Abstract
Among individuals with posttraumatic stress disorder (PTSD), verbal learning and memory are areas of weakness compared with other cognitive domains (e.g., visuospatial memory). In this study, previously deployed military veterans completed clinical assessments of word memory and vocabulary (n = 243) and a laboratory task measuring encoding, free recall, repetition priming, and recognition of words (n = 147). Impaired verbal memory was selectively related to reexperiencing symptoms of PTSD but was not associated with other symptom groupings or blast-induced traumatic brain injury. Implicit priming of response times following word repetition was also unrelated to clinical symptoms. Instead, slowed response times during encoding explained associations between reexperiencing and memory performance. These findings are consistent with alterations in attentional control explaining PTSD-related verbal-memory deficits. Such findings have implications for understanding trauma-focused psychotherapy and recovery, which may depend on efficient attentional processing of words to alter posttraumatic reexperiencing symptoms.
Keywords: posttraumatic stress disorder, PTSD, brain injury, verbal memory, implicit priming, attention, encoding
Memories and decision-making surrounding trauma are important for understanding the nature of posttraumatic stress disorder (PTSD) and related symptoms. There is evidence for disruptions in the management of autobiographical memory content in PTSD (Brewin, 2003; McNally et al., 1994, 1995), but it remains less clear how specific cognitive processes supporting memory function are tied to the symptoms of PTSD. Given disparities between rates of trauma exposure (≈50%–60%) and lifetime prevalence of PTSD (≈8%; Kilpatrick et al., 2013), individual differences in particular aspects of memory formation and retrieval may play a role in how trauma can lead to chronic posttraumatic stress symptoms. In this study of previously deployed U.S. military veterans, we investigated functions that support verbal memory and tested whether deficits in specific cognitive processes were associated with particular types of PTSD symptoms.
Individuals with PTSD commonly describe troubles with cognitive functioning (American Psychiatric Association, 2013; Binder et al., 1999; Jacobs & Iacopino, 2001), and several meta-analyses of PTSD have revealed impairments across most performance-based neuropsychological tests (Brewin et al., 2007; Johnsen & Asbjørnsen, 2008; Polak et al., 2012). Verbal memory, processing speed, and attention/executive functioning are areas of weakness relative to visuospatial functioning and visual memory domains (Scott et al., 2015). Specifically, initial verbal learning as well as later verbal recall after extended retention periods are often impaired in PTSD. In addition, verbal learning deficits appear to increase in proportion to the overall severity of posttraumatic stress symptoms and cannot be accounted for by exposure to past trauma alone (i.e., not present among trauma-exposed control participants; Scott et al., 2015), which suggests that impaired verbal performance may be important to the formation and maintenance of symptoms. One possibility is that in the months and years after psychological trauma, individuals with greater posttraumatic stress symptoms may experience fundamental cognitive inefficiencies when initially learning and encoding verbal material (Johnsen & Asbjørnsen, 2009). Rather than a selective problem with retention or recall affecting memory retrieval only, attentional disruption during initial encoding may be the key feature of impaired verbal memory among this clinical population.
During learning paradigms, implicit memory is activated in parallel with explicit memory processes (Donaldson et al., 2001; Squire & Knowlton, 2000). In practical terms, implicit memory facilitates a gist-level familiarity for previously presented stimuli, which enables more effective behavioral responding without evoking full conscious recollection. Several theoretical models predict preserved or even enhanced implicit memory functioning among individuals with PTSD (Brewin, 2014; Ehlers & Clark, 2000), but these predictions are difficult to directly evaluate. Implicit memory is usually inferred according to differences in uncued free-recall and cued-recognition performance. However, recognition tasks are generally easier than free-recall tasks and poorly separate explicit and implicit memory within conventional memory instruments. Using response times (RTs) during word tasks without explicit memory requirements is a more direct means of assessing implicit memory (Marsolek, 2003; Wagner & Koutstaal, 2002). Responses will generally be faster for recently processed words compared with new words because of implicit memory. This approach has been employed to evaluate implicit memory among people with schizophrenia (Sponheim et al., 2004). Similar evaluations for individuals with PTSD symptoms could test a potential mechanistic role of automatic implicit processes for verbal-memory impairments.
The heterogeneity of PTSD symptoms adds additional complexity when studying memory deficits. PTSD symptoms span a wide range of emotional and behavioral experiences and often present on a continuum across individuals with and without the categorical diagnosis of PTSD (Ruscio et al., 2002). An exclusive focus on categorical definitions of PTSD may obscure which symptom domains are predictive of functional outcomes and limit statistical power for detecting associations with cognitive mechanisms (Cuthbert, 2005; Grove, 1991). Factor analyses suggest the existence of separate PTSD symptom dimensions: intrusive reexperiencing, avoidance, dysphoric mood, and hyperarousal (Yufik & Simms, 2010), which appear to more closely track with brain-based systems than the PTSD diagnosis alone (Lieberman et al., 2017; Marquardt et al., 2018, 2021). Therefore, investigating how specific symptom dimensions of PTSD map onto memory deficits may clarify what aspects of the disorder relate to the consolidation and retrieval of verbal material.
For some individuals, traumatic events can lead to especially complex effects on functioning. People experiencing significant PTSD symptoms often meet criteria for other psychiatric diagnoses (Brady, 1997) and frequently report physical injuries sustained from traumatic experiences (Blanchard et al., 1995; Rasmussen et al., 2007). For recent U.S. military service members, mild traumatic brain injuries (mTBIs) associated with wartime explosive blasts are common (Warden, 2006). Blast exposures powerful enough to induce brain injury often are psychologically traumatic, which contributes to confusion regarding the exact origin of dysfunction after deployment (Hoge et al., 2008; Kennedy et al., 2007; Vanderploeg et al., 2009). Veterans with blast mTBI histories can present with clinical complaints resembling aspects of posttraumatic stress, such as impaired concentration, mood changes, and fatigue (Sayer, 2012), thereby making it difficult for frontline clinicians to determine the source of the cognitive deficits. For this reason, it is important to evaluate the degree to which PTSD symptoms account for verbal-memory impairment in veterans compared with mTBI. Understanding the likely source of memory impairment will help clarify whether PTSD symptoms or neurologically based injuries are the most appropriate treatment targets.
In the present study, we implemented widely used clinical neuropsychological measures in concert with a laboratory task to understand how specific facets of word-memory processing may be associated with symptoms of PTSD. Given the frequent comorbidity of brain injury and PTSD in military samples, we recruited a sample with a wide range of postdeployment clinical presentations to evaluate effects of posttraumatic symptoms and mTBI. Crucially, all individuals experienced similar deployments to war zones, which allowed for differentiation of current PTSD symptoms from the effects of common stressors associated with overseas deployments and military service. There are relatively few studies investigating relationships between symptom groupings of PTSD and verbal-memory deficits. We sought to clarify how separate components of verbal learning and memory relate to distinct domains of posttraumatic symptoms while considering the influence of mTBI. Clarifying which symptom domains are relevant to specific aspects of memory impairment could inform the personalization of PTSD treatment and guide innovation for psychotherapies involving reconsolidation of memories via word processing.
Method
Participants
Participants were U.S. military veterans with previous deployments to combat zones in Afghanistan and/or Iraq during Operations Enduring and/or Iraqi Freedom. To capture the range of typical clinical presentations among this population, we emphasized recruitment of individuals with PTSD symptoms and blast-related mTBIs. Participant sampling took place within a longitudinal study of Minnesota National Guard members and outpatients at the Minneapolis Veterans Affairs Health Care System (VAHCS). Individuals who reported similar deployment experiences but denied significant PTSD symptoms or a history of blast-related mTBI were also recruited from the same sources and included as a trauma-exposed comparison control group. In accordance with protocol approval by the Minneapolis VAHCS and University of Minnesota Institutional Review Boards, all participants provided written consent. Two separate study samples with similar inclusion and exclusion criteria were used for this report (for study details, see the Supplemental Material available online). Participants from Study 1 completed both the neuropsychological and laboratory assessments and denied a predeployment history of Axis I psychopathology as defined in the fourth edition, text revision of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR; Gilmore et al., 2018; Marquardt et al., 2018; Nelson et al., 2012). Study 2 participants completed only the neuropsychological evaluation and were not excluded for history of predeployment mental illness (besides psychotic disorders and attention-deficit conditions; Disner et al., 2017, 2018). In total, 243 veterans participated in the verbal functioning assessments.
