Abstract
The genetic diversity of symbiotic Xenorhabdus and Photorhabdus bacteria associated with entomopathogenic nematodes was examined by a restriction fragment length polymorphism analysis of PCR-amplified 16S rRNA genes (rDNAs). A total of 117 strains were studied, most of which were isolated from the Caribbean basin after an exhaustive soil sampling. The collection consisted of 77 isolates recovered from entomopathogenic nematodes in 14 Caribbean islands and of 40 reference strains belonging to Xenorhabdus and Photorhabdus spp. collected at various localities worldwide. Thirty distinctive 16S rDNA genotypes were identified, and cluster analysis was used to distinguish the genus Xenorhabdus from the genus Photorhabdus. The genus Xenorhabdus appears more diverse than the genus Photorhabdus, and for both genera the bacterial genotype diversity is in congruence with the host-nematode taxonomy. The occurrence of symbiotic bacterial genotypes was related to the ecological distribution of host nematodes.
Xenorhabdus and Photorhabdus spp. (Enterobacteriaceae) are symbiotically associated with the entomopathogenic nematodes (EPNs). Both partners of each bacterial-helminthic complex act together to kill insect prey by producing toxins and septicemia. Nematodes reproduce in the insect cadaver, feeding on the produced bacterial biomass and the insect tissues metabolized by the bacteria (4). When nematodes are harvested freshly from soil samples, all of the bacterial isolations from the intestinal contents of infective juveniles showed the presence of Xenorhabdus spp. in Steinernema spp. and Photorhabdus spp. in Heterorhabditis spp. It has been postulated that this high specificity is mainly due to the effect of a series of antimicrobial end products excreted by the symbiont itself during the multiplication of the nematodes in the parasitized insects (12). When infective juveniles escape the insect cadaver, they harvest symbiont cells in their intestine, securing among the generations the perenniality of the symbiotic association between both partners.
Axenic nematodes and symbionts are generally entomopathogenic by themselves, but the bacterial partner requires assistance from the nematode to achieve inoculation (the 50% lethal dose is usually less than 50 cells, depending on the test insects) (16). In natural conditions, symbionts are inoculated into the insect hemolymph by their host nematode, which acts as a living syringe on the target insects. In some symbioses, both partners must participate after inoculation to achieve pathogenesis, as with, for instance, the Steinernema glaseri-Xenorhabdus poinarii symbiosis (3).
Xenorhabdus and Photorhabdus spp. appear to display a high and monophyletic diversity: five Xenorhabdus species have been described (X. nematophilus, X. poinarii, X. bovienii, X. beddingii, and X. japonicus [6, 23]), and only one Photorhabdus species has been described (P. luminescens [11]). However, within the P. luminescens species several genomic groups have been recognized by DNA-DNA hybridization (7) and suggested by 16S rDNA sequencing (20, 31). Thus, the number of species could be underestimated.
In both genera, identification of new bacterial isolates or species is difficult because most strains are phenotypically very similar and fail to give positive results in many classical tests for identification (10) and because of a lack of sufficient members per taxon. Consequently, only a few species have been described, and some of these are represented by only a few isolates (6). Ecological data relating bacterial symbionts with their nematode host or their environment remain weak. Thus, studies on the taxonomic diversity and distribution of members of Xenorhabdus and Photorhabdus spp. are needed. As a first step, we recently used a PCR-restriction fragment length polymorphism (RFLP) method applied to the 16S rRNA gene to rapidly identify new isolates of both genera of symbionts (13). This approach distinguished Xenorhabdus and Photorhabdus species and identified groups as effectively as did DNA-DNA hybridization (7, 13). The method was applied on a limited number of bacterial strains obtained after isolation from nematode collections. In the current study, a larger and more comprehensive sampling of symbionts was undertaken in order to learn more about their ecological distribution relative to host taxonomy and environmental factors.
An exhaustive sampling was performed among 14 islands in the Caribbean basin. Caribbean islands are interesting because they are assumed to present limited soil imports and are subject to the same climate and because previous studies on native EPNs are available (8, 21, 27). The sampling is based on two surveys: (i) one is an exhaustive soil collection based on a grid map covering all the seven Guadeloupe islands (Grande Terre and Basse Terre, Marie-Galante, La Désirade, Petite Terre, Les Saintes, Saint-Martin, and Saint-Barthélemy) from which nematodes were trapped to study their distribution (14), and (ii) the other is a collection of nematodes recovered at random from seven other neighboring Caribbean islands (Martinique, Saint-Vincent, Cuba, Jamaica, Puerto Rico, the Dominican Republic, and Trinidad and Tobago) (19). The purpose of this study was to examine the diversity of EPN symbionts by PCR ribotyping (13) and to correlate the results to nematode taxonomy throughout the Caribbean islands and to the sampling environment (soil type, rainfall, elevation, and vegetal covering or crops) in the Guadeloupe islands. Finally, the isolates were compared to additional Xenorhabdus and Photorhabdus strains isolated from nematodes widely distributed throughout the rest of the world and from human clinical samples in order to determine whether Caribbean bacterial symbionts represent distinct genotypes of Xenorhabdus and Photorhabdus.
MATERIALS AND METHODS
Exhaustive nematode sampling in the Guadeloupe islands.
Nematodes were collected from the seven Guadeloupe islands. Exhaustive soil sampling was conducted between February and December of 1996. To harvest each sample, three portions of soil were chosen randomly in a 100-m2 area. Each portion consisted of a core 5.5 cm in diameter at a 25-cm depth (ca. 0.6 dm3 of soil). The three portions were mixed, giving 1.8 dm3 from which 0.6 dm3 was used for recovering the nematodes as previously described (14). In all, 538 soil samples were collected according to a square grid with points spaced at 2-km intervals and covering the whole surface of the Guadeloupe islands. EPNs were isolated by using the Galleria trap technique (9) and identified by using morphological criteria, isozyme analysis, and satellite DNA probes (17, 19). The presence of nematodes was related to site location, elevation, rainfall, soil type, and vegetation (14).
Random sampling in other Caribbean islands.
Nematodes were also collected from the seven other Caribbean islands listed above. In contrast to the exhaustive Guadeloupe survey, these samples were collected randomly from a variety of ecosystems, including croplands, orchards, grasslands, salt marches, and forests. On Martinique, Saint-Vincent, Trinidad and Tobago, and Jamaica, samples were collected by the Institut National de la Recherche Agronomique (INRA) laboratory in Guadeloupe. From the three other Caribbean islands, nematodes were collected and identified by E. Arteaga and M. Montes (Ministerio de Agricultura, Estación Nacional de Sanidad de los Cítricos, La Habana, Cuba), W. Figueroa (University of Puerto Rico, Río Pedras, Puerto Rico), and L. Garrido and A. Carro (Universidad Autónoma de Santo Domingo, Engombe, Santo Domingo). After delivery of the biological material, the taxonomic position of this nematode collection was checked again (19) by using the methods mentioned above.
