179. doi: 10.1016/j.pt.2010.01.004 [DOI] [PubMed] [Google Scholar]
- Maciel P. M., Sabogal-Paz L. P. (2016). Removal of Giardia spp. and Cryptosporidium spp. from water supply with high turbidity: analytical challenges and perspectives. J. Water Health 14, 369–378. doi: 10.2166/wh.2015.227 [DOI] [PubMed] [Google Scholar]
- Mendoza R., Otranto D. (2023). Zoonotic parasites associated with predation by dogs and cats. Parasit Vectors. 16, 55. doi: 10.1186/s13071-023-05670-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X. Z., MY L., Lyu C., YF Q., ZY Z., XB Y., et al. (2021). The global prevalence and risk factors of Cryptosporidium infection among cats during 1988–2021: A systematic review and meta-analysis. Microb. Pathog. 158, 105096. doi: 10.1016/j.micpath.2021.105096 [DOI] [PubMed] [Google Scholar]
- Moon D. C., JH C., Boby N., SJ K., HJ S., HS P., et al. (2022). Prevalence of bacterial species in skin, urine, diarrheal stool, and respiratory samples in cats. Pathogens. 11, 324. doi: 10.3390/pathogens11030324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh M., KB K., SC C., Ko J.-A., Ryu Y. (2021). Jeju Animal Shelter abandoned animals status and actual condition analysis. Korean J. Vet. Serv. 44, 175–183. doi: 10.7853/kjvs.2021.44.4.175 [DOI] [Google Scholar]
- Onder Z., Yetişmiş G., Pekmezci D., Kökçü N. D., Zafer G., Pekmezci AÇ., et al. (2021). Investigation of zoonotic Cryptosporidium and Giardia intestinalis species and genotypes in cats (Felis catus). Turkiye Parazitol Derg. 45, 252–256. doi: 10.4274/tpd.galenos.2021.46320 [DOI] [PubMed] [Google Scholar]
- Pallant L., Barutzki D., Schaper R., Thompson R. C. (2015). The epidemiology of infections with Giardia species and genotypes in well cared for dogs and cats in Germany. Parasit Vectors. 8, 2. doi: 10.1186/s13071-014-0615-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer C. S., Traub R. J., Robertson I. D., Devlin G., Rees R., Thompson R. C. (2008). Determining the zoonotic significance of Giardia and Cryptosporidium in Australian dogs and cats. Vet. Parasitol. 154, 142–147. doi: 10.1016/j.vetpar.2008.02.031 [DOI] [PubMed] [Google Scholar]
- Power M. L., Holley M., UM R., Worden P., Gillings M. R. (2011). Identification and differentiation of Cryptosporidium species by capillary electrophoresis single-strand conformation polymorphism. FEMS Microbiol. Lett. 314, 34–41. doi: 10.1111/j.1574-6968.2010.02134.x [DOI] [PubMed] [Google Scholar]
- Procesi G. I., Carnio A., Berrilli F., Montalbano Di Filippo M., Scarito A., Amoruso C., et al. (2022). Giardia duodenalis in colony stray cats from Italy. Zoonoses Public Health 69 (1), 46–54. doi: 10.1111/zph.12894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambozzi L., Menzano A., Mannelli A., Romano S., Isaia M. C. (2007). Prevalence of cryptosporidian infection in cats in Turin and analysis of risk factors. J. Feline Med. Surg. 9, 392–396. doi: 10.1016/j.jfms.2007.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Lopez L., Elwin K., Chalmers R. M., Enemark H. L., Beser J., Troell K. (2020). Development of a gp60-subtyping method for Cryptosporidium felis. Parasit Vectors. 13, 39. doi: 10.1186/s13071-020-3906-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorza A. V., Tyrrell P., Wennogle S. A., Chandrashekar R., Lappin M. R. (2021). Experimental infection of cats with Cystoisospora felis. J. Vet. Intern. Med. 35, 269–272. doi: 10.1111/jvim.16012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiei R., Najjari M., Kargar Kheirabad A. K., Hatam G. (2016). Severe diarrhea due to Cystoisospora belli infection in an HTLV-1 woman. Iran J. Parasitol. 11, 121–125. [PMC free article] [PubMed] [Google Scholar]
- Sulaiman I. M., Fayer R., Bern C., Gilman R. H., Trout J. M., Schantz P. M., et al. (2003). Triosephosphate isomerase gene characterization and potential zoonotic transmission of Giardia duodenalis. Emerg. Infect. Dis. 9, 1444–1452. doi: 10.3201/eid0911.030084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghipour A., Khazaei S., Ghodsian S., Shajarizadeh M., Olfatifar M., Foroutan M., et al. (2021). Global prevalence of Cryptosporidium spp. in cats: a systematic review and meta-analysis. Res. Vet. Sci. 137, 77–85. doi: 10.1016/j.rvsc.2021.04.015 [DOI] [PubMed] [Google Scholar]
- Tangtrongsup S., Scorza A. V., Reif J. S., Ballweber L. R., Lappin M. R., Salman M. D. (2020). Seasonal distributions and other risk factors for Giardia duodenalis and Cryptosporidium spp. infections in dogs and cats in Chiang Mai, Thailand. Prev. Vet. Med. 174, 104820. doi: 10.1016/j.prevetmed.2019.104820 [DOI] [PubMed] [Google Scholar]
- Tzannes S., Batchelor D. J., Graham P. A., Pinchbeck G. L., Wastling J., German A. J. (2008). Prevalence of Cryptosporidium, Giardia and Isospora species infections in pet cats with clinical signs of gastrointestinal disease. J. Feline Med. Surg. 10, 1–8. doi: 10.1016/j.jfms.2007.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Gonzalez-Moreno O., Roellig D. M., Oliver L., Huguet J., Guo Y., et al. (2019). Epidemiological distribution of genotypes of Giardia duodenalis in humans in Spain. Parasit Vectors. 12, 432. doi: 10.1186/s13071-019-3692-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Yao L., Chen H., Zhang W., Jiang Y., Yang F., et al. (2022). Giardia duodenalis in patients with diarrhea and various animals in northeastern China: prevalence and multilocus genetic characterization. Parasit Vectors. 15, 165. doi: 10.1186/s13071-022-05269-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Ying J. L. J., Monis P., Ryan U. (2015). Molecular characterisation of Cryptosporidium and Giardia in cats (Felis catus) in Western Australia. Exp. Parasitol. 155, 13–18. doi: 10.1016/j.exppara.2015.05.001 [DOI] [PubMed] [Google Scholar]
- Youn H., Cho M.-R., Lim Y.-S., KH K., Bae B.-K., Shin N., et al. (2012). The prevalence of feline parasites in Suwon, Korea. Korean J. Vet. Res. 52, 65–68. doi: 10.14405/kjvr.2012.52.2.065 [DOI] [Google Scholar]
- Zahedi A., Paparini A., Jian F., Robertson I., Ryan U. (2016). Public health significance of zoonotic Cryptosporidium species in wildlife: critical insights into better drinking water management. Int. J. Parasitol. Parasites Wildl. 5, 88–109. doi: 10.1016/j.ijppaw.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data presented in this study are available upon request from the corresponding author. Representative DNA sequences from the present study were deposited in the GenBank database under the accession numbers OQ598555-OQ598563 for gp60 of C. felis, OQ473126, OQ473172-OQ473185, OQ534549, and OQ534551-OQ534555 for ITS-1 of Cystoisospora, OQ442978-OQ442994 for tpi of G. duodenalis, OQ442958-OQ442969 for b-giardin of G. duodenalis, and OQ442970-OQ442977 for gdh of G. duodenalis.