Assessment
Clinical interview
Interview assessments were completed by research assistant and clinical psychology doctoral student staff under supervision from a licensed, doctoral-level clinical psychologist. The Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I; First et al., 2002) and the Clinician Administered PTSD Scale for DSM-IV (CAPS; Blake et al., 1995) were used to assess mental illness diagnoses. Individuals were classified in the PTSD group if they met DSM-IV-TR criteria for PTSD or qualified for subthreshold PTSD defined as endorsing at least one PTSD symptom in each of the DSM-IV-TR symptom groupings (B–D). This diagnostic threshold (i.e., full PTSD plus subthreshold PTSD combined together) is consistent with alternative rating schemes aimed at increasing sensitivity for clinically meaningful presentations of PTSD symptoms (Cukor et al., 2010; Marshall et al., 2001; Weathers et al., 1999, 2001; Zlotnick et al., 2002). CAPS symptom frequency scores of at least 1 and intensity scores of at least 2 were required to meet a PTSD symptom (Weathers et al., 1999). Because of an emphasis on assessing symptoms specific to PTSD in Study 1, the CAPS interview was discontinued if Criterion A (trauma exposure) and Criterion B (reexperiencing) were not met (see Table 1 and Table S1 in the Supplemental Material). Thus, a subset of Study 1 participants was not evaluated on Criterion C and D symptoms (for a tabulation of cases, see Table 1 and Table S1 in the Supplemental Material). Study 2 did not have a discontinuation criterion, and all participants completed the full CAPS. Diagnostic consensus for each participant was completed by at least two doctoral-level psychologists or advanced graduate students following review of the interview materials and available medical records.
Table 1.
Demographics and Clinical Characteristics for Combined Studies 1 and 2
| Variable | Participant status | |||
|---|---|---|---|---|
| No PTSD | PTSD | |||
| No mTBI | mTBI | No mTBI | mTBI | |
| Total | 89 | 40 | 47 | 67 |
| Study 1 | 31 | 18 | 21 | 42 |
| Study 2 | 58 | 22 | 26 | 25 |
| Female | 7 | 0 | 5 | 4 |
| Minority | 11 | 1 | 10 | 9 |
| Age (years) a | M = 34.2 (SD = 8.8) | M = 33.1 (SD = 8.4) | M = 35.2 (SD = 9.0) | M = 31.3 (SD = 6.3) |
| Education (years) a | M = 14.9 (SD = 1.6) | M = 14.5 (SD = 1.8) | M = 14.7 (SD = 2.5) | M = 13.9 (SD = 1.6) |
| Time since blast mTBI (months) a | M = 57.6 (SD = 29.6) | M = 61.2 (SD = 26.4) | ||
| Depressive disorder b | 8 | 4 | 13 | 26 |
| Alcohol dependence c | 7 | 6 | 12 | 17 |
| Full CAPS completed | 60 | 27 | 47 | 66 |
Note: Values are ns unless otherwise specified. PTSD = posttraumatic stress disorder; mTBI = mild traumatic brain injury; CAPS = Clinician-Administered PTSD Scale for DSM-IV (Blake et al., 1995).
These are group mean values using all available participant data. bCurrent major depressive disorder or dysthymia according to the fourth edition text revision of the Diagnostic and Statistical Manual of Mental Disorder (DSM-IV-TR). cCurrent alcohol dependence according to the DSM-IV-TR.
Deployment-related blast exposure and head injury sequelae were evaluated using the semistructured Minnesota Blast Exposure Screening Tool (MN-BEST; Nelson et al., 2011). Each participant’s three most significant blast events were assessed according to severity of acute-stage signs and symptoms (e.g., loss of consciousness, duration of postblast amnesia, neurological signs) and plausibility (e.g., reported proximity with the blast detonation site) using American Congress of Rehabilitation Medicine criteria (Kay et al., 1993). Consensus for mTBI was achieved using neuropsychological assessment teams, which included at least one licensed clinical neuropsychologist. Participants from both Study 1 and Study 2 were then assigned to one of four groups: no PTSD/no mTBI, no PTSD/mTBI, PTSD/no mTBI, and PTSD/mTBI (see Table 1).
Neuropsychological evaluation of verbal functioning
Participants completed the California Verbal Learning Test–Second Edition (CVLT-II; Delis, 2000) as a component of a larger battery of cognitive assessments (Disner et al., 2017; Nelson et al., 2012, 2020). The CVLT-II is a widely used measure of word list learning and memory with multiple indices of performance relevant for recall and recognition of verbal content (Delis, 2000; Rabin et al., 2016). Experimenters read all word stimuli aloud and provided queries to participants to assess performance. Confirmatory factor analyses have identified four latent factors underlying CVLT-II performance (DeJong & Donders, 2009; Donders, 2008): Attention Span indexes effectiveness at maintaining information in mind for immediate use, Learning Efficiency reflects the degree to which strategic learning approaches were enacted (e.g., semantic clustering), Delayed Recall captures how much verbal information was retained and later accessed via recall and recognition, and Inaccurate Recall reflects the presence of word intrusions and false positives during recollection. Composite scores in each domain were generated and converted to z scores using the age- and sex-stratified normative information published within the CVLT-II manual (Delis, 2000). To assess the specificity of associations between CVLT-II verbal-memory performance deficits and postdeployment clinical conditions, we also tested for statistical relationships with the Wechsler Test of Adult Reading (WTAR; Wechsler, 2001) scores. The WTAR contains words with irregular pronunciations that exhibit increasing phonetic difficulty. Pronunciation ability for WTAR words may reflect past verbal learning histories and lifetime cognitive abilities that are typically resistant to recent disease processes or neurologic insult (Green et al., 2008). T scores based on the original WTAR normative sample were generated for study participants. Participants were excluded from any study analysis when they failed one or more effort measures in the larger test battery (for additional details, see Disner et al., 2017).
Laboratory procedure
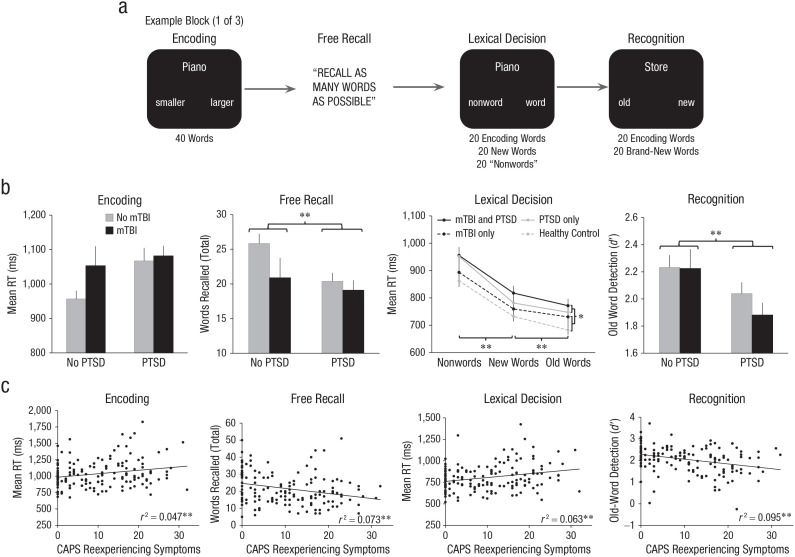
Participants from Study 1 completed an experimental, laboratory-based, verbal-memory task designed to distinguish between explicit and implicit memory abilities and generate more nuanced metrics of core cognitive processes contributing to memory performance (Longenecker et al., 2018; Paller et al., 1995; Sponheim et al., 2004). All stimuli were presented via computer monitor. The task consisted of three blocks, each of which was composed of discrete encoding, free-recall, lexical-decision, and recognition periods (Fig. 1a). During encoding, 40 words for various objects were displayed individually for 244 ms; 4,651-ms intertrial intervals and 3,700-ms response windows began 300 ms after each word presentation. Participants responded via button box to make size judgments about whether the named object (e.g., COTTAGE) was smaller or larger than the computer monitor on which the words were displayed. After encoding, participants performed uncued free recall aloud for as many words as possible from the just-completed encoding period. This was followed by a lexical-decision condition to assess implicit memory through a measurement of repetition priming. The lexical-decision period involved 20 old words from the preceding encoding period, 20 new words, and 20 pronounceable nonwords. Each word was displayed for 244 ms; intertrial intervals were 3,667 ms, and response windows were 2,700 ms. Participants were told to indicate whether each stimulus was a word or a nonword as quickly as possible. The difference in RTs between the old words and new words was used as a measure of repetition priming and implicit memory function. The recognition period involved presentation of 20 words during the encoding period and 20 new words using timing windows identical to the lexical-decision trials. The 20 words presented from the encoding period were those that were not presented during the lexical-decision period, thus ensuring that recognition was not confounded by a prior presentation of words during the lexical-decision period. No trauma-related word content was included within the word lists.
