Abstract
A novel glucosyltransferase which catalyzed the transfer of glucose from UDP-glucose to positions 2′ and 5′ of partially methylated flavonols was isolated from the shoots of Chrysosplenium americanum Schwein ex Hooker. It was purified 225-fold by ammonium sulfate precipitation and successive chromatography on Sephadex G-100, hydroxyapatite, and polybuffer ion exchanger. This glucosyltransferase appeared to be a single polypeptide with an apparent molecular weight of 42,000 daltons, pH optimum of 7.5 to 8.0, and an isoelectric point of 5.1. It had low but similar Km values for the 2′ and 5′ positions of flavonol substrates and the cosubstrate UDP-glucose and was inhibited by both reaction products, the glucosides formed, and UDP.
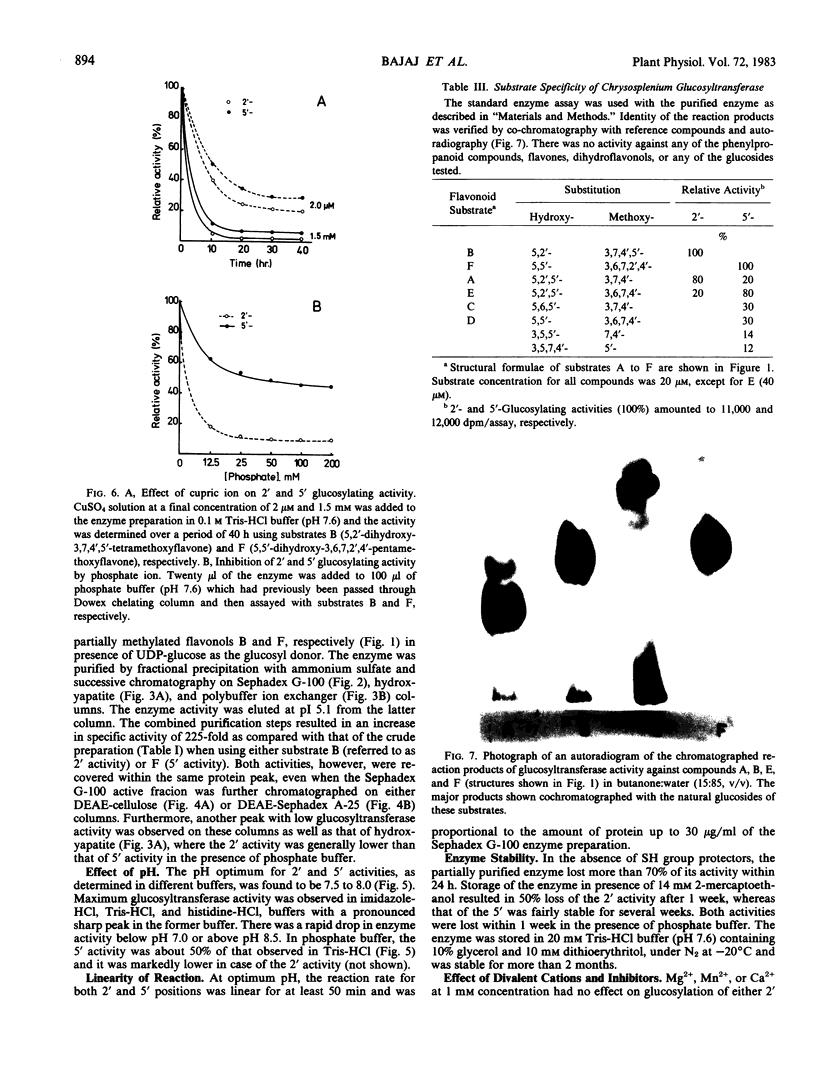
Glucosyltransferase activity was independent of divalent cations, was not inhibited by EDTA, but showed requirement for SH groups. The differential effect on enzyme activity of metal ions, especially cupric ion, and various SH group reagents seemed to indicate the involvement of two active sites in the glucosylation reaction; the site specific for 2′ activity being more susceptible than that of the 5′ activity. The substrate specificity expressed by this glucosyltransferase and the requirement of at least two para-oriented B-ring substituents (at 2′ and 5′) for activity support this view.
