Abstract
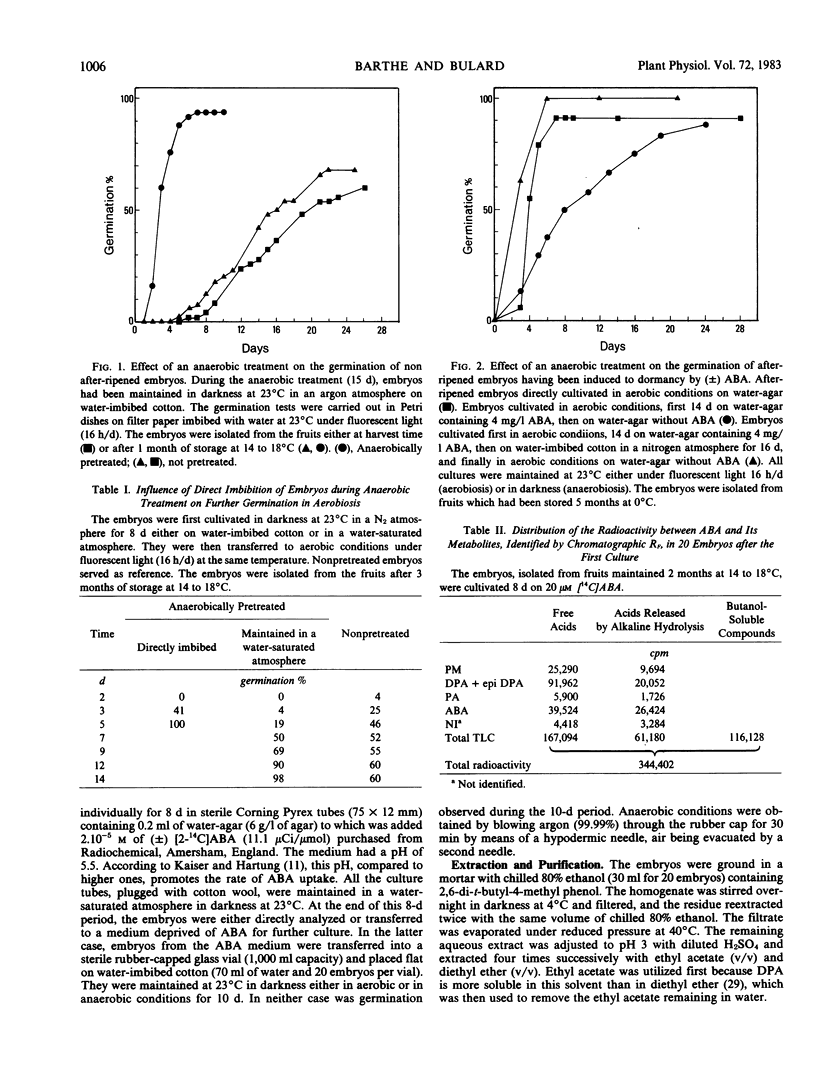
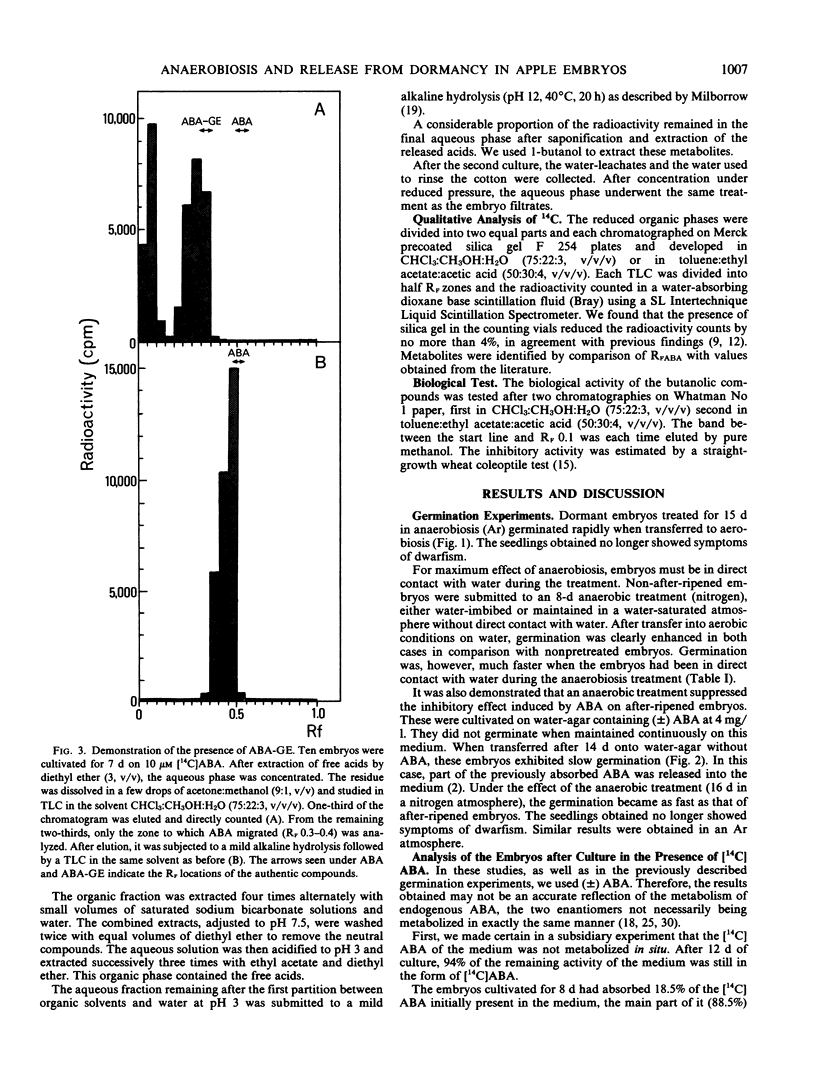
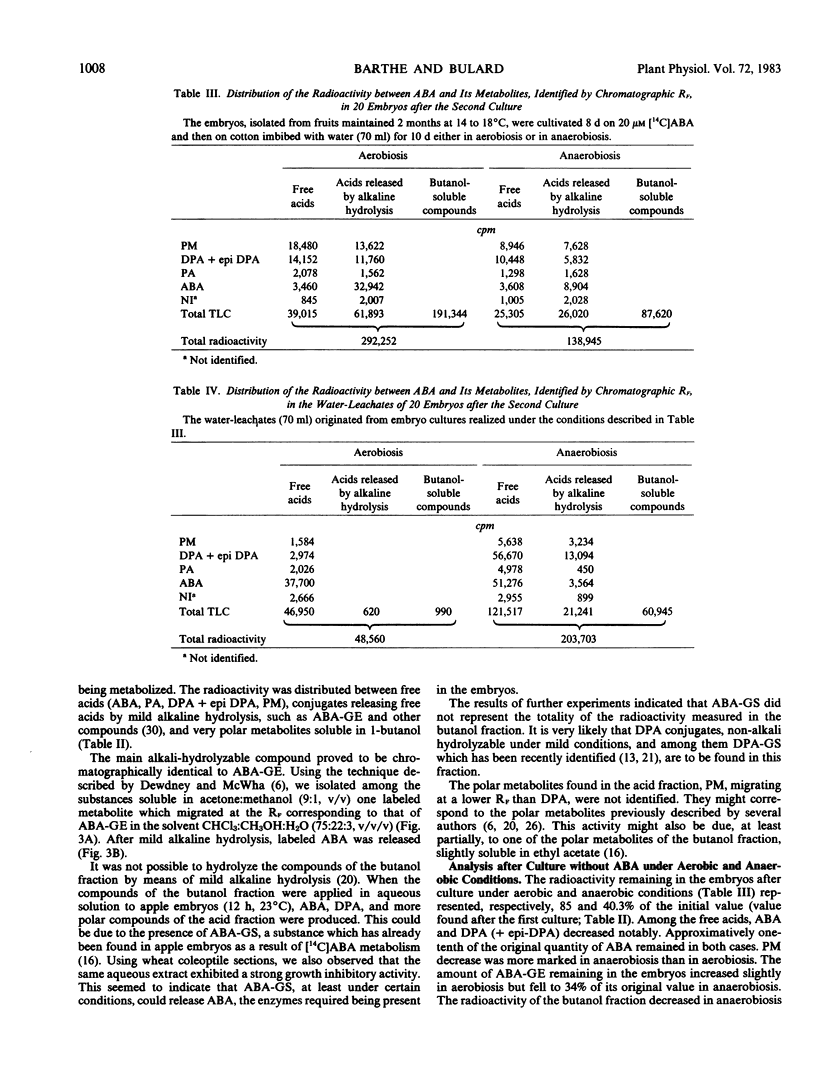
An anaerobic treatment released Pyrus malus L. cv Golden Delicious embryos from their primary dormancy. It also suppressed the inhibitory effect induced by exogenous abscisic acid (ABA) on after-ripened embryos. For the study of ABA metabolism, a two-step culture method was developed. Embryos in primary dormancy were cultivated aerobically in the presence of [14C]ABA (first culture). Some were directly analyzed to evaluate metabolism of absorbed ABA. The remaining embryos were cultivated on moist cotton without ABA, either in aerobic or anaerobic conditions (second culture). The amounts of ABA and its metabolites were measured both in the embryos and the water-leachates. After the second culture, the embryos showed a spectacular decrease in ABA content, with no difference between anaerobic and aerobic cultures. The amount of ABA glucose ester increased slightly in aerobiosis but diminished markedly in anaerobiosis. Radioactivity of the butanol fraction, which corresponded to polar conjugates, decreased considerably in anaerobiosis, whereas it increased in aerobiosis.