Fig. 1.
Laboratory verbal-memory task design and results. In (a), examples are given of each portion of the task during one block of the laboratory verbal-memory paradigm. Categorical results are given in (b): Task performance is graphed separately for categories of posttraumatic stress disorder (PTSD) and mild traumatic brain injury (mTBI). Dimensional results are given in (c): The scatterplots (with best-fitting regression lines) show the associations between symptom dimensions and task-performance measures (n = 147). Asterisks indicate significant differences between groups (*p < .05; **p < .01). CAPS = Clinician Administered PTSD Scale (Blake et al., 1995); RT = response time.
Encoding accuracy and mean RT were calculated using the total number of correct size judgments across all blocks. Free-recall scores were the total number of correctly self-generated words across all blocks; repeated words and words remembered from different blocks were not counted. Lexical-decision accuracy and mean RT were computed for correct word and nonword identifications across all blocks. Implicit memory was measured as described above (see Data Analysis section). During recognition, hits, false alarms, misses, and correct rejections were characterized by treating old words as targets and new words as nontargets. Signal detection indices were generated using recommended corrections for perfect performance (Hautus, 1995; Stanislaw & Todorov, 1999). Discrimination between target and nontarget words as a measure of recognition memory was characterized using d′. Higher d′ scores indicated better recognition. Two participants were excluded because encoding and lexical-decision accuracy scores were more than 5 SD below the sample mean. Furthermore, the signal detection metric of C was examined to assess for response bias and appraise task engagement during recognition (see the Supplemental Material). One individual with C scores more than 5 SD from the sample mean was excluded. A total of 147 individuals from Study 1 were included in analyses of laboratory verbal memory (Table 1).
Data analysis
We performed two sets of analyses to statistically predict memory functioning. First, we used categorical designations of PTSD and mTBI. Then, we repeated the analyses using dimensional characterizations of PTSD symptoms and mTBI severity. All analyses were computed using IBM SPSS (Version 25).
Analysis of categorical variables
Demographics characteristics of groups were compared with Pearson χ2 tests, t tests, and 2 (PTSD: PTSD, no PTSD) × 2 (mTBI: mTBI, no mTBI) between-subjects analyses of variance (ANOVAs). To test for verbal functioning deficits using the combined Study 1 and Study 2 samples, we conducted 2 (PTSD: PTSD, no PTSD) × 2 (mTBI: mTBI, no mTBI) between-subjects ANOVAs on the following neuropsychological scores: Attention Span, Learning Efficiency, Delayed Recall, Inaccurate Recall, and WTAR total score. Similar two-way ANOVAs were constructed for the following Study 1 verbal-memory laboratory task measures: encoding accuracy, encoding mean RT, total free recall, lexical-decision accuracy, and recognition d′.
To assess implicit priming effects on mean RT during the lexical-decision phase, we conducted a 2 (PTSD: PTSD, no PTSD; between subjects) × 2 (mTBI: mTBI, no mTBI; between subjects) × 3 (trial type: old word, new word, nonword; within subjects) multivariate analysis of variance (for follow-up analyses, see the Supplemental Material). Post hoc analyses of within-subjects effects were completed using a significance threshold that was corrected for false-discovery rate (FDR; q = .05) for multiple comparisons (Benjamini & Hochberg, 1995). We expected individuals with heightened PTSD symptoms to have impaired free recall of word stimuli and slower overall lexical-decision RTs but intact repetition priming and implicit memory.
Analysis of dimensional variables
We completed a second set of analyses using partial correlations to examine linear associations between specific PTSD symptom groupings and verbal-performance indices while covarying for past-blast mTBI severity. We used the four symptom groupings from the Dysphoria model supported by factor-analytic evidence (Yufik & Simms, 2010). Symptom frequency (0–4) and intensity (0–4) ratings from the CAPS were summed to generate severity scores for the following groupings: Reexperiencing (B1–B5), Avoidance (C1–C2), Dysphoria (C3–D3), and Hyperarousal (D4–D5). Blast mTBI severity scores were generated using the MN-BEST adaptation of the Ruff and Richardson (1999) rating scheme. An FDR-corrected significance threshold (q = .05) was used across the 20 partial correlations for the neuropsychological measures and the 24 partial correlations for the laboratory measures. Overall lexical-decision RT was calculated using average RT across the old-word, new-word, and nonword conditions.
Data from the experimental verbal-memory laboratory task were subject to follow-up mediation analyses to evaluate which cognitive mechanisms may explain the link between intrusive reexperiencing and memory performance. Three sets of mediation models were generated with the PROCESS 3.3 extension for SPSS (six total models; Hayes, 2017). Models included memory performance (either total free recall or d′ recognition) as the consequent (i.e., dependent variable), reexperiencing severity as the antecedent (i.e., independent variable), blast mTBI severity as the covariate, and cognitive performance (i.e., accuracy) and efficiency (i.e., mean RT during correct trials) as mediators. First, encoding accuracy and RT were entered to assess indirect effects between reexperiencing and memory performance via earlier attentional control, specifically during initial exposure to the word stimuli in the encoding period. Second, lexical-decision accuracy and overall RT were used to assess whether similar indirect associations could also be observed by way of more general impairments in attentional control occurring outside of the encoding period. Finally, we tested lexical-decision mediators of old-word RTs while including new-word RTs as a covariate to model implicit priming as a potential explanatory factor for recall and recognition-memory performance deficits. Statistical inferences about indirect effects (products of the a and b path coefficients) were made using bootstrapped (5,000 resamples) 95% confidence intervals (CIs).
Results
Demographics and clinical characteristics
Participant characteristics are reported in Table 1. For the combined Study 1 and Study 2 sample, clinical groups did not differ by gender or ethnic minority status, ps ≥ .086. There was an effect of mTBI for age, F(1, 239) = 5.37, p = .021, η p 2 = .022. Individuals with blast mTBI histories were 2.6 years younger than participants without those histories. There were no main or interaction effects with PTSD for age, ps ≥ .201. Furthermore, there was a main effect of mTBI for years of education, F(1, 233) = 5.81, p = .017, η p 2 = .024. On average, blast mTBI was associated with 0.6 fewer years of education. There were no main or interaction effects with PTSD for years of education, ps ≥ .214. Veterans with blast-related mTBI did not differ according to PTSD status in terms of months since the most recent blast event, p = .524. Individuals with PTSD were more likely to meet criteria for a comorbid depressive disorder (i.e., major depressive disorder [MDD] or dysthymic disorder), χ2 = 22.64, p < .001, Cramér’s V = .305, and for comorbid alcohol dependence, χ2 = 9.99, p = .002, Cramér’s V = .203. Individuals with blast-related mTBI histories were also more likely to meet criteria for a depressive disorder, χ2 = 5.73, p = .017, Cramér’s V = .154, but there were no group differences in rates of comorbid alcohol dependence, p = .124. Similar analyses were repeated for Study 1 participants with valid laboratory data and are reported in the Supplemental Material.
Neuropsychological evaluation of verbal functioning
CVLT-II verbal memory
We examined CVLT-II scores to assess verbal deficits across various learning and memory-performance domains. There was a main effect of PTSD for Delayed Recall, F(1, 239) = 5.227, p = .023, η p 2 = .021. Veterans with either full or subthreshold PTSD scored an average of 1.4 SD below comparison control participants when attempting to remember previously presented word material. Blast-related mTBI did not exhibit main or interaction effects for Delayed Recall (ps ≥ .279). In addition, no main or interaction effects for PTSD or blast-related mTBI were noted for the CVLT-II measures of Attention Span, Learning Efficiency, or Inaccurate Recall (ps ≥ .182). When using continuous measures of posttraumatic stress symptoms, Reexperiencing exhibited a partial correlation with Delayed Recall after covarying for blast TBI severity and correcting for multiple comparisons (Table 2). Veterans reporting greater reexperiencing symptoms remembered fewer previously presented word stimuli. This association was specific for Delayed Recall because no additional significant partial correlations were observed between other PTSD symptom groupings and indices of Attention Span, Learning Efficiency, or Inaccurate Recall.