The names of the nematode species cited in this study follow recent modifications of the International Code of Zoological Nomenclature. Therefore, unlike the previous descriptions, the following names will be used in this study: S. affine, S. arenarium, S. cubanum, S. intermedium, S. puertoricense, S. rarum, S. riobrave, and H. indica instead of S. affinis, S. anomalae, S. cubana, S. intermedia, S. puertoricencis, S. rara, S. riobravis, and H. indicus, respectively (18).
Bacterial isolates and reference strains.
Individual bacterial colonies were isolated from the infective stages by the hanging-drop technique (25). We examined 77 isolates from the Caribbean area, including 31 strains from the Guadeloupe islands and 46 strains from the remaining Caribbean islands (Table 1). To identify the isolates, their phenotypic properties and RFLP patterns of amplified 16S ribosomal DNA (rDNA) were compared to those of 27 reference strains previously studied (10, 13). Thirteen other reference strains, including seven Xenorhabdus and six Photorhabdus strains, were added (Table 2). The seven new Xenorhabdus strains included strain USFL52 of X. bovienii, strain SK72 of X. poinarii, and five other Xenorhabdus spp. that originated from different parts of the world and from new species of host nematodes, i.e., Steinernema monticolum (29), S. scapterisci, S. serratum, S. kushidai, and S. riobrave (Table 2). Five opportunistic Photorhabdus spp. isolated from human patients at the Centers for Diseases Control (Atlanta, Ga.) (15) and strain Q614, which is the only known nonluminescent Photorhabdus strain, were also examined (5).
TABLE 1.
List of the 77 bacterial isolates used in this study
| Island group and isolatea | Geographical origin | Host nematode origin | Source of nematodesb | 16S rDNA genotypec |
|---|---|---|---|---|
| Guadeloupe islands | ||||
| Photorhabdus isolates | ||||
| FRG03-17 | Grande Terre | H. indica | H. Mauléon | 12 |
| FRG19 | Basse Terre | H. indica | H. Mauléon | 12 |
| FRG20 | Saint-Barthélemy | H. indica | H. Mauléon | 12 |
| FRG24-25 | Saint-Martin | H. indica | H. Mauléon | 12 |
| FRG28 | La Désirade | H. indica | H. Mauléon | 12 |
| FRG33 | Les Saintes | H. indica | H. Mauléon | 12 |
| FRG35 | Marie Galante | H. indica | H. Mauléon | 12 |
| FRG01 | Basse Terre | H. bacteriophora | H. Mauléon | 13 |
| FRG02 | Grande Terre | H. bacteriophora | H. Mauléon | 13 |
| FRG18 | Petite Terre | H. indica | H. Mauléon | 27 |
| FRG21-23 | Saint-Barthélemy | H. indica | H. Mauléon | 27 |
| FRG27 | Saint-Martin | H. indica | H. Mauléon | 27 |
| FRG26 | Saint-Martin | Heterorhabditis sp.d | H. Mauléon | 28 |
| FRG29 | Basse Terre | H. bacteriophora | H. Mauléon | 28 |
| Xenorhabdus isolates | None | |||
| Other Caribbean islands | ||||
| Photorhabdus isolates | ||||
| P2M | Cuba | H. indica | E. Arteaga | 12 |
| DO02-04 and -07; DO09-10, -12, and -14; and DO18-21 and 23-25 | Dominican Republic | H. indica | L. Garrido | 12 |
| DO13 | Dominican Republic | Heterorhabditis sp.d | L. Garrido | 12 |
| FRM03 | Martinique | H. indica | H. Mauléon | 12 |
| PR02-B, PR05, PR06-C, PR14, PR16 to PR19, PR21 and PR22, PR27-A, PR27-B, PR38, PR54, PR60, and PR63 | Puerto Rico | H. indica | W. Figueroa | 12 |
| DO01 and DO08 | Dominican Republic | H. bacteriophora | L. Garrido | 13 |
| PR02-A | Puerto Rico | H. bacteriophora | W. Figueroa | 13 |
| TT01 and TT03 | Trinidad | H. bacteriophora | H. Mauléon | 13 |
| JM12 | Jamaica | H. indica | H. Mauléon | 27 |
| FRM05 | Martinique | H. indica | H. Mauléon | 27 |
| Xenorhabdus isolates | ||||
| CU01 | Cuba | S. cubanum | E. Arteaga | 3 |
| JM26 | Jamaica | S. bicornutum | H. Mauléon | 25 |
| PR06-A | Puerto Rico | S. puertoricense | W. Figueroa | 19 |
| VC01 | Saint-Vincent | Steinernema sp. 1e | H. Mauléon | 20 |
| FRM16 | Martinique | Steinernema sp. 2e | H. Mauléon | 21 |
Names are given according to an international nomenclature that indicates first the international code of the home country followed by a number; strain P2M, however, is a strain designation kept due to several previous descriptions (CU, Cuba; DO, Dominican Republic; FRG, France, Guadeloupe [corresponds to HG used in Constant et al. (14)]; FRM, France, Martinique; JM, Jamaica; PR, Puerto Rico; TT, Trinidad and Tobago; and VC, Saint-Vincent).
E. Arteaga, Estación de Sanidad de los Cítricos, Ministerio de Agricultura, Ciudad Habana, Cuba; L. Garrido, Universidad Autónoma de Santo Domingo, Santo Domingo, Dominican Republic; W. Figueroa, University of Puerto Rico, Río Piedras, Puerto Rico; and H. Mauléon, INRA, Unité de Recherches en Productions Végétales, Guadeloupe, France.
16S rDNA genotypes are defined in Table 3.
Heterorhabditis sp. not yet identified.
New species of Steinernema not yet described.
TABLE 2.