Full text
PDF
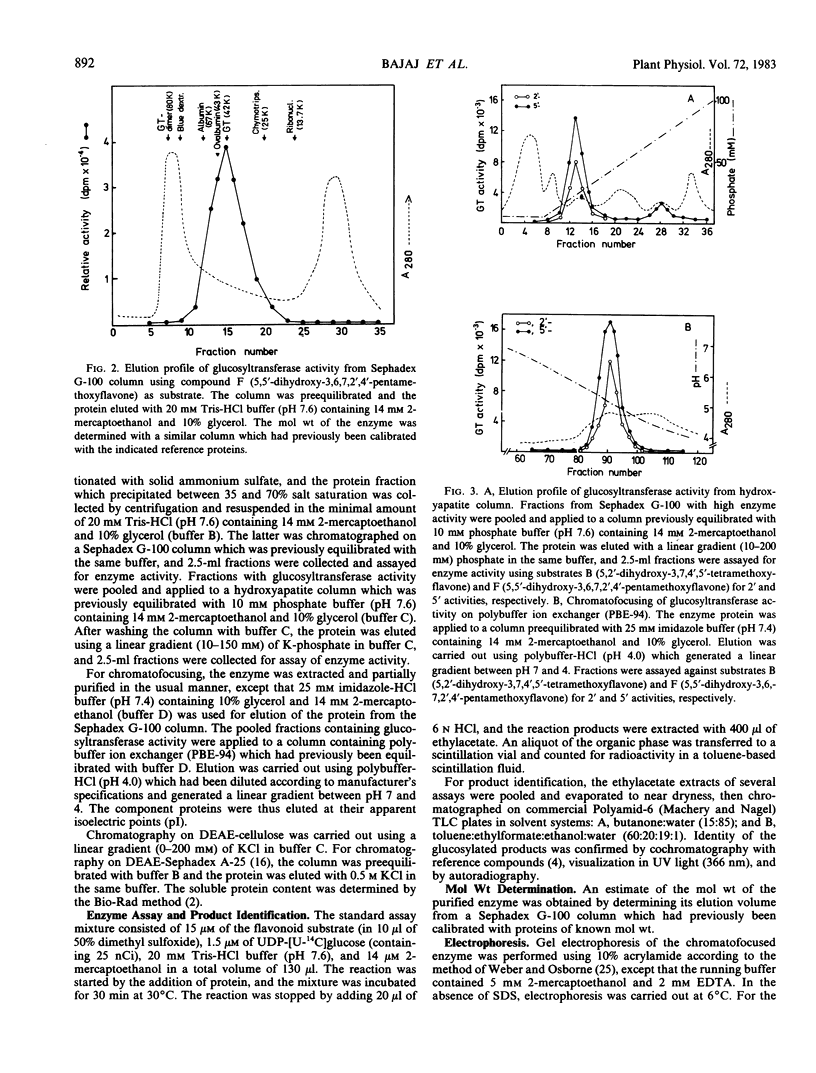
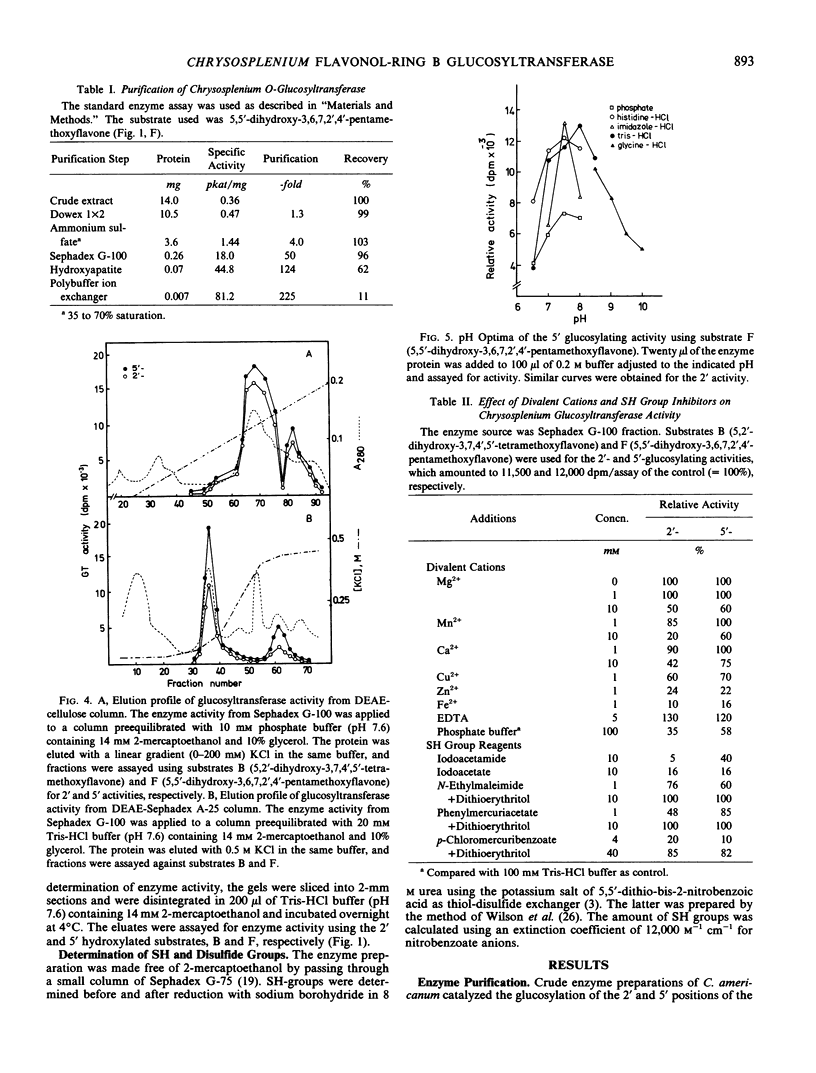

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boothe R. L., Folk J. E. A reversible, calcium-dependent, copper-catalyzed inactivation of guinea pig liver transglutaminase. J Biol Chem. 1969 Jan 25;244(2):399–405. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cavallini D., Graziani M. T., Dupré S. Determination of disulphide groups in proteins. Nature. 1966 Oct 15;212(5059):294–295. doi: 10.1038/212294a0. [DOI] [PubMed] [Google Scholar]
- Ibrahim R. K., Grisebach H. Purification and properties of UDP-glucose: coniferyl alcohol glucosyltransferase from suspension culturesof Paul's scarlet rose. Arch Biochem Biophys. 1976 Oct;176(2):700–708. doi: 10.1016/0003-9861(76)90214-9. [DOI] [PubMed] [Google Scholar]
- Ireland R. J., Deluca V., Dennis D. T. Isoenzymes of pyruvate kinase in etioplasts and chloroplasts. Plant Physiol. 1979 May;63(5):903–907. doi: 10.1104/pp.63.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan P. S., Mansell R. L. Isolation and partial characterization of three glucosyl transferases involved in the biosynthesis of flavonol triglucosides in Pisum sativum L. Arch Biochem Biophys. 1982 Feb;213(2):434–443. doi: 10.1016/0003-9861(82)90569-0. [DOI] [PubMed] [Google Scholar]
- Köster J., Barz W. UDP-glucose:isoflavone 7-O-glucosyltransferase from roots of chick pea (Cicer arietinum L.). Arch Biochem Biophys. 1981 Nov;212(1):98–104. doi: 10.1016/0003-9861(81)90347-7. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Sutter A., Grisebach H. UDP-glucose: flavonol 3-0-glucosyltransferase from cell suspension cultures of parsley. Biochim Biophys Acta. 1973 Jun 6;309(2):289–295. doi: 10.1016/0005-2744(73)90027-2. [DOI] [PubMed] [Google Scholar]
- Sutter A., Ortmann R., Grisebach H. Purification and properties of an enzyme from cell suspension cultures of parsley catalyzing the transfer of D-glucose from UDP-D-glucose to flavonoids. Biochim Biophys Acta. 1972 Jan 20;258(1):71–87. doi: 10.1016/0005-2744(72)90967-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]