Table 2.
Partial Correlations Between Verbal Performance Measures and Posttraumatic Stress Symptom Domains
| Measure | CAPS symptom-severity scores | |||
|---|---|---|---|---|
| Reexperiencing | Avoidance | Dysphoria | Hyperarousal | |
| Neuropsychological | ||||
| WTAR | −0.057 | 0.134 | 0.138 | 0.010 |
| CVLT-II Attention Span | −0.109 | 0.061 | 0.005 | −0.070 |
| CLVT-II Learning Efficiency | −0.108 | 0.026 | −0.008 | −0.071 |
| CVLT-II Delayed Recall | −0.197 | −0.048 | −0.082 | −0.119 |
| CVLT-II Inaccurate Recall | 0.041 | −0.046 | −0.025 | 0.070 |
| Laboratory task | ||||
| Encoding (overall accuracy) | −0.062 | 0.026 | 0.086 | −0.021 |
| Encoding (overall RT) | 0.226 | 0.119 | −0.004 | 0.061 |
| Free recall (total) | −0.243 | 0.147 | −0.014 | −0.084 |
| Lexical (overall accuracy) | −0.018 | 0.165 | 0.158 | 0.128 |
| Lexical (overall RT) | 0.262 | 0.175 | 0.078 | 0.090 |
| Recognition (d′) | −0.314 | −0.097 | −0.134 | −0.191 |
Note: Partial correlations displayed between study measures of verbal performance for the conventional neuropsychological measures as well as the laboratory task measures. Boldface type indicates significant associations after applying a q = .05 false discovery rate for multiple comparisons across the 20 neuropsychological and 25 laboratory task measures associations. CAPS = Clinician-Administered PTSD Scale for DSM-IV (Blake et al., 1995); WTAR = Wechsler Test of Adult Reading (Wechsler, 2001); CVLT-II = California Verbal Learning Test–Second Edition (Delis, 2000); RT = response time.
WTAR overall verbal abilities
When veterans were evaluated on phonetic pronunciation using the WTAR, there were no main or interaction effects of PTSD or blast-related mTBI (ps ≥ .309). Partial correlations between posttraumatic stress symptom groupings and WTAR scores while covarying for blast TBI severity were not significant (Table 2). Thus, impairments in CVLT-II Delayed Recall scores could not be explained by general verbal ability or vocabulary.
Laboratory verbal-processing task performance
Encoding
Veterans from Study 1 completed the laboratory verbal-memory task to quantify performance using more nuanced metrics (Fig. 1). When we performed the initial size judgments for word stimuli, there were no main or interaction effects of PTSD or blast-related mTBI for encoding accuracy, ps ≥ .100. In addition, there were no main or interaction effects of PTSD or blast-related mTBI for encoding RTs, ps ≥ .063. Thus, there were negligible differences between the categorical groups on performance during the encoding period. However, partial correlations between PTSD symptom-severity scores and encoding efficiency revealed specific associations with Reexperiencing while covarying for blast TBI severity and correcting for multiple comparisons (Table 2). Individuals who reported elevated levels of reexperiencing symptoms produced slower word size judgments during the encoding period. There were no additional significant associations with encoding accuracy or RT for any other PTSD symptom grouping.
Free recall
For free recall of encoded words, there was a main effect of PTSD (Fig. 1), F(1, 143) = 4.80, p = .030, η p 2 = .032. Individuals with full or subthreshold PTSD recalled an average of 3.6 fewer words from the encoding period than comparison control participants. There were no main or interaction effects for blast-related mTBI, ps ≥ .062. A follow-up partial correlation between Reexperiencing and total free-recall performance was significant when covarying for blast TBI severity and correcting for multiple comparisons (Table 2). Greater reexperiencing symptoms predicted worse recall of word stimuli. There were no other associations between free recall and PTSD symptom groupings.
Lexical-decision priming effects
When veterans made lexical decisions about whether the presented text was a word or nonword, there were no main or interaction effects of PTSD or blast-related mTBI for accuracy, ps ≥ .072. For mean RT during correct trials, there was a within-subjects effect of trial type (Fig. 1), F(2, 142) = 127.95, p < .001, η p 2 = .643. Veterans were quickest for previously viewed words, intermediate for new words, and slowest for nonwords (ps ≤ .001 for all comparisons). This was consistent with the expected implicit memory priming effect. There was no interaction between PTSD and word condition, p = .616. Thus, there was little support for implicit priming deficits among participants with either full or subthreshold PTSD. However, there was a main effect of PTSD for RT, F(1, 143) = 4.81, p = .030, η p 2 = .033. Veterans with full or subthreshold PTSD were slower overall to complete lexical decisions regardless of the specific task condition. In addition, there were no main or interaction effects for blast-related mTBI on lexical-decision RTs, ps ≥ .140. As follow-up for these findings, we computed average overall RT collapsed across all lexical-decision trials. Overall RT was associated with Reexperiencing when covarying for blast TBI severity and correcting for multiple comparisons (Table 2). Similar to the encoding period, individuals with greater symptoms of trauma-related reexperiencing were slower at making lexical decisions. This was true regardless of past exposure to the words or semantic meaning of the text. There were no other associations between lexical-decision overall RT and other PTSD symptom groupings.
Recognition
There was a main effect of PTSD on recognition of previously displayed word stimuli as measured by d′ (Fig. 1), F(1, 143) = 7.14, p = .008, η p 2 = .048. Veterans with full or subthreshold PTSD demonstrated impaired ability to discriminate between previously displayed old words and new words relative to comparison control participants. There were no main or interaction effects for blast-related mTBI (ps ≥ .331). In a similar way as with free recall, Reexperiencing exhibited a partial correlation with recognition d′ when covarying for blast TBI severity and correcting for multiple comparisons (Table 2). Thus, greater reexperiencing severity was associated with worse recognition of word stimuli. There were no significant partial correlations between recognition-memory performance and any other PTSD symptom grouping.
Indirect mediation effects for memory performance
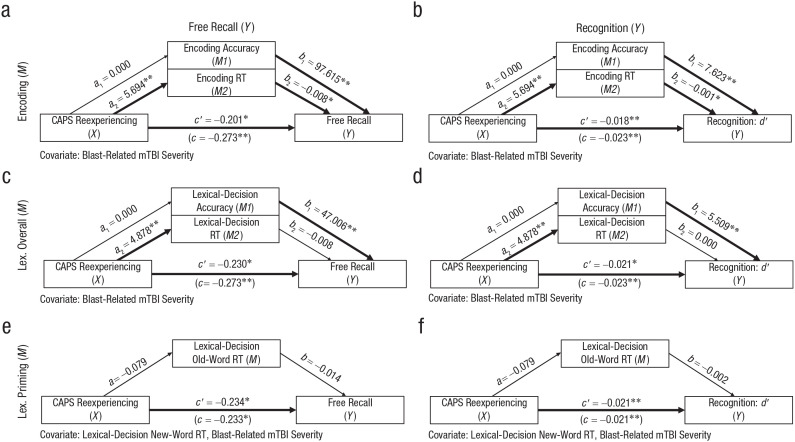
To test whether attentional control during encoding could explain the observed associations between verbal-memory performance and reexperiencing symptoms, we constructed mediation models (Figs. 2a and 2b; see Table S3 in the Supplemental Material). Reexperiencing symptom severity was indirectly related to free-recall performance through its relationship with encoding RT (a2b2 = −0.046, 95% confidence interval (CI) = [−0.107, −0.003]). Although encoding accuracy independently predicted free recall, this indirect effect was not significantly different from zero (a1b1 = −0.026, 95% CI = [−0.101, 0.043]). A second model using recognition d′ as the dependent variable also revealed an indirect effect between reexperiencing by way of encoding RT (a2b2 = −0.0030, 95% CI = [−0.0063, −0.0003]). Encoding accuracy predicted recognition performance, but the associated indirect effect was not different from zero (a1b1 = −0.002, 95% CI = [−0.008, 0.003]).
Fig. 2.
