Abstract
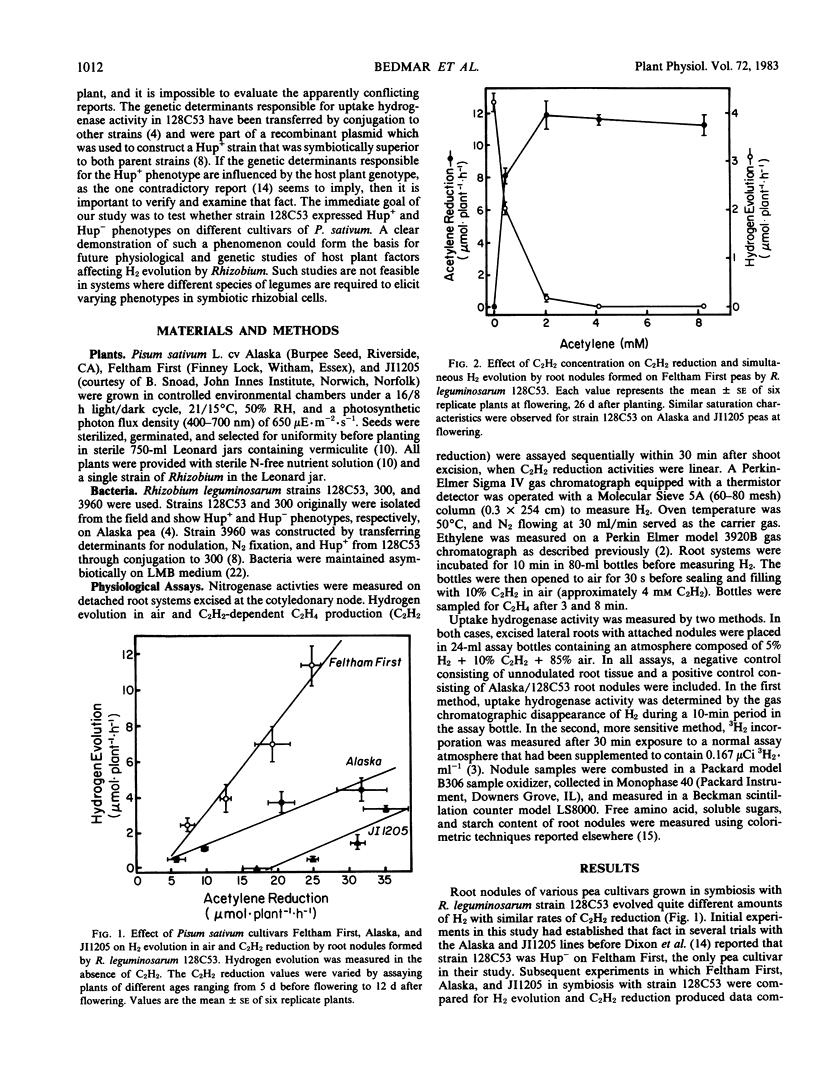
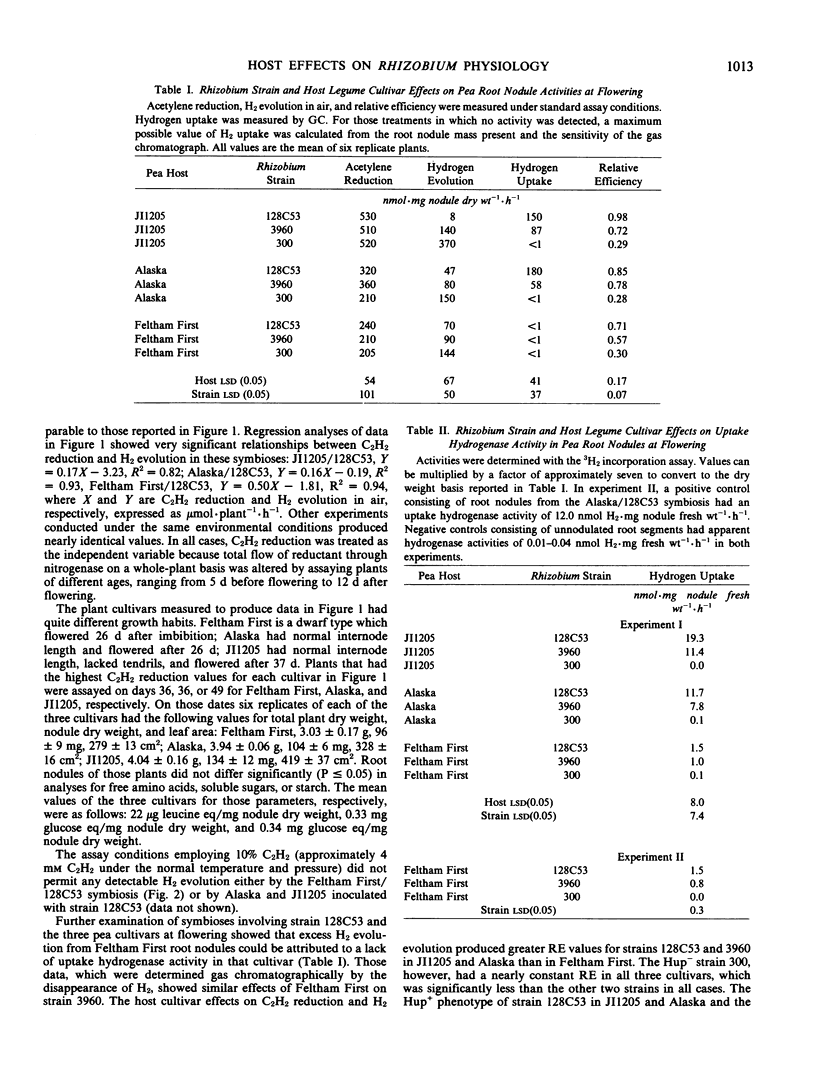
The effect of host plant cultivar on H2 evolution by root nodules was examined in symbioses between Pisum sativum L. and selected strains of Rhizobium leguminosarum. Hydrogen evolution from root nodules containing Rhizobium represents the sum of H2 produced by the nitrogenase enzyme complex and H2 oxidized by any uptake hydrogenase present in those bacterial cells. Relative efficiency (RE) calculated as RE = 1 − (H2 evolved in air/C2 H2 reduced) did not vary significantly among `Feltham First,' `Alaska,' and `JI1205' peas inoculated with R. leguminosarum strain 300, which lacks uptake hydrogenase activity (Hup−). That observation suggests that the three host cultivars had no effect on H2 production by nitrogenase. However, RE of strain 128C53 was significantly (P ≤ 0.05) greater in symbiosis with cultivar JI1205 than in root nodules of Feltham First. At a similar rate of C2H2 reduction on a whole-plant basis, nearly 24 times more H2 was evolved from the Feltham First/128C53 symbiosis than from the JI1205/128C53 association. Root nodules from the Alaska/128C53 symbiosis had an intermediate RE over the entire study period, which extended from 21 to 36 days after planting. Direct assays of uptake hydrogenase by two methods showed significant (P ≤ 0.05) host cultivar effects on H2 uptake capacity of both strain 128C53 and the genetically related strain 3960. The 3H2 incorporation assay showed that strains 128C53 and 3960 in symbiosis with Feltham First had about 10% of the uptake hydrogenase activity measured in root nodules of Alaska or JI1205. These data are the first demonstration of significant host plant effects on rhizobial uptake hydrogenase in a single plant species.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht S. L., Maier R. J., Hanus F. J., Russell S. A., Emerich D. W., Evans H. J. Hydrogenase in Rhizobium japonicum Increases Nitrogen Fixation by Nodulated Soybeans. Science. 1979 Mar 23;203(4386):1255–1257. doi: 10.1126/science.203.4386.1255. [DOI] [PubMed] [Google Scholar]
- Bethlenfalvay G. J., Phillips D. A. Ontogenetic Interactions between Photosynthesis and Symbiotic Nitrogen Fixation in Legumes. Plant Physiol. 1977 Sep;60(3):419–421. doi: 10.1104/pp.60.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlenfalvay G. J., Phillips D. A. Variation in nitrogenase and hydrogenase activity of alaska pea root nodules. Plant Physiol. 