List of the 43 Xenorhabdus, Photorhabdus, and other Enterobacteriaceae strains used as references in RFLP analysis of 16S rRNA genes
| Straina | Host nematode origin | Geographical origin | Source | 16S rDNA genotypeb |
|---|---|---|---|---|
| X. nematophilus | ||||
| AN6 (ATCC 19061)T | S. carpocapsae | Georgia | R. Akhurst | 1 |
| A24 | S. carpocapsae | Russia | R. Akhurst | 2 |
| F1 | S. carpocapsae | France | C. Laumond | 2 |
| X. poinarii | ||||
| G6 (ATCC 49121)T | S. glaseri | North Carolina | R. Akhurst | 3 |
| NC33 | S. glaseri | North Carolina | R. Akhurst | 3 |
| SK72 | S. glaseri | Florida | N. Simões | 3 |
| X. beddingii Q58 (UQM 2872)T | Steinernema sp.3c | Queensland, Australia | R. Akhurst | 5 |
| X. bovienii | ||||
| T228 (UQM 2211)T | S. feltiae | Tasmania, Australia | R. Akhurst | 6 |
| USFL52 | S. feltiae | Florida | G. Smart | 6 |
| Si | S. intermedium | South Carolina | R. Akhurst | 7 |
| F3 | S. affine | France | C. Laumond | 8 |
| SK2 | S. kraussei | Czech Republic | Z. Mrácek | 8 |
| F5 | S. feltiae | France | C. Laumond | 8 |
| X. japonicus JP02 | S. kushidai | Japan | N. Ishibashi | 18 |
| Xenorhabdus spp. | ||||
| K77 | S. rarum | Argentina | M. De Doucet | 4 |
| SaV | S. arenarium | Russia | R. Akhurst | 9 |
| KR1 | S. monticolum | Korea | P. Stock | 22 |
| UY61 | S. scapterisci | Uruguay | G. Smart | 23 |
| CN01 | S. serratum | South China | J. Liu | 24 |
| USTX62 | S. riobrave | Texas | E. Cabanillas | 26 |
| P. luminescens | ||||
| Hb (ATCC 29999)T | H. bacteriophora | Victoria, Australia | R. Akhurst | 10 |
| Hm | Heterorhabditis sp.d | Wisconsin | K. Nealson | 10 |
| C8406 | Heterorhabditis sp.d | Haï Nan Island, China | R. Akhurst | 11 |
| IS5 | H. indica | Israel | I. Glazer | 12 |
| K80 | Heterorhabditis sp.d | Argentina | M. De Doucet | 13 |
| HP88 | H. bacteriophora | Ohio | R. Akhurst | 13 |
| X1Nach | H. megidis (NWE group)e | Russia | R. Akhurst | 14 |
| HW79 | H. megidis (NWE group)e | The Netherlands | P. Westerman | 14 |
| ItH211 | Heterorhabditis sp. | Italy | K. Deseo | 14 |
| HL81 | H. megidis (NWE group)e | The Netherlands | P. Westerman | 14 |
| Meg | H. megidis | Ohio | R. Akhurst | 15 |
| C1 (ATCC 29304) | H. bacteriophora (H. heliothidis) | California | R. Akhurst | 16 |
| NZH | H. zealandica | New Zealand | W. Wouts | 17 |
| D1 | H. indica | North Territory, Australia | R. Akhurst | 27 |
| 1216-79 | Clinical (ATCC 43948) | CDC, Atlanta, Ga | J. Farmer | 29 |
| 2407-88 | Clinical (ATCC 43952) | CDC, Atlanta, Ga. | J. Farmer | 29 |
| 2617-87 | Clinical (ATCC 43951) | CDC, Atlanta, Ga. | J. Farmer | 29 |
| 3105-77 | Clinical (ATCC 43949) | CDC, Atlanta, Ga. | J. Farmer | 29 |
| 3265-86 | Clinical (ATCC 43950) | CDC, Atlanta, Ga. | J. Farmer | 29 |
| Q614 | Heterorhabditis sp.d | Queensland, Australia | R. Akhurst | 30 |
| Other Enterobacteriaceae | ||||
| Proteus vulgaris CIP 58.60T | Institut Pasteur, Paris, France | 31 | ||
| Escherichia coli CIP 54.8T | Institut Pasteur, Paris, France | 32 | ||
| Serratia marcescens CIP 103235T | Institut Pasteur, Paris, France | 33 |
Names are given according to the usual nomenclature of the strains as described in previous studies, except for the new designations, which indicate first the international code of the home country followed by a number (CN, China; JP, Japan; KR, Korea; USFL, United States, Florida; USTX, United States, Texas; and UY, Uruguay). Abbreviations: ATCC, American Type Culture Collection (Rockville, Md.); CIP, Collection de l’Institut Pasteur (Paris, France); UQM, Culture Collection of the University of Queensland Department of Microbiology (Brisbane, Australia). A superscript “T” indicates a type strain.
16S rDNA genotypes are defined in Table 3.
New species of Steinernema not yet described.
Heterorhabditis sp. not yet identified.
NWE, Nematodes belonging to the North West European group.
Phenotypic characterization.
To verify in a preliminary step that the isolates belonged to the Xenorhabdus and Photorhabdus genera, we used conventional phenotypic criteria (10) and compared the results with reference strains. All of the tests were conducted at 28°C. Cellular morphology and motility were assessed by microscopic examination of 24-h-old nutrient broth cultures. Dye adsorption of bromothymol blue was tested on nutrient agar supplemented with 0.004% (wt/vol) triphenyltetrazolium chloride and 0.0025% (wt/vol) bromothymol blue (NBTA medium) for Xenorhabdus isolates (2), and dye adsorption of neutral red was tested on MacConkey agar for Photorhabdus isolates (10). Antimicrobial activity was determined by the method of Akhurst (1) with Micrococcus luteus as the indicator microorganism. Other tests included the use of API 20E and API 20NE strips (Biomerieux, Craponne, France); catalase; bioluminescence; phospholipase (lecithinase); lipolysis on Tween 20, 40, 60, 80, and 85; and pigmentation (10).
Nucleic acid extraction.
Cells were grown on nutrient agar plates for 48 h at 28°C, scraped off in TE8 buffer (50 mM Tris-HCl, 20 mM EDTA; pH 8), and centrifuged in a microcentrifuge tube for 2 min at 10,000 × g. The cell pellets were washed twice in TE8 buffer and stored at −20°C. DNA extraction was performed with the nucleic acid extraction kit Isoquick (ORCA Research, Inc., Bothell, Wash.) according to the rapid DNA extraction protocol of the manufacturer. The DNA pellets were dissolved in 100 μl of pure water and diluted 20- to 100-fold to be used as templates for PCR.
16S rDNA restriction analysis.
16S rDNAs were amplified by using the primers and reaction conditions previously described (13). For each isolate, 6 to 17 μl of amplified 16S rDNA was digested overnight with 5 U of restriction endonuclease (GIBCO BRL, Cergy-Pontoise, France). PCR products of Xenorhabdus isolates and reference strains were digested separately with six tetrameric endonucleases previously found to produce polymorphic digests (13): CfoI, HinfI, DdeI, AluI, HaeIII, and MspI. Amplified DNAs from the Photorhabdus isolates were digested with three endonucleases (AluI, CfoI, and HaeIII) which were sufficient to generate all the genotypes of Photorhabdus strains previously studied (13). DNA digests were then analyzed by horizontal electrophoresis at 6 V/cm in 3% (wt/vol) Nusieve or Metaphor agarose (Tebu, Le Perray en Yvelines, France) gels in 0.5× TBE buffer (44.6 mM Tris-base; 44.6 mM boric acid; 1 mM EDTA; pH 8) containing 0.5 mg of ethidium bromide per liter. The gels were visualized under UV light with an imager (The Imager, software version 2.03; Appligene, Inc., Strasbourg, France). Genetic relationships between two amplified 16S rRNA genes were evaluated by determining the presence or absence of DNA restriction fragments of a given length. Dice’s similarity coefficient, based on the proportion of shared restriction fragments, was calculated, and the distance matrix was determined by the Nei and Li method (22). Distance values were displayed as a dendrogram by using the unweighted-pair-group method with arithmetic means (UPGMA) (28).
RESULTS
Isolation and phenotypic characterization of bacterial symbionts.
All bacterial isolates from the Guadeloupe and other Caribbean island surveys shared the common phenotypic properties with all reference strains (data not shown) and therefore belonged to Xenorhabdus and Photorhabdus genera (10).