Indirect effect models predicting word-memory performance. Shown are parallel mediation models testing whether encoding accuracy, encoding reaction time (RT), lexical-decision accuracy, and lexical-decision RT mediate the relationship between reexperiencing symptoms and free-recall/recognition performance (n = 147). c represents the total effect of Clinician Administered PTSD Scale for DSM-IV intrusions (X) on the dependent variable of interest (Y; e.g., free recall), and c′ represents the direct effect of X on Y when indirect effects of encoding or lexical-decision performance are accounted for in the model. a1 and a2 represent the unstandardized coefficients describing the relationship between X and the mediating variables of interest (M1, M2; e.g., encoding accuracy, encoding RT). Likewise, b1 and b2 represent the unstandardized coefficients describing the relationship between M1/M2 and Y. All mediation models included blast-related mild traumatic brain injury severity as a covariate. Asterisks indicate significant paths (*p < .05, **p < .01).
We generated comparison models using lexical-decision accuracy and overall RT as mediators to assess whether general verbal-processing inefficiencies outside of the encoding period could explain memory impairments (Figs. 2c and 2d; see Table S3 in the Supplemental Material). Neither indirect paths through lexical-decision accuracy (a1b1 = −0.005, 95% CI = [−0.062, 0.036]) nor through lexical-decision overall RT (a2b2 = −0.039, 95% CI = [−0.090, 0.003]) mediated the free-recall findings. In addition, neither indirect paths through lexical-decision accuracy (a1b1 = −0.001, 95% CI = [−0.006, 0.004]) nor through lexical-decision overall RT (a2b2 = −0.002, 95% CI = [−0.005, 0.002]) mediated an association between Reexperiencing and recognition d′.
Finally, we tested models with lexical old-word RT as the mediator and lexical new-word RT as a covariate to isolate the implicit priming effect (Figs. 2e, 2f; see Table S3 in the Supplemental Material). Reexperiencing did not significantly predict old-word RT, and old-word RT did not significantly predict free-recall or recognition performance. Consequently, indirect paths to free recall (ab = 0.001, 95% CI = [−0.002, 0.002]) and recognition (ab = 0.002, 95% CI = [−0.032, 0.027]) included zero.
In summary, our findings support an indirect association between reexperiencing symptoms and explicit verbal memory by way of encoding inefficiency. General word-processing speed and implicit memory functioning could not explain those same verbal-memory deficits.
Discussion
We investigated the verbal-memory performance of military combat veterans using conventional neuropsychological instruments and an experimental laboratory task. Individuals with full or subthreshold PTSD exhibited specific impairments in delayed word recall for the CVLT-II. Intrusive reexperiencing symptoms predicted those recall impairments as well. Note that WTAR scores related to general verbal ability or vocabulary were unrelated to posttraumatic stress symptoms. On the laboratory verbal-memory task, veterans with full and subthreshold PTSD displayed impaired free recall, worse recognition, and slower RTs. Similar to the CVLT-II, reexperiencing symptoms predicted laboratory indices of verbal-memory performance. Mediation analyses revealed indirect effects between reexperiencing symptom severity and later free-recall and recognition performance by way of initial encoding inefficiency (i.e., slower RTs). Moreover, our analyses with laboratory-task data revealed intact implicit memory repetition priming for veterans with significant symptoms of PTSD. The findings together suggest that inefficient attentional control during initial encoding of verbal material may be central to verbal-memory impairments in PTSD.
Dimensional assessments of psychopathology have several advantages over categorical diagnoses for revealing associations with psychopathology (Cuthbert, 2005; Grove, 1991). In the present study, we repeatedly observed stronger effects of symptom dimensions on verbal-memory processes than when using a categorical diagnosis of PTSD. In addition, the associations were with intrusive reexperiencing and not with other dimensions of PTSD (e.g., dysphoric mood). This is in line with previous findings suggesting that impaired verbal memory (relative to visuospatial-memory performance) cannot be explained by comorbid depression with PTSD (Scheiner et al., 2014; Scott et al., 2015). Indeed, MDD alone is not associated with selective deficits in verbal memory such as those observed with PTSD (Bora et al., 2013). Reexperiencing of traumatic events, a collection of symptoms not represented within the MDD criteria, may exhibit a special relationship with the cognitive processes evoked during verbal-memory tasks. Attentional control deficits during the encoding of words may be a differentiating feature among individuals with intrusive reexperiencing symptoms relative to other forms of internalizing psychopathology.
By demonstrating connections between word memory and specific symptoms, this work adds to emerging theoretical models of PTSD. Word-based encoding may be required for adaptive consolidation of traumatic memories. Maladaptive posttraumatic stress may persist in part because of superficial verbal encoding within autobiographical memory stores (Ehlers & Clark, 2000). At the time of traumatic events, emotionally salient information is effortlessly encoded using perceptual memory representations (Brewin, 2003, 2014). To create verbal representations of those same events, effortful reprocessing is required. In everyday circumstances, reexperiencing symptoms of PTSD may be the consequence of perceptual cuing (e.g., encountering trauma reminders) without experiencing verbal representations during those same events (Brewin, 2014). Verbal representations of traumatic events may help limit or interfere with full emotional responses to distressing perceptual cues (Brewin et al., 2007; Chin & Schooler, 2008). Thus, recurrences of reexperiencing symptoms may stem from incomplete verbal encoding and reconsolidation of emotionally activating perceptual memories.
For the military veterans assessed several years postdeployment, there may be a connection between attentional control deficits and the chronic nature of their symptoms. In ideal circumstances, trauma reminders experienced in conditions of safety are opportunities for adaptive processing and reconsolidation. When effectively executed, elaborative verbal encoding may allow a person to metaphorically “file away” trauma content within appropriate autobiographical memory stores. Some individuals may be more adept at naturally completing this type of word-based meaning making following psychological stressors (Park, 2010), which may be facilitated by attentional control for verbal material. For example, higher verbal-learning performance prospectively predicts fewer reexperiencing symptoms in the months after trauma exposure (Parslow & Jorm, 2007). Therefore, when a person has less effective verbal learning skills, they may be more vulnerable to trauma effects because of a decreased capacity to engage in reconsolidation. This is relevant to clinical interventions because verbal memory is also a predictor of trauma-focused psychotherapy outcomes (Etkin et al., 2019; Nijdam et al., 2015; Scott et al., 2017). Sufficient attentional control may be a prerequisite for reconsolidation of perceptually based traumatic memories and adaptive long-term outcomes.
Given the lack of associations between repetition priming and PTSD, the functioning of implicit verbal processes may not be critical for explaining recovery after trauma. This interpretation is thematically consistent with theorizing about the mechanisms of action of psychotherapies (Ecker et al., 2012; Neimeyer, 2002; Park & Ai, 2006; Schnyder et al., 2015; Steger & Park, 2012). Passive and repetitive exposure to trauma content may bring about only short-term emotional habituation. Without deeper restructuring of posttraumatic memories, additional intrusive reexperiencing symptoms to novel trauma cues (e.g., spontaneous recovery) are possible. The present study highlights the need for innovations to improve encoding for individuals with weaknesses in word learning. It may be possible to enhance trauma-focused psychotherapy outcomes by emphasizing meaning making over and above prolonged exposure to trauma cues alone. Furthermore, it may be possible to target weaknesses in verbal encoding directly through cognitive remediation training (Jak et al., 2019).
Blast-related mTBI was unrelated to measures of verbal processing. There is a growing consensus regarding the negligible long-term impact of mild brain injury on cognitive functioning (Sweet et al., 2013). Maladaptive trajectories in emotional and cognitive functioning after military deployments can often be accounted for by psychopathology including PTSD even in the context of mTBI (Disner et al., 2017; Nelson et al., 2012, 2020; Polusny et al., 2011; Wilk et al., 2012). Partial correlations between symptom groupings and cognitive performance while covarying for blast injury severity revealed potential cognitive markers of posttraumatic reexperiencing that were independent of mTBI. Nevertheless, the current study is limited by a lack of battlefield collateral information about brain injury sequelae at the time of the explosive blasts. We sought to address this weakness by employing semistructured interview tools and consensus diagnosis teams including neuropsychologists (Nelson, Davenport, et al., 2015). As with most studies conducted in veteran samples, the longitudinal reliability of self-reported mTBI in the current sample is unknown. Potential reporting inconsistencies years after the blast events may obscure accurate estimation of the effects of mTBI on later cognitive functions and psychopathology (Nelson, Anderson, et al., 2015).