1979 May;63(5):816–820. doi: 10.1104/pp.63.5.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulen W. A., LeComte J. R. The nitrogenase system from Azotobacter: two-enzyme requirement for N2 reduction, ATP-dependent H2 evolution, and ATP hydrolysis. Proc Natl Acad Sci U S A. 1966 Sep;56(3):979–986. doi: 10.1073/pnas.56.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter K. R., Jennings N. T., Hanus J., Evans H. J. Hydrogen evolution and uptake by nodules of soybeans inoculated with different strains of Rhizobium japonicum. Can J Microbiol. 1978 Mar;24(3):307–311. doi: 10.1139/m78-051. [DOI] [PubMed] [Google Scholar]
- Dejong T. M., Phillips D. A. Nitrogen Stress and Apparent Photosynthesis in Symbiotically Grown Pisum sativum L. Plant Physiol. 1981 Aug;68(2):309–313. doi: 10.1104/pp.68.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. O. Hydrogenase in legume root nodule bacteroids: occurrence and properties. Arch Mikrobiol. 1972;85(3):193–201. doi: 10.1007/BF00408844. [DOI] [PubMed] [Google Scholar]
- Dixon R. O. Hydrogenase in pea root nodule bacterioids. Arch Mikrobiol. 1968;62(3):272–283. doi: 10.1007/BF00413898. [DOI] [PubMed] [Google Scholar]
- Edie S. A., Phillips D. A. Effect of the host legume on acetylene reduction and hydrogen evolution by Rhizobium nitrogenase. Plant Physiol. 1983 May;72(1):156–160. doi: 10.1104/pp.72.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisbrenner G., Evans H. J. Spectral evidence for a component involved in hydrogen metabolism of soybean nodule bacteroids. Plant Physiol. 1982 Dec;70(6):1667–1672. doi: 10.1104/pp.70.6.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerich D. W., Ruiz-Argüeso T., Ching T. M., Evans H. J. Hydrogen-dependent nitrogenase activity and ATP formation in Rhizobium japonicum bacteroids. J Bacteriol. 1979 Jan;137(1):153–160. doi: 10.1128/jb.137.1.153-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman R. V., Burris R. H. Electron allocation to alternative substrates of Azotobacter nitrogenase is controlled by the electron flux through dinitrogenase. Biochim Biophys Acta. 1980 Jun 10;591(1):63–75. doi: 10.1016/0005-2728(80)90220-0. [DOI] [PubMed] [Google Scholar]
- Keyser H. H., van Berkum P., Weber D. F. A Comparative Study of the Physiology of Symbioses Formed by Rhizobium japonicum with Glycine max, Vigna unguiculata, and Macroptilium atropurpurem. Plant Physiol. 1982 Dec;70(6):1626–1630. doi: 10.1104/pp.70.6.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustu S., Burton D