Analysis of the water-leachates indicated that, compared to aerobic conditions, anaerobiosis increased total leaching of radioactive materials (× 4.2) as well as leaching of ABA (× 1.4). In addition, anaerobiosis induced leaching of conjugates, such as ABA glucose ester and butanol-soluble metabolites. We concluded that the anaerobic treatment affects mainly membrane permeability.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christiansen M. N., Carns H. R., Slyter D. J. Stimulation of Solute Loss from Radicles of Gossypium hirsutum L. by Chilling, Anaerobiosis, and Low pH. Plant Physiol. 1970 Jul;46(1):53–56. doi: 10.1104/pp.46.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt A. J., Lowe R. H. Loss of organic acids, amino acids, k, and cl from barley roots treated anaerobically and with metabolic inhibitors. Plant Physiol. 1967 Dec;42(12):1731–1736. doi: 10.1104/pp.42.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W. M., Hartung W. Uptake and Release of Abscisic Acid by Isolated Photoautotrophic Mesophyll Cells, Depending on pH Gradients. Plant Physiol. 1981 Jul;68(1):202–206. doi: 10.1104/pp.68.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H., Handley R., Overstreet R. Potassium Loss and Changes in the Fine Structure of Corn Root Tips Induced by H-ion. Plant Physiol. 1966 Dec;41(10):1725–1735. doi: 10.1104/pp.41.10.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish D. J., Davies P. J. Emergent growth: an auxin-mediated response. Plant Physiol. 1977 Apr;59(4):745–749. doi: 10.1104/pp.59.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondheimer E., Galson E. C., Chang Y. P., Walton D. C. Asymmetry, its importance to the action and metabolism of abscisic Acid. Science. 1971 Nov 19;174(4011):829–831. doi: 10.1126/science.174.4011.829. [DOI] [PubMed] [Google Scholar]
- Sondheimer E., Galson E. C., Tinelli E., Walton D. C. The Metabolism of Hormones during Seed Germination and Dormancy: IV. The Metabolism of (S)-2-C-Abscisic Acid in Ash Seed. Plant Physiol. 1974 Dec;54(6):803–808. doi: 10.1104/pp.54.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton D. C., Sondheimer E. Metabolism of 2-C-(+/-)-abscisic Acid in excised bean axes. Plant Physiol. 1972 Mar;49(3):285–289. doi: 10.1104/pp.49.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]