Another potential caveat includes the cross-sectional nature of this investigation. We cannot conclusively determine whether the observed effects would be better conceptualized as markers of trauma exposure or risk factors relevant for understanding the individual impact of trauma. In addition, a larger sample of participants had CAPS reexperiencing symptoms assessed compared with other PTSD symptom dimensions because of the discontinued Study 1 clinical interviews if reexperiencing criteria were not met. However, correlations for reexperiencing symptoms are larger than other symptom dimensions, and the findings from the laboratory task were replicated using neuropsychological measurements of verbal memory from a combined sample of Study 1 and Study 2 participants. Also note that the similar associations with reexperiencing symptoms were observed using auditory and written word stimuli from the CVLT-II and laboratory task, respectively. Future studies of verbal memory may benefit from the inclusion of both emotionally neutral and trauma-relevant words to tease apart subtle effects related to attentional bias toward threat (Ashley et al., 2013).
In the present study, we identified impaired explicit verbal memory but intact implicit verbal memory in military veterans with symptoms of PTSD. Note that intrusive reexperiencing symptoms were associated with a range of cognitive processes involved in verbal memory, including slowed RTs at encoding and worse recollection of word stimuli at recall. According to the mediation analyses, inefficiency during encoding explained the connection between reexperiencing symptoms and worse verbal-recall performance. We propose that altered attentional control for word stimuli may explain aspects of explicit verbal-memory impairments in previously deployed veterans with maladaptive long-term outcomes. Such findings point toward the importance of bolstering use of attentional control systems to facilitate reconsolidation of trauma-related content and improve functioning in people with significant posttraumatic stress symptoms.
Supplemental Material
Supplemental material, sj-pdf-1-cpx-10.1177_21677026211025018 for Inefficient Attentional Control Explains Verbal-Memory Deficits Among Military Veterans With Posttraumatic Reexperiencing Symptoms by Craig A. Marquardt, Victor J. Pokorny, Seth G. Disner, Nathaniel W. Nelson, Kathryn A. McGuire and Scott R. Sponheim in Clinical Psychological Science
Acknowledgments
We thank the veteran participants in this study.
Footnotes
ORCID iD: Craig A. Marquardt  https://orcid.org/0000-0003-2714-6168
https://orcid.org/0000-0003-2714-6168
Supplemental Material: Additional supporting information can be found at http://journals.sagepub.com/doi/suppl/10.1177/21677026211025018
Transparency
Action Editor: Erin B. Tone
Editor: Kenneth J. Sher
Author Contributions
S. R. Sponheim and N. W. Nelson secured funding for the study and led data collection. N. W. Nelson, K. A. McGuire, and S. R. Sponheim designed the task protocol, and C. A. Marquardt and S. R. Sponheim developed the study concept. C. A. Marquardt, N. W. Nelson, K. A. McGuire, S. G. Disner, and S. R. Sponheim performed diagnostic consensus evaluations using participant interview materials. C. A. Marquardt and V. J. Pokorny performed the data analysis under supervision from S. G. Disner and S. R. Sponheim. All the authors contributed interpretations of the findings. C. A. Marquardt, V. J. Pokorny, S. G. Disner, and S. R. Sponheim drafted the manuscript, and N. W. Nelson and K. A. McGuire provided revisions. V. J. Pokorny created the figures. All of the authors approved the final manuscript for submission.
The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Funding: The Department of Veterans Affairs Rehabilitation R&D Program (S. R. Sponheim; I01RX000622), the Congressionally Directed Medical Research Program (S. R. Sponheim), the Department of Defense (S. R. Sponheim; PT074550), and the Minnesota Veterans Research Institute (N. W. Nelson) provided funds for this work. Funding sources were not involved in the choice of topics, study design, data analysis or interpretation, or preparation/submission of this manuscript. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the Department of Veterans Affairs or the Department of Defense.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). [Google Scholar]
- Ashley V., Honzel N., Larsen J., Justus T., Swick D. (2013). Attentional bias for trauma-related words: Exaggerated emotional Stroop effect in Afghanistan and Iraq war veterans with PTSD. BMC Psychiatry, 13, Article 86. 10.1186/1471-244X-13-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57(1), 289–300. [Google Scholar]
- Binder L. M., Storzbach D., Anger W. K., Campbell K. A., Rohlman D. S., Center H. R. (1999). Subjective cognitive complaints, affective distress, and objective cognitive performance in Persian Gulf War veterans. Archives of Clinical Neuropsychology, 14(6), 531–536. [PubMed] [Google Scholar]
- Blake D. D., Weathers F. W., Nagy L. M., Kaloupek D. G., Gusman F. D., Charney D. S., Keane T. M. (1995). The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress, 8(1), 75–90. 10.1002/jts.2490080106 [DOI] [PubMed] [Google Scholar]
- Blanchard E. B., Hickling E. J., Mitnick N., Taylor A. E., Loos W. R., Buckley T. C. (1995). The impact of severity of physical injury and perception of life threat in the development of post-traumatic stress disorder in motor vehicle accident victims. Behaviour Research and Therapy, 33(5), 529–534. [DOI] [PubMed] [Google Scholar]
- Bora E., Harrison B., Yücel M., Pantelis C. (2013). Cognitive impairment in euthymic major depressive disorder: A meta-analysis. Psychological Medicine, 43(10), 2017–2026. [DOI] [PubMed] [Google Scholar]
- Brady K. T. (1997). Posttraumatic stress disorder and comorbidity: Recognizing the many faces of PTSD. The Journal of Clinical Psychiatry, 58(Suppl. 9), 12–15 [PubMed] [Google Scholar]
- Brewin C. R. (2003). Posttraumatic stress disorder: Malady or myth? Yale University Press. [Google Scholar]
- Brewin C. R. (2014). Episodic memory, perceptual memory, and their interaction: Foundations for a theory of posttraumatic stress disorder. Psychological Bulletin, 140(1), 69–97. 10.1037/a0033722 [DOI] [PubMed] [Google Scholar]
- Brewin C. R., Kleiner J. S., Vasterling J. J., Field A. P. (2007). Memory for emotionally neutral information in posttraumatic stress disorder: A meta-analytic investigation. Journal of Abnormal Psychology, 116(3), 448–463. [DOI] [PubMed] [Google Scholar]
- Chin J. M., Schooler J. W. (2008). Why do words hurt? Content, process, and criterion shift accounts of verbal overshadowing. European Journal of Cognitive Psychology, 20(3), 396–413. [Google Scholar]
- Cukor J., Wyka K., Jayasinghe N., Difede J. (2010). The nature and course of subthreshold PTSD. Journal of Anxiety Disorders, 24(8), 918–923. [DOI] [PubMed] [Google Scholar]
- Cuthbert B. N. (2005). Dimensional models of psychopathology: Research agenda and clinical utility. Journal of Abnormal Psychology, 114(4), 565–569. 10.1037/0021-843X.114.4.565 [DOI] [PubMed] [Google Scholar]
- DeJong J., Donders J. (2009). A confirmatory factor analysis of the California Verbal Learning Test—Second Edition (CVLT-II) in a traumatic brain injury sample. Assessment, 16(4), 328–336. [DOI] [PubMed] [Google Scholar]
- Delis D. C. (2000). California verbal learning test [Adult version. Manual]. Psychological Corporation. [Google Scholar]
- Disner S. G., Kramer M. D., Nelson N. W., Lipinski A. J., Christensen J. M., Polusny M. A., Sponheim S. R. (2017). Predictors of postdeployment functioning in combat-exposed US military veterans. Clinical Psychological Science, 5(4), 650–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disner S. G., Marquardt C. A., Mueller B. A., Burton P. C., Sponheim S. R. (2018). Spontaneous neural activity differences in posttraumatic stress disorder: A quantitative resting-state meta-analysis and fMRI validation. Human Brain Mapping, 39(2), 837–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson D. I., Petersen S. E., Buckner R. L. (2001). Dissociating memory retrieval processes using fMRI: Evidence that priming does not support recognition memory. Neuron, 31(6), 1047–1059. [DOI] [PubMed] [Google Scholar]
