Abstract
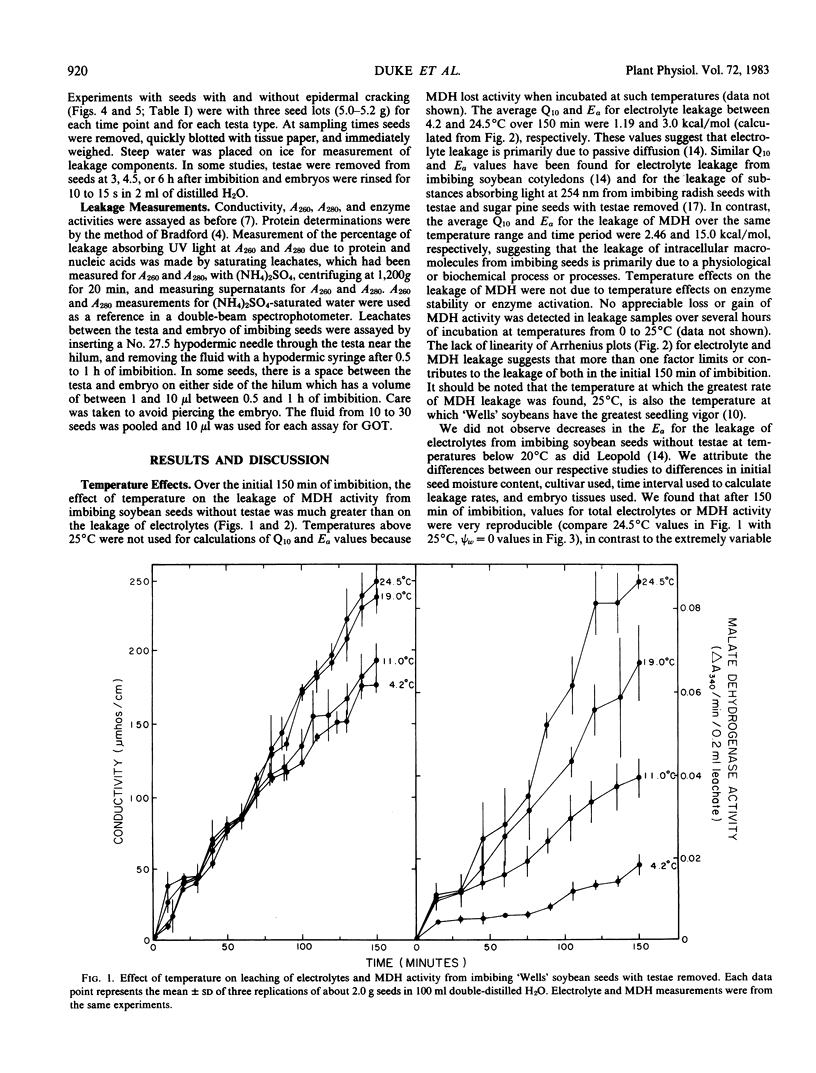
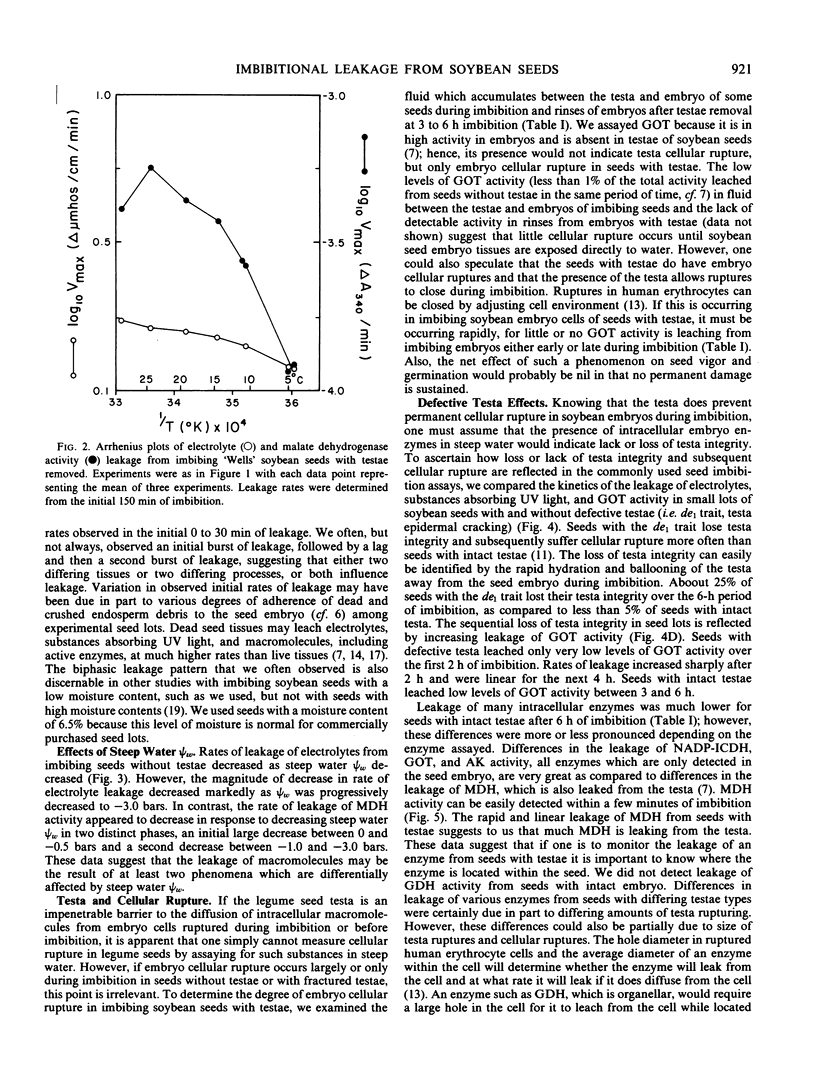
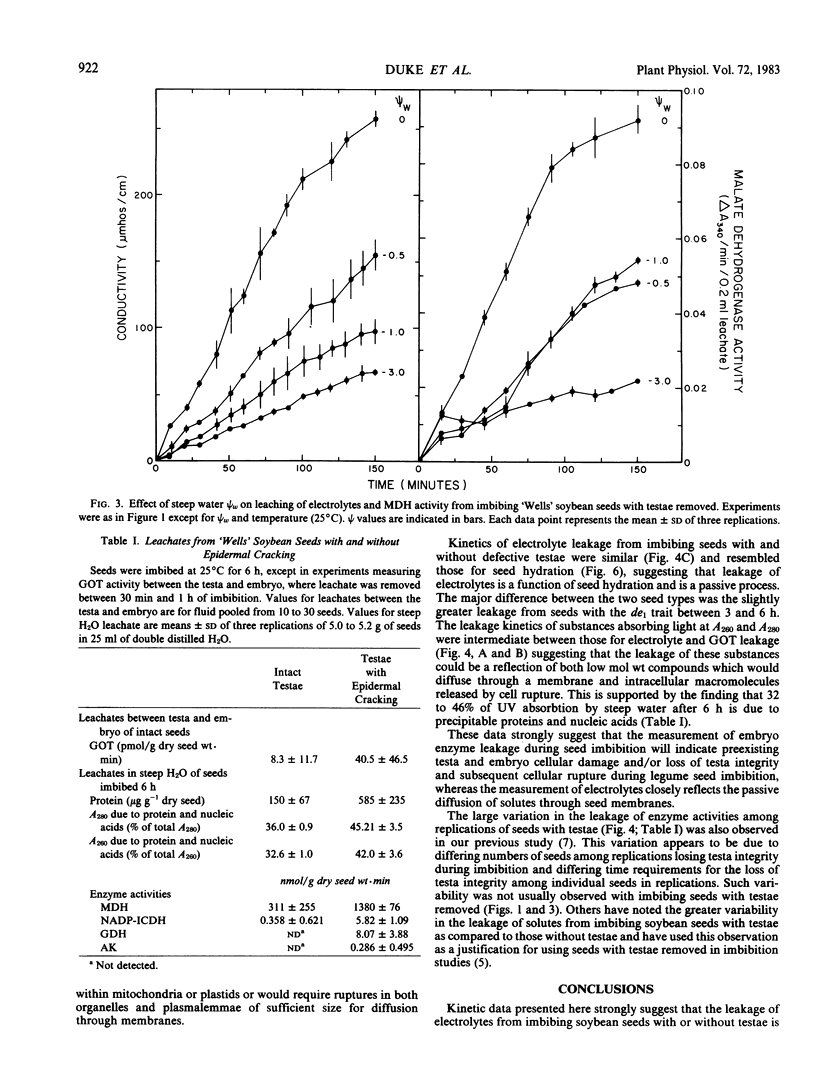
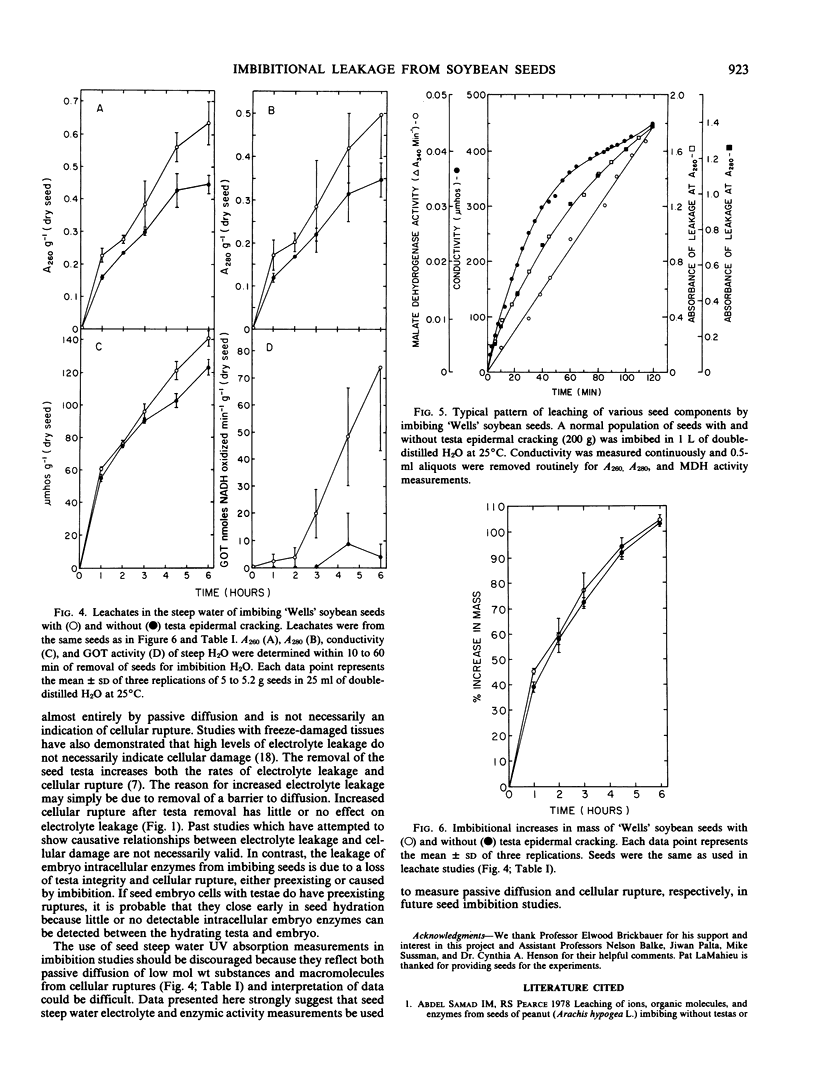
Leakage of electrolytes, substances absorbing UV light, and enzymic activities from imbibing soybean (Glycine max [L.] Merr.) seeds were compared to determine the extent that passive diffusion and cellular rupture contribute to each. Imbibing seeds with testae removed had average Arrhenius energies of activation (5 to 25°C) of 3.0 and 15.8 kilocalories per mole, respectively, for the leakage of electrolytes and embryo malate dehydrogenase activity. Leakage of embryo enzymes from imbibing seeds was dependent on loss of testa integrity and subsequent loss of cellular membrane integrity or inability to seal preexisting membrane discontinuities. These data suggest that electrolyte leakage from imbibing seeds is primarily by passive diffusion, whereas the diffusion of intracellular macromolecules is primarily dependent on physiological phenomena affecting membrane integrity. Kinetic data and examination of the composition of seed leachates indicated that the leakage of substances absorbing UV light during imbibition is due to both passive diffusion of low molecular weight solutes