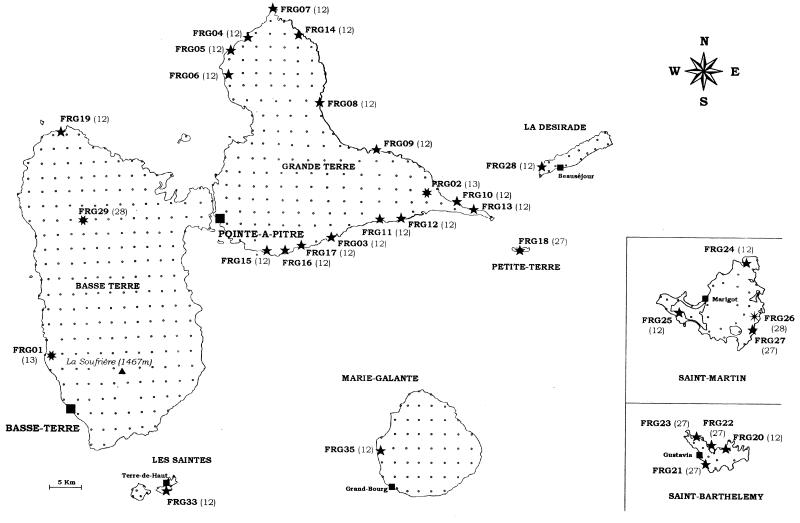
Of the 538 soil samples collected from the seven Guadeloupe islands, 31 (5.8%) contained Heterorhabditis nematodes (Fig. 1), from which 31 Photorhabdus strains were isolated (Table 1). No Steinernema spp. were found, and therefore no Xenorhabdus spp. were obtained from the Guadeloupe islands. Of the Heterorhabditis nematodes, 27 were H. indica (87%), three were H. bacteriophora (10%), and one was not identified (3%) (Table 1). H. indica was mainly located in coastal areas and rarely inland (Fig. 1): 25 isolates originated from soil marshes, sandy beaches, and the slopes of a limestone cliff (soil pH, 8.0 to 9.3; elevation, 0 to 75 m), and 2 isolates originated from pastures (vertisol at pH 6.5 to 7.5; elevation, ≤240 m). The three H. bacteriophora were found in cropland soil (vertisol at pH 6.5 to 7.5; elevation, ≤25 m), orchard soil (sand at pH 9; elevation, ≤25 m), and rainforest soil (oxisol at pH 5.5; elevation, ≤350 m). No other inland areas provided any EPNs (Fig. 1).
FIG. 1.
Map grid showing the exhaustive soil sampling in Guadeloupe islands (small points). The sites where entomopathogenic bacterium-nematode complexes were harvested are indicated by symbols identifying the nematode species: ★, H. indica; ✷, H. bacteriophora; and ✠, Heterorhabditis sp. The symbiont strain and genotype number are shown beside each symbol.
From the seven other Caribbean islands, 46 symbionts were isolated, including 41 Photorhabdus and five Xenorhabdus spp. Photorhabdus sp. was found on all of the islands except Saint-Vincent (Table 1). Of the Photorhabdus isolates, 35 were isolated from H. indica, 5 were from H. bacteriophora, and 1 was from an unidentified Heterorhabditis sp. Of the five Xenorhabdus isolates, one originated from S. cubanum (in Cuba), one originated from S. bicornutum (in Jamaica), one originated from S. puertoricense (in Puerto Rico), and two were from two new species of Steinernema not yet described (in Martinique and Saint-Vincent) (Table 1). In the Dominican Republic and Puerto Rico, a species prevalence similar to that for the Guadeloupe islands was observed, allowing us to determine that H. indica was also dominant (15 of 18 isolates in the Dominican Republic and 16 of 17 isolates in Puerto Rico) and that H. bacteriophora was rarely represented (2 of 18 isolates and 1 of 17 isolates, respectively). However, in contrast to the Guadeloupe survey, H. indica and consequently its Photorhabdus symbiont were isolated from the inland part of Puerto Rico and the Dominican Republic and were not restricted to the coastal areas.
Amplified 16S rDNAs and RFLP data.
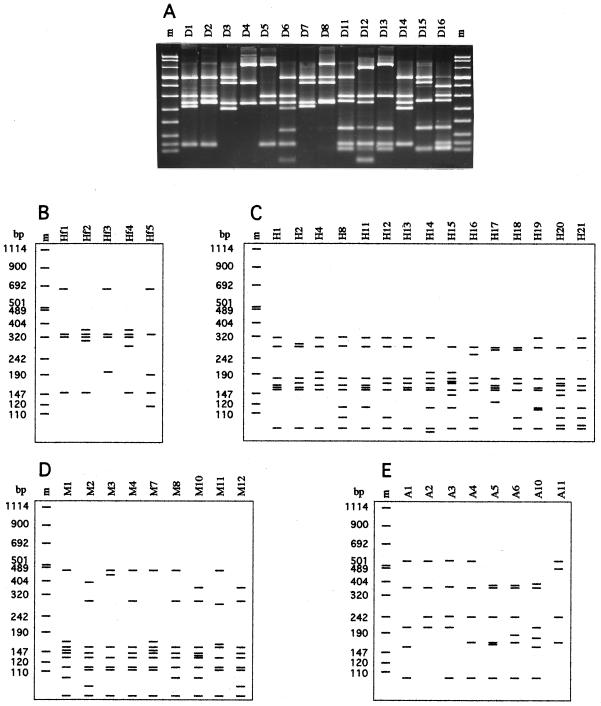
In all, 72 Photorhabdus, 5 Xenorhabdus, and 43 reference strains (including 20 Xenorhabdus and 20 Photorhabdus spp. and 3 other genera of Enterobacteriaceae) were further investigated by PCR ribotyping. 16S rDNA genes of all 120 strains were amplified by using PCR primers representing regions of the 16S rDNA conserved in bacteria. All of the strains produced a single band of about 1,600 bp. Polymorphic restriction patterns were obtained with the six endonucleases used. Results of the Photorhabdus and Xenorhabdus patterns are presented in Fig. 2 except for the CfoI patterns that were the same as those shown previously (13). By combining all of the restriction patterns, the 120 strains could be grouped into 33 genotypes (Table 3).
FIG. 2.
Photographic (A) and schematic (B to E) restriction patterns of PCR-amplified 16S rRNA genes from Xenorhabdus and Photorhabdus strains digested with the following enzymes: DdeI (A), HinfI (B), HaeIII (C), MspI (D), and AluI (E). The lane assignments correspond to the patterns given in Table 3. Lanes m, molecular-weight marker VIII (Boehringer Mannheim).
TABLE 3.
Genotypes and restriction patterns revealed by RFLP analysis of PCR-amplified 16S rRNA genes
| RFLP genotypea | Representative strain | Species | Restriction pattern of amplified 16S rRNA genes digested with:
|
|||||
|---|---|---|---|---|---|---|---|---|
| CfoI | HinfI | HaeIII | MspI | DdeI | AluI | |||
| 1 | AN6T | X. nematophilus | C1 | Hf1 | H1 | M1 | D4 | A1 |
| 2 | A24 | X. nematophilus | C1 | Hf1 | H1 | M7 | D4 | A1 |
| 3 | G6T | X. poinarii | C1 | Hf1 | H8 | M2 | D5 | A3 |
| 4 | K77 | Xenorhabdus sp. | C1 | Hf1 | H4 | M2 | D5 | A3 |
| 5 | Q58T | X. beddingii | C1 | Hf1 | H1 | M8 | D5 | A2 |
| 6 | T228T | X. bovienii | C2 | Hf1 | H11 | M2 | D3 | A1 |
| 7 | Si | X. bovienii | C2 | Hf2 | H11 | M2 | D3 | A1 |
| 8 | F3 | X. bovienii | C1 | Hf1 | H11 | M2 | D3 | A1 |
| 9 | SaV | Xenorhabdus sp. | C8 | Hf4 | H8 | M2 | D11 | A1 |
| 10 | HbT | P. luminescens | C5 | Hf3 | H12 | M3 | D1 | A5 |
| 11 | C8406 | P. luminescens | C5 | Hf3 | H12 | M4 | D1 | A3 |
| 12 | IS5 | P. luminescens | C4 | Hf3 | H12 | M3 | D6 | A3 |
| 13 | HP88 | P. luminescens | C1 | Hf3 | H13 | M3 | D14 | A3 |
| 14 | X1Nach | P. luminescens | C3 | Hf1 | H13 | M4 | D2 | A4 |
| 15 | Meg | P. luminescens | C1 | Hf1 | H2 | M4 | D7 | A6 |
| 16 | C1 | P. luminescens | C4 | Hf1 | H2 | M4 | D7 | A6 |
| 17 | NZH | P. luminescens | C1 | Hf1 | H14 | M4 | D7 | A4 |
| 18 | JP02 | Xenorhabdus sp. | C1 | Hf2 | H1 | M1 | D4 | A1 |
| 19 | PR06-A | Xenorhabdus sp. | C1 | Hf1 | H4 | M2 | D4 | A1 |
| 20 | VC01 | Xenorhabdus sp. | C1 | Hf1 | H4 | M2 | D13 | A1 |
| 21 | FRM16 | Xenorhabdus sp. | C1 | Hf1 | H4 | M4 | D8 | A10 |
| 22 | KR1 | Xenorhabdus sp. | C1 | Hf2 | H19 | M2 | D15 | A1 |
| 23 | UY61 | Xenorhabdus sp. | C1 | Hf1 | H20 | M12 | D16 | A1 |
| 24 | CN01 | Xenorhabdus sp. | C1 | Hf1 | H21 | M2 | D11 | A3 |
| 25 | JM26 | Xenorhabdus sp. | C1 | Hf5 | H15 | M10 | D12 | A3 |
| 26 | USTX62 | Xenorhabdus sp. | C1 | Hf5 | H15 | M10 | D5 | A3 |
| 27 | JM12 | P. luminescens | C4 | Hf3 | H16 | M3 | D6 | A3 |
| 28 | FRG26 | P. luminescens | C1 | Hf3 | H12 | M11 | D14 | A3 |
| 29 | 1216-79 | P. luminescens | C1 | Hf1 | H18 | M4 | D1 | A11 |
| 30 | Q614 | P. luminescens | C4 | Hf3 | H17 | M4 | D1 | A4 |
| 31 | CIP 5860T | Proteus vulgaris | C7 | Hf2 | H6 | M6 | D9 | A8 |
| 32 | CIP 548T | Escherichia coli | C6 | Hf1 | H5 | M5 | D10 | A7 |
| 33 | CIP 103235T | Serratia marcescens | C1 | Hf1 | H7 | M5 | D8 | A9 |
RFLP genotypes numbered 1 to 17 and 31 to 33 were previously identified (13), and RFLP genotypes numbered 18 to 30 were defined in this study.
RFLP analysis of Photorhabdus isolates and reference strains.
The 72 Caribbean Photorhabdus strains were divided into four genotypes (numbered 12, 13, 27, and 28 in Table 1) based on their RFLP patterns (Fig. 2).
A subsample of eight Photorhabdus isolates belonging to each of the four genotypes defined above was typed with three additional tetrameric enzymes (HinfI, MspI, and DdeI). No more polymorphism was observed.
RFLP patterns from the Caribbean strains were compared to those of 20 Photorhabdus reference strains, of which 14 had been previously typed (13). The six new reference strains (1216-79, 2407-88, 2617-87, 3105-77, 3265-86, and Q614) were separated into two newly defined genotypes (Table 2): genotype 29 included five opportunistic Photorhabdus clinical strains, which were all identical, and genotype 30 included the unique strain Q614 from Australia. Thus, among all the P. luminescens strains tested, 12 genotypes were defined (genotypes 10 to 17 and 27 to 30 [Table 3]), 4 of which were novel. Three restriction enzymes (AluI, CfoI, and HaeIII) were sufficient to resolve them.
Three of the four genotypes observed in Caribbean Photorhabdus isolates were also observed among Photorhabdus reference strains originating from other parts of the world: genotype 12 was represented by the reference strain IS5, a symbiont of H. indica from Israel; genotype 27 matched the genotype of strain D1, a symbiont of H. indica from Australia; and genotype 13 corresponded to those of the symbiotic strains HP88 and K80 from H. bacteriophora and Heterorhabditis sp., respectively. In contrast, genotype 28 was specific to two Photorhabdus isolates from the Guadeloupe islands and was not observed among the reference strains.
RFLP analysis of Xenorhabdus isolates and reference strains.
The amplified 16S rDNAs from the five Caribbean Xenorhabdus isolates (CU01, JM26, PR06-A, VC01, and FRM16) were analyzed with six endonucleases and were compared to the genotypes of 20 Xenorhabdus reference strains, of which 13 had been analyzed previously and shown to belong to nine 16S rDNA genotypes (13). By combining the different restriction patterns (Fig. 2; Table 3), each of the five Caribbean isolates belonged to a distinct 16S rDNA genotype, four of which were novel (Table 1). The seven newly investigated Xenorhabdus reference strains (SK72, USFL52, JP02, KR1, UY61, CN01, and USTX62) were grouped into seven different 16S rDNA genotypes (named genotypes 3, 6, 18, 22 to 24, and 26), five of which were novel (Table 2). In all, 18 Xenorhabdus genotypes were defined, half of which were novel (Table 3). The Caribbean isolate CU01, obtained from S. cubanum, and the reference strain SK72, a symbiont of S. glaseri, were identical to the type strain (G6T) of the species X. poinarii. The new reference strain USFL52 from Florida exhibited the same pattern as T228T, the type strain of X. bovienii.
Genetic relationships between amplified 16S rRNA genes.