- Donders J. (2008). A confirmatory factor analysis of the California Verbal Learning Test—Second Edition (CVLT-II) in the standardization sample. Assessment, 15(2), 123–131. [DOI] [PubMed] [Google Scholar]
- Ecker B., Ticic R., Hulley L. (2012). Unlocking the emotional brain: Eliminating symptoms at their roots using memory reconsolidation. Routledge. [Google Scholar]
- Ehlers A., Clark D. M. (2000). A cognitive model of posttraumatic stress disorder. Behaviour Research and Therapy, 38(4), 319–345. [DOI] [PubMed] [Google Scholar]
- Etkin A., Maron-Katz A., Wu W., Fonzo G. A., Huemer J., Vértes P. E., Patenaude B., Richiardi J., Goodkind M. S., Keller C. J., Ramos-Cejudo J., Zaiko Y. V., Peng K. K., Shpigel E., Longwell P., Toll R. T., Thompson A., Zack S., Gonzalez B., . . . O’Hara R. (2019). Using fMRI connectivity to define a treatment-resistant form of post-traumatic stress disorder. Science Translational Medicine, 11(486), eaal3236. 10.1126/scitranslmed.aal3236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M., Spitzer R., Gibbon M., Williams J. (2002). Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P). New York State Psychiatric Institutes, Biometric Research. [Google Scholar]
- Gilmore C. S., Marquardt C. A., Kang S. S., Sponheim S. R. (2018). Reduced P3b brain response during sustained visual attention is associated with remote blast mTBI and current PTSD in US military veterans. Behavioural Brain Research, 340, 174–182. 10.1016/j.bbr.2016.12.002 [DOI] [PubMed] [Google Scholar]
- Green R. E., Melo B., Christensen B., Ngo L.-A., Monette G., Bradbury C. (2008). Measuring premorbid IQ in traumatic brain injury: An examination of the validity of the Wechsler Test of Adult Reading (WTAR). Journal of Clinical and Experimental Neuropsychology, 30(2), 163–172. [DOI] [PubMed] [Google Scholar]
- Grove W. M. (1991). When is a diagnosis worth making? A statistical comparison of two prediction strategies. Psychological Reports, 69(1), 3–17. [PubMed] [Google Scholar]
- Hautus M. J. (1995). Corrections for extreme proportions and their biasing effects on estimated values of d′. Behavior Research Methods, Instruments, & Computers, 27(1), 46–51. [Google Scholar]
- Hayes A. F. (2017). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach (Vol. 2). Guilford Publications. [Google Scholar]
- Hoge C. W., McGurk D., Thomas J. L., Cox A. L., Engel C. C., Castro C. A. (2008). Mild traumatic brain injury in US soldiers returning from Iraq. New England Journal of Medicine, 358(5), 453–463. [DOI] [PubMed] [Google Scholar]
- Jacobs U., Iacopino V. (2001). Torture and its consequences: A challenge to clinical neuropsychology. Professional Psychology: Research and Practice, 32(5), 458–464. [Google Scholar]
- Jak A. J., Jurick S., Crocker L. D., Sanderson-Cimino M., Aupperle R., Rodgers C. S., Thomas K. R., Boyd B., Norman S. B., Lang A. J., Keller A. V., Schiehser D. M., Twamley E. W. (2019). SMART-CPT for veterans with comorbid post-traumatic stress disorder and history of traumatic brain injury: A randomised controlled trial. Journal of Neurology, Neurosurgery and Psychiatry, 90(3), 333–341. 10.1136/jnnp-2018-319315 [DOI] [PubMed] [Google Scholar]
- Johnsen G. E., Asbjørnsen A. E. (2008). Consistent impaired verbal memory in PTSD: A meta-analysis. Journal of Affective Disorders, 111(1), 74–82. [DOI] [PubMed] [Google Scholar]
- Johnsen G. E., Asbjørnsen A. E. (2009). Verbal learning and memory impairments in posttraumatic stress disorder: The role of encoding strategies. Psychiatry Research, 165(1–2), 68–77. [DOI] [PubMed] [Google Scholar]
- Kay T., Harrington D., Adams R., Anderson T., Berrol S., Cicerone K., Dahlberg C., Gerber D., Goka R., Harley P., Hilt J., Horn L., Lehmkuhl D., Malec J. (1993). Definition of mild traumatic brain injury. Journal of Head Trauma Rehabilitation, 8(3), 86–87. [Google Scholar]
- Kennedy J., Jaffee M. S., Leskin G., Stokes J. W., Leal F. O., Fitzpatrick P. (2007). Posttraumatic stress disorder and posttraumatic stress disorder-like symptoms and mild traumatic brain injury. Journal of Rehabilitation Research and Development, 44(7), 895–920. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. G., Resnick H. S., Milanak M. E., Miller M. W., Keyes K. M., Friedman M. J. (2013). National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. Journal of Traumatic Stress, 26(5), 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman L., Gorka S. M., Funkhouser C. J., Shankman S. A., Phan K. L. (2017). Impact of posttraumatic stress symptom dimensions on psychophysiological reactivity to threat and reward. Journal of Psychiatric Research, 92, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker J. M., Venables N. C., Kang S. S., McGuire K. A., Sponheim S. R. (2018). Brain responses at encoding predict limited verbal memory retrieval by persons with schizophrenia. Archives of Clinical Neuropsychology, 33(4), 477–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt C. A., Goldman D. J., Cuthbert B. N., Lissek S., Sponheim S. R. (2018). Symptoms of posttraumatic stress rather than mild traumatic brain injury best account for altered emotional responses in military veterans. Journal of Traumatic Stress, 31(1), 114–124. [DOI] [PubMed] [Google Scholar]
- Marquardt C. A., Pokorny V. J., Kang S. S., Cuthbert B. N., Sponheim S. R. (2021). Posttraumatic stress symptom dimensions and brain responses to startling auditory stimuli in combat veterans. Journal of Abnormal Psychology, 130(5), 455–467. 10.1037/abn0000552. [DOI] [PubMed] [Google Scholar]
- Marshall R. D., Olfson M., Hellman F., Blanco C., Guardino M., Struening E. L. (2001). Comorbidity, impairment, and suicidality in subthreshold PTSD. American Journal of Psychiatry, 158(9), 1467–1473. [DOI] [PubMed] [Google Scholar]
- Marsolek C. J. (2003). What is priming and why. In Bowers J. S., Marsolek C. J. (Eds.), Rethinking implicit memory (pp. 41–64). Oxford University Press. [Google Scholar]
- McNally R. J., Lasko N. B., Macklin M. L., Pitman R. K. (1995). Autobiographical memory disturbance in combat-related posttraumatic stress disorder. Behaviour Research and Therapy, 33(6), 619–630. [DOI] [PubMed] [Google Scholar]
- McNally R. J., Litz B. T., Prassas A., Shin L. M., Weathers F. W. (1994). Emotional priming of autobiographical memory in post-traumatic stress disorder. Cognition & Emotion, 8(4), 351–367. [Google Scholar]
- Neimeyer R. A. (2002). Traumatic loss and the reconstruction of meaning. Journal of Palliative Medicine, 5(6), 935–943. 10.1089/10966210260499177 [DOI] [PubMed] [Google Scholar]
- Nelson N. W., Anderson C. R., Thuras P., Kehle-Forbes S. M., Arbisi P. A., Erbes C. R., Polusny M. A. (2015). Factors associated with inconsistency in self-reported mild traumatic brain injury over time among military personnel in Iraq. The British Journal of Psychiatry, 206(3), 237–244. [DOI] [PubMed] [Google Scholar]
- Nelson N. W., Davenport N. D., Sponheim S. R., Anderson C. R. (2015). Blast-related mild traumatic brain injury: Neuropsychological evaluation and findings. In Kobeissy F. H. (Ed.), Brain neurotrauma: Molecular, neuropsychological, and rehabilitation aspects (pp. 451–469). CRC Press. [PubMed] [Google Scholar]
- Nelson N. W., Disner S. G., Anderson C. R., Doane B. M., McGuire K., Lamberty G. J., Hoelzle J., Sponheim S. R. (2020). Blast concussion and posttraumatic stress as predictors of postcombat neuropsychological functioning in OEF/OIF/OND veterans. Neuropsychology, 34, 116–126. 10.1037/neu0000594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N. W., Hoelzle J. B., Doane B. M., McGuire K. A., Ferrier-Auerbach A. G., Charlesworth M. J., Lamberty G. J., Polusny M. A., Arbisi P. A., Sponheim S. R. (2012). Neuropsychological outcomes of US veterans with report of remote blast-related concussion and current psychopathology. Journal of the International Neuropsychological Society, 18(5), 845–855. 10.1017/S1355617712000616 [DOI] [PubMed] [Google Scholar]