and macromolecules released from ruptured cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becwar M. R., Stanwood P. C., Roos E. E. Dehydration effects on imbibitional leakage from desiccation-sensitive seeds. Plant Physiol. 1982 May;69(5):1132–1135. doi: 10.1104/pp.69.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bramlage W. J., Leopold A. C., Parrish D. J. Chilling Stress to Soybeans during Imhibition. Plant Physiol. 1978 Apr;61(4):525–529. doi: 10.1104/pp.61.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke S. H., Kakefuda G. Role of the testa in preventing cellular rupture during imbibition of legume seeds. Plant Physiol. 1981 Mar;67(3):449–456. doi: 10.1104/pp.67.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke S. H., Schrader L. E., Miller M. G. Low Temperature Effects on Soybean (Glycine max [L.] Merr. cv. Wells) Mitochondrial Respiration and Several Dehydrogenases during Imbibition and Germination. Plant Physiol. 1977 Nov;60(5):716–722. doi: 10.1104/pp.60.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson L. A. The effect soaking pea seeds with or without seedcoats has on seedling growth. Plant Physiol. 1968 Feb;43(2):255–259. doi: 10.1104/pp.43.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold A. C. Temperature effects on soybean imbibition and leakage. Plant Physiol. 1980 Jun;65(6):1096–1098. doi: 10.1104/pp.65.6.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber M. R., Steck T. L. A description of the holes in human erythrocyte membrane ghosts. J Biol Chem. 1982 Oct 10;257(19):11651–11659. [PubMed] [Google Scholar]
- McKersie B. D., Stinson R. H. Effect of Dehydration on Leakage and Membrane Structure in Lotus corniculatus L. Seeds. Plant Physiol. 1980 Aug;66(2):316–320. doi: 10.1104/pp.66.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. B., Noland T. L. Temperature effects on seed imbibition and leakage mediated by viscosity and membranes. Plant Physiol. 1982 Feb;69(2):428–431. doi: 10.1104/pp.69.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palta J. P., Levitt J., Stadelmann E. J. Freezing injury in onion bulb cells: I. Evaluation of the conductivity method and analysis of ion and sugar efflux from injured cells. Plant Physiol. 1977 Sep;60(3):393–397. doi: 10.1104/pp.60.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish D. J., Leopold A. C. Transient changes during soybean imbibition. Plant Physiol. 1977 Jun;59(6):1111–1115. doi: 10.1104/pp.59.6.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]