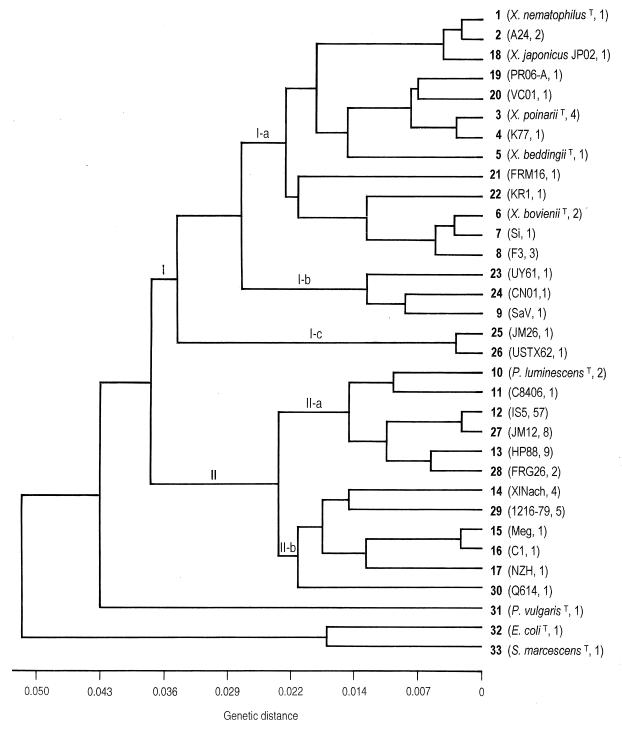
Comparison of the restriction profiles obtained with Photorhabdus and Xenorhabdus isolates revealed 30 distinctive genotypes. The three additional genotypes in Table 3 correspond to the other Enterobacteriaceae. To estimate the genetic relationships between PCR-amplified 16S rDNAs, we calculated a matrix of pairwise genetic distances for the 33 genotypes defined with the six restriction enzymes (Table 3). A mean of 33 restriction fragments per genotype was analyzed. The distance matrix was used to construct a dendrogram based on a UPGMA algorithm (Fig. 3). Twenty-three of the 16S rDNA genotypes were represented by only one to two strains, and the remaining seven were represented by multiple strains (Fig. 3). Two major groups (I and II) were delineated at a genetic distance of 0.038 and corresponded to Xenorhabdus and Photorhabdus genera, respectively. As expected, the three other Enterobacteriaceae genera branched apart from these two groups.
FIG. 3.
Cluster analysis (UPGMA) of the 33 PCR-RFLP genotypes of 16S rDNA defined in Table 3. The name of the representative strain and the number of strains which had the same genotype are in parentheses.
Within group I, three subgroups (I-a, I-b, and I-c) were shown (Fig. 3). The first one, I-a, comprised 20 of the 25 Xenorhabdus strains examined, including the 5 described species. Strain JP02, from S. kushidai, was grouped with the X. nematophilus species at a very low genetic distance of 0.005. Xenorhabdus isolates PR06-A, from S. puertoricense (Puerto Rico), and VC01, from Steinernema sp. (Saint-Vincent), clustered near the type strain of X. poinarii and strain K77 from S. rarum. Strain KR1, from S. monticolum, clustered near the X. bovienii. Strain FRM16, a Xenorhabdus isolate from Martinique, could not be grouped specifically with any other strain. The second subgroup, I-b, included three genotypes, each represented by a single strain isolated from the nematodes: S. scapterisci, S. serratum, and S. arenarium. A relatively high genetic divergence of 1.3% was found among them. The third separate subgroup, I-c, branched far apart from subgroups I-a and I-b at the limit of the genus Xenorhabdus. It was composed of only two strains: JM26, from S. bicornutum (Jamaica), and USTX62, from S. riobrave (Texas), which were closely related (0.35% divergence).
Group II of the Photorhabdus bacteria was divided into two subgroups, II-a and II-b (Fig. 3). Within subgroup II-a the type strain of P. luminescens clustered with the four genotypes encountered in the Caribbean basin (genotypes 12, 13, 27, and 28). Among them, genotypes 12 and 27, from symbionts of H. indica, were closely related (0.2% divergence). Genotypes 13 and 28 from symbionts of H. bacteriophora were less related (0.6% divergence). Subgroup II-b encompassed more divergent genotypes, including symbionts of H. megidis and H. zealandica (genotypes 14 to 17), the clinical strains (genotype 29), and the strain Q614 (genotype 30).
Bacterial genotype distribution in relation to host nematodes and geography.
We isolated 72 Photorhabdus and 5 Xenorhabdus spp. from the Caribbean basin. Among the Photorhabdus spp. four genotypes were identified. All of the 63 isolates of the genotypes 12 and 27 originated from H. indica. The two reference strains IS5 and D1, isolated from H. indica, also shared these genotypes. Genotype 12 was therefore the most prevalent (56 of 63 isolates) of the Photorhabdus genotypes, and it occurred throughout the Caribbean region. It was restricted to the coastal areas in the Guadeloupe islands (Fig. 1), but it also occurred inland in the Dominican Republic and Puerto Rico. Genotype 27 was rare and found only in Petite Terre, Jamaica, Martinique, and in the northern Guadeloupe islands (Saint-Martin and Saint-Barthélemy).
All seven isolates of genotype 13 originated from H. bacteriophora and matched the reference H. bacteriophora strain HP88. Three other genotypes (genotypes 10, 16, and 28) also were found in isolates from H. bacteriophora (strains Hb, C1, and FRG29). Genotype 28 was new, was restricted to Guadeloupe, and was not detected among the reference strains collected in the rest of the world.
Steinernema spp. were very scarce in the Caribbean basin and therefore could not be related to geographical origins. The five Xenorhabdus isolates corresponded to five different genotypes, and each of them was isolated from a different species of Steinernema: S. cubanum, S. bicornutum, S. puertoricense, Steinernema sp.1, and Steinernema sp.2, the two latter being new species not yet described.
DISCUSSION
Based on RFLP analysis of 16S rDNA from bacterial symbionts, host nematode characterization, and the geographical distribution of genetic bacterial groups, we described here the genetic composition of EPN symbiotic bacteria in the Caribbean basin and compared them to symbiotic strains collected at various localities throughout the world.
The sampling intensity allowed us to estimate a higher degree of diversity compared with the Xenorhabdus and Photorhabdus strains studied previously. Thirteen new genotypes were detected and successfully added to the 17 previously defined ones (13); this was done without substantially altering the phylogenetic relationships established previously by clustering analysis. Thus, PCR-RFLP analysis applied to 16S rDNA proved to be a rapid and sensitive typing method for distinguishing strains of the Xenorhabdus and Photorhabdus genera. Because we found new genotypes and new restriction patterns among Xenorhabdus and Photorhabdus spp., the number of endonucleases required to generate all of the genotypes has to be reconsidered. HaeIII, CfoI, and AluI have to be used to differentiate all of the Photorhabdus genotypes, and five restriction enzymes (CfoI, HinfI, MspI, HaeIII, and DdeI) are necessary in order to distinguish all of the Xenorhabdus genotypes.
The addition of new genotypes in the 16S rDNA clustering analysis revealed an unusually high level of genetic diversity within the Xenorhabdus genus compared to previous descriptions (13, 20, 26, 30, 31). This development likely resulted from the large number of studied strains originating from 16 identified Steinernema species and from various localities worldwide. For instance, S. arenarium, S. bicornutum, S. scapterisci, and S. serratum, whose symbionts were not previously typed, proved to harbor divergent Xenorhabdus symbionts that were distantly related to described species. Most of the genotypes were so divergent that they may represent new species. However, due to a lack of similar bacterial isolates, new Xenorhabdus species could not be described. Compared to 16S ribosomal sequencing studies (20, 30, 31), the phylogenetic position of the symbiont of S. kushidai, which is closely related to X. nematophilus, was corroborated, whereas the phylogenetic position of the symbiont of S. riobrave was different. Because novel Xenorhabdus strains were detected, the complete 16S rRNA genes should be sequenced in order to refine the phylogenetic tree of the Xenorhabdus genus.
Clustering analysis of the Photorhabdus genotypes revealed two major subgroups corresponding to the host nematodes and their ecological data. The first subgroup, II-a, included symbionts of H. indica and H. bacteriophora, which were found in the Caribbean and other tropical regions, whereas the second subgroup, II-b, included symbionts of H. megidis and H. zealandica, which were limited to the temperate regions. Previous 16S rDNA sequencing analyses corroborate the delineation between symbionts of H. indica and H. bacteriophora and the symbionts of H. megidis (20, 31). Moreover, within subgroup II-a, the similarity between symbionts of H. indica and H. bacteriophora is also corroborated by ribosomal sequencing (20).
Because of a higher number of isolates and a precise identification of their symbiotic nematodes that were not available in our previous data (13), a clear relationship between 16S rDNA genotypes and Heterorhabditis species origins was detected. Thus, Photorhabdus genotypes 12 and 27 were exclusively associated with H. indica, whereas Photorhabdus genotypes 13 and 28 were only associated with H. bacteriophora. Yet in two cases (strains HbT and C1) an inconsistency was observed. HbT and C1 were isolated from nematodes initially named H. bacteriophora but which are now known to differ from the typical H. bacteriophora represented by HP88 (18). The native host-nematodes of HbT and C1 may belong to two distinct species or subspecies that are different from the species of H. bacteriophora associated with genotypes 13 and 28. Because the identification of Heterorhabditis spp. is difficult, the characterization of their bacterial symbionts may help resolve some difficult taxonomic questions regarding their hosts.
Geographical grouping of the Photorhabdus genotypes was linked to nematode distribution. Bacterial genotypes associated with H. indica are restricted to tropical areas as is their host H. indica (24), whereas genotypes associated with H. bacteriophora seem to be more homogeneously distributed, as is their host H. bacteriophora (18). Furthermore, in the Guadeloupe islands, most of the H. indica isolates were found in coastal sandy soils, and all of the H. bacteriophora were found in the vertisols of croplands and the oxisols of forests. These results agree with previous studies indicating that Heterorhabditis spp. mainly occur in coastal areas (18). However, some H. indica nematodes were isolated from inland soils in Puerto Rico and the Dominican Republic, possibly as a result of soil material transfer on these relatively developed islands. Both genotypes associated with H. indica (genotypes 12 and 27) were spread throughout the Caribbean basin, suggesting that the host species is the predominant determinant of geographic distribution. To evaluate more accurately the selective pressure applied by the host nematode versus those that might be applied by soil factors on symbiont populations, further studies on the possible occurrence of free-living Xenorhabdus and Photorhabdus isolates in soil are required.
The high degree of Xenorhabdus diversity is congruent with the wide diversity of associated Steinernema nematodes and, with only one exception, the genotypes reflect the nematode host species. This exception is represented by the genotype of X. poinarii, which is associated with two nematode species: S. glaseri and S. cubanum. These two nematode species are closely related because they share morphological and ITS-based similarities (18). A complementary study of the symbionts by phenotypic characterization and DNA-DNA hybridization is in progress to confirm this finding. If verified, this would be the second reported case of a Xenorhabdus species associated with different Steinernema species, X. bovienii associated with S. feltiae, S. kraussei, and S. affine that occur in the same region and environment (11).
Molecular tools, such as 16S rDNA PCR-RFLP analysis for bacterial typing, along with satellite DNA probes and isozyme analysis for EPN identification, are fast and accurate ways of comparing bacterium-nematode associations on a large geographical scale. A high level of taxonomic congruence has been detected by using this approach between symbiont pairs, a finding that supports an early coevolution of these symbioses. The perenniality of the association may have resulted from the vertical transmission of symbiotic bacteria during monoxenic sepsis produced during parasitism and from an early intestinal contamination of infective juveniles escaping insect cadavers. Each species of nematode seems to secure a very restricted microbial niche that is more or less specific to a particular Xenorhabdus or a Photorhabdus species.
ACKNOWLEDGMENTS
The technical assistance of Eliane Bonifassi and Anne Lanois for the isolation and first characterization of the reference symbionts is gratefully acknowledged. We also appreciate being able to use the biological material provided by Eva Arteaga, Enrique Cabanillas, Agueda Carro, Wilfredo Figueroa, Luis Garrido, Nelson Simões, Grover Smart, and Patricia Stock. We thank Alan Kirk for revising the English of the manuscript.
This work was supported by the MENRT grant 95-5-10697.
REFERENCES
- 1.Akhurst R J. Morphological and functional dimorphism in Xenorhabdus spp., bacteria symbiotically associated with the insect pathogenic nematodes Neoaplectana and Heterorhabditis. J Gen Microbiol. 1980;121:303–309. [Google Scholar]
- 2.Akhurst R J. Antibiotic activity of Xenorhabdus spp., bacteria symbiotically associated with insect pathogenic nematodes of the families Heterorhabditidae and Steinernematidae. J Gen Microbiol. 1982;128:3061–3065. doi: 10.1099/00221287-128-12-3061. [DOI] [PubMed] [Google Scholar]
- 3.Akhurst R J. Xenorhabdus nematophilus subsp. poinarii: its interaction with insect pathogenic nematodes. Syst Appl Microbiol. 1986;8:142–147. [Google Scholar]
- 4.Akhurst R J, Boemare N E. Biology and taxonomy of Xenorhabdus. In: Gaugler R, Kaya H K, editors. Entomopathogenic nematodes in biological control. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 75–90. [Google Scholar]
- 5.Akhurst R J, Boemare N E. A non-luminescent strain of Xenorhabdus luminescens (Enterobacteriaceae) J Gen Microbiol. 1986;132:1917–1922. [Google Scholar]
- 6.Akhurst R J, Boemare N E. A numerical taxonomic study of the genus Xenorhabdus (Enterobacteriaceae) and proposed elevation of the subspecies of X. nematophilus to species. J Gen Microbiol. 1988;134:1835–1845. doi: 10.1099/00221287-134-7-1835. [DOI] [PubMed] [Google Scholar]
- 7.Akhurst R J, Mourant R G, Baud L, Boemare N E. Phenotypic and DNA relatedness study between nematode symbionts and clinical strains of the genus Photorhabdus (Enterobacteriaceae) Int J Syst Bacteriol. 1996;46:1034–1041. doi: 10.1099/00207713-46-4-1034. [DOI] [PubMed] [Google Scholar]
- 8.Arteaga-Hernandez E M, Mrácek Z. Heterorhabditis heliothidis, a parasite of insect pests in Cuba. Folia Parasitol. 1984;31:11–17. [Google Scholar]
- 9.Bedding R A, Akhurst R J. A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica. 1975;21:109–110. [Google Scholar]
- 10.Boemare N E, Akhurst R J. Biochemical and physiological characterization of colony form variants in Xenorhabdus spp. (Enterobacteriaceae) J Gen Microbiol. 1988;134:751–761. doi: 10.1099/00221287-134-7-1835. [DOI] [PubMed] [Google Scholar]
- 11.Boemare N E, Akhurst R J, Mourant R G. DNA relatedness between Xenorhabdus spp. (Enterobacteriaceae), symbiotic bacteria of entomopathogenic nematodes, and a proposal to transfer Xenorhabdus luminescens to a new genus, Photorhabdus gen. nov. Int J Syst Bacteriol. 1993;43:249–255. [Google Scholar]
- 12.Boemare N E, Givaudan A, Brehélin M, Laumond C. Symbiosis and pathogenicity of nematode-bacterium complexes. Symbiosis. 1997;22:21–45. [Google Scholar]
- 13.Brunel B, Givaudan A, Lanois A, Akhurst R J, Boemare N. Fast and accurate identification of Xenorhabdus and Photorhabdus species by restriction analysis of PCR-amplified 16S rRNA genes. Appl Environ Microbiol. 1997;63:574–580. doi: 10.1128/aem.63.2.574-580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Constant, P., L. Marchay, M. Fischer-Le Saux, S. Panoma, and H. Mauléon. Natural occurrence of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) in Guadeloupe islands. Fund. Appl. Nematol., in press.