- Nelson N. W., Hoelzle J. B., McGuire K. A., Ferrier-Auerbach A. G., Charlesworth M. J., Sponheim S. R. (2011). Neuropsychological evaluation of blast-related concussion: Illustrating the challenges and complexities through OEF/OIF case studies. Brain Injury, 25(5), 511–525. 10.3109/02699052.2011.558040 [DOI] [PubMed] [Google Scholar]
- Nijdam M. J., de Vries G.-J., Gersons B. P., Olff M. (2015). Response to psychotherapy for posttraumatic stress disorder: The role of pretreatment verbal memory performance. The Journal of Clinical Psychiatry, 76(8), e1023–e1028. 10.4088/JCP.14m09438 [DOI] [PubMed] [Google Scholar]
- Paller K. A., Kutas M., McIsaac H. K. (1995). Monitoring conscious recollection via the electrical activity of the brain. Psychological Science, 6(2), 107–111. [Google Scholar]
- Park C. L. (2010). Making sense of the meaning literature: An integrative review of meaning making and its effects on adjustment to stressful life events. Psychological Bulletin, 136(2), 257–301. 10.1037/a0018301 [DOI] [PubMed] [Google Scholar]
- Park C. L., Ai A. L. (2006). Meaning making and growth: New directions for research on survivors of trauma. Journal of Loss and Trauma, 11(5), 389–407. [Google Scholar]
- Parslow R. A., Jorm A. F. (2007). Pretrauma and posttrauma neurocognitive functioning and PTSD symptoms in a community sample of young adults. American Journal of Psychiatry, 164(3), 509–515. [DOI] [PubMed] [Google Scholar]
- Polak A. R., Witteveen A. B., Reitsma J. B., Olff M. (2012). The role of executive function in posttraumatic stress disorder: A systematic review. Journal of Affective Disorders, 141(1), 11–21. [DOI] [PubMed] [Google Scholar]
- Polusny M. A., Kehle S. M., Nelson N. W., Erbes C. R., Arbisi P. A., Thuras P. (2011). Longitudinal effects of mild traumatic brain injury and posttraumatic stress disorder comorbidity on postdeployment outcomes in National Guard soldiers deployed to Iraq. Archives of General Psychiatry, 68(1), 79–89. 10.1001/archgenpsychiatry.2010.172 [DOI] [PubMed] [Google Scholar]
- Rabin L. A., Paolillo E., Barr W. B. (2016). Stability in test-usage practices of clinical neuropsychologists in the United States and Canada over a 10-year period: A follow-up survey of INS and NAN members. Archives of Clinical Neuropsychology, 31(3), 206–230. [DOI] [PubMed] [Google Scholar]
- Rasmussen A., Rosenfeld B., Reeves K., Keller A. S. (2007). The effects of torture-related injuries on long-term psychological distress in a Punjabi Sikh sample. Journal of Abnormal Psychology, 116(4), 734–740. 10.1037/0021-843X.116.4.734 [DOI] [PubMed] [Google Scholar]
- Ruff R., Richardson A. M. (1999). Mild traumatic brain injury. In Sweet J. J. (Ed.), Forensic neuropsychology: Fundamentals and practice (pp. 315–338). CRC Press. [Google Scholar]
- Ruscio A. M., Ruscio J., Keane T. M. (2002). The latent structure of posttraumatic stress disorder: A taxometric investigation of reactions to extreme stress. Journal of Abnormal Psychology, 111(2), 290–301. [PubMed] [Google Scholar]
- Sayer N. A. (2012). Traumatic brain injury and its neuropsychiatric sequelae in war veterans. Annual Review of Medicine, 63, 405–419. [DOI] [PubMed] [Google Scholar]
- Scheiner D. L., Keilp J., Mindt M. R., Burke A. K., Oquendo M. A., Mann J. J. (2014). Verbal learning deficits in posttraumatic stress disorder and depression. Journal of Traumatic Stress, 27(3), 291–298. [DOI] [PubMed] [Google Scholar]
- Schnyder U., Ehlers A., Elbert T., Foa E. B., Gersons B. P., Resick P. A., Shapiro F., Cloitre M. (2015). Psychotherapies for PTSD: What do they have in common? European Journal of Psychotraumatology, 6(1), Article 28186. 10.3402/ejpt.v6.28186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. C., Harb G., Brownlow J. A., Greene J., Gur R. C., Ross R. J. (2017). Verbal memory functioning moderates psychotherapy treatment response for PTSD-Related nightmares. Behaviour Research and Therapy, 91, 24–32. [DOI] [PubMed] [Google Scholar]
- Scott J. C., Matt G. E., Wrocklage K. M., Crnich C., Jordan J., Southwick S. M., Krystal J. H., Schweinsburg B. C. (2015). A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychological Bulletin, 141(1), 105–140. 10.1037/a0038039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponheim S. R., Steele V. R., McGuire K. A. (2004). Verbal memory processes in schizophrenia patients and biological relatives of schizophrenia patients: Intact implicit memory, impaired explicit recollection. Schizophrenia Research, 71(2–3), 339–348. [DOI] [PubMed] [Google Scholar]
- Squire L. R., Knowlton B. J. (2000). The medial temporal lobe, the hippocampus, and the memory systems of the brain. The New Cognitive Neurosciences, 2, 756–776. [Google Scholar]
- Stanislaw H., Todorov N. (1999). Calculation of signal detection theory measures. Behavior Research Methods, Instruments, & Computers, 31(1), 137–149. [DOI] [PubMed] [Google Scholar]
- Steger M. F., Park C. L. (2012). The creation of meaning following trauma: Meaning making and trajectories of distress and recovery. In McMackin R. A., Newman E., Fogler J. M., Keane T. M. (Eds.), Trauma therapy in context: The science and craft of evidence-based practice (pp. 171–191). American Psychological Association. [Google Scholar]
- Sweet J. J., Goldman D. J., Breting L. M. G. (2013). Traumatic brain injury: Guidance in a forensic context from outcome, dose–response, and response bias research. Behavioral Sciences and the Law, 31(6), 756–778. [DOI] [PubMed] [Google Scholar]
- Vanderploeg R. D., Belanger H. G., Curtiss G. (2009). Mild traumatic brain injury and posttraumatic stress disorder and their associations with health symptoms. Archives of Physical Medicine and Rehabilitation, 90(7), 1084–1093. [DOI] [PubMed] [Google Scholar]
- Wagner A. D., Koutstaal W. (2002). In Ramachandran V. S. (Ed.), Priming encyclopedia of the human brain (Vol. 4, pp. 27–46). Elsevier. [Google Scholar]
- Warden D. (2006). Military TBI during the Iraq and Afghanistan wars. Journal of Head Trauma Rehabilitation, 21(5), 398–402. [DOI] [PubMed] [Google Scholar]
- Weathers F. W., Keane T. M., Davidson J. R. T. (2001). Clinician-Administered PTSD Scale: A review of the first ten years of research. Depression and Anxiety, 13(3), 132–156. 10.1002/da.1029 [DOI] [PubMed] [Google Scholar]
- Weathers F. W., Ruscio A. M., Keane T. M. (1999). Psychometric properties of nine scoring rules for the Clinician-Administered Posttraumatic Stress Disorder Scale. Psychological Assessment, 11(2), 124–133. 10.1037/1040-3590.11.2.124 [DOI] [Google Scholar]
- Wechsler D. (2001). Wechsler test of adult reading: WTAR. Psychological Corporation. [Google Scholar]
- Wilk J. E., Herrell R. K., Wynn G. H., Riviere L. A., Hoge C. W. (2012). Mild traumatic brain injury (concussion), posttraumatic stress disorder, and depression in U.S. soldiers involved in combat deployments: Association with postdeployment symptoms. Psychosomatic Medicine, 74(3), 249–257. 10.1097/PSY.0b013e318244c604 [DOI] [PubMed] [Google Scholar]
- Yufik T., Simms L. J. (2010). A meta-analytic investigation of the structure of posttraumatic stress disorder symptoms. Journal of Abnormal Psychology, 119(4), 764–776. 10.1037/a0020981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnick C., Franklin C. L., Zimmerman M. (2002). Does “subthreshold” posttraumatic stress disorder have any clinical relevance? Comprehensive Psychiatry, 43(6), 413–419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-cpx-10.1177_21677026211025018 for Inefficient Attentional Control Explains Verbal-Memory Deficits Among Military Veterans With Posttraumatic Reexperiencing Symptoms by Craig A. Marquardt, Victor J. Pokorny, Seth G. Disner, Nathaniel W. Nelson, Kathryn A. McGuire and Scott R. Sponheim in Clinical Psychological Science