- 15.Farmer J J, III, Jorgensen J H, Grimont P A D, Akhurst R J, Poinar G O, Jr, Ageron E, Pierce G V, Smith J A, Carter G P, Wilson K L, Hickman-Brenner F W. Xenorhabdus luminescens (DNA hybridization group 5) from human clinical specimens. J Clin Microbiol. 1989;27:1594–1600. doi: 10.1128/jcm.27.7.1594-1600.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forst S, Dowds B, Boemare N, Stackebrandt E. Xenorhabdus spp. and Photorhabdus spp.: bugs that kill bugs. Annu Rev Microbiol. 1997;51:47–72. doi: 10.1146/annurev.micro.51.1.47. [DOI] [PubMed] [Google Scholar]
- 17.Grenier E, Bonifassi E, Abad P, Laumond C. Use of species-specific satellite DNAs as diagnostic probes in the identification of Steinernematidae and Heterorhabditidae entomopathogenic nematodes. Parasitology. 1996;113:483–489. doi: 10.1017/s0031182000081555. [DOI] [PubMed] [Google Scholar]
- 18.Hominick W M, Briscoe B R, Garcia del Pino F, Heng J, Hunt D J, Kozodoy E, Mracek Z, Nguyen K B, Reid A P, Spiridonov S, Stock P, Sturhan D, Waturu C, Yoshida M. Biosystematics of entomopathogenic nematodes: current status, protocols and definitions. J Helminthol. 1997;71:271–298. doi: 10.1017/s0022149x00016096. [DOI] [PubMed] [Google Scholar]
- 19.Laumond, C., H. Mauléon, W. Figueroa, E. Arteaga, and L. Garrido. Identification of Heterorhabditis and Steinernema from Caribbean islands. Nematologica, in press.
- 20.Liu J, Berry R, Poinar G, Moldenke A. Phylogeny of Photorhabdus and Xenorhabdus species and strains as determined by comparison of partial 16S rRNA gene sequences. Int J Syst Bacteriol. 1997;47:948–951. doi: 10.1099/00207713-47-4-948. [DOI] [PubMed] [Google Scholar]
- 21.Mauléon H, Barré N, Panoma S. Pathogenicity of 17 isolates of entomophagous nematodes (Steinernematidae and Heterorhabditidae) for the ticks Amblyomma variegatum (Fabricius), Boophilus microplus (Canestrini) and Boophilus annulatus (Say) Exp Appl Acarol. 1993;17:831–838. doi: 10.1007/BF00225856. [DOI] [PubMed] [Google Scholar]
- 22.Nei M, Li W H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA. 1979;76:5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura Y, Hagiwara A, Suzuki T, Yamanaka S. Xenorhabdus japonicus sp. nov. associated with the nematode Steinernema kushidai. World J Microbiol Biotechnol. 1994;10:207–210. doi: 10.1007/BF00360889. [DOI] [PubMed] [Google Scholar]
- 24.Poinar G O, Jr, Karunakar G K, David H. Heterorhabditis indicus n.sp. (Rhabditida: Nematoda) from India: separation of Heterorhabditis spp. by infective juveniles. Fund Appl Nematol. 1992;15:467–472. [Google Scholar]
- 25.Poinar G O, Jr, Thomas G M. Significance of Achromobacter nematophilus Poinar and Thomas (Achromobacteriaceae: Eubacteriales) in the development of the nematode DD-136 (Neoaplectana sp., Steinernematidae) Parasitology. 1966;56:385–390. doi: 10.1017/s0031182000070980. [DOI] [PubMed] [Google Scholar]
- 26.Rainey F A, Ehlers R-U, Stackebrandt E. Inability of the polyphasic approach to systematics to determine the relatedness of the genera Xenorhabdus and Photorhabdus. Int J Syst Bacteriol. 1995;45:379–381. doi: 10.1099/00207713-45-2-379. [DOI] [PubMed] [Google Scholar]
- 27.Román J, Figueroa W. Steinernema puertoricencis n.sp. (Rhabditida: Steinernematidae), a new entomopathogenic nematode from Puerto Rico. J Agric Univ P R. 1994;78:167–175. [Google Scholar]
- 28.Sneath P H A, Sokal R R. Numerical taxonomy. The principles and practice of numerical classification. San Francisco, Calif: W. H. Freeman & Co.; 1973. [Google Scholar]
- 29.Stock S P, Choo H Y, Kaya H K. An entomopathogenic nematode, Steinernema monticolum sp.n. (Rhabditida: Steinernematidae) from Korea with a key to other species. Nematologica. 1997;43:15–29. [Google Scholar]
- 30.Suzuki T, Yabusaki H, Nishimura Y. Phylogenetic relationships of entomopathogenic nematophilic bacteria: Xenorhabdus spp. and Photorhabdus sp. J Basic Microbiol. 1996;36:351–354. doi: 10.1002/jobm.3620360509. [DOI] [PubMed] [Google Scholar]
- 31.Szállás E, Koch C, Fodor A, Burghardt J, Buss O, Szentirmai A, Nealson K H, Stackebrandt E. Phylogenetic evidence for the taxonomic heterogeneity of Photorhabdus luminescens. Int J Syst Bacteriol. 1997;47:402–407. doi: 10.1099/00207713-47-2-402. [DOI] [PubMed] [Google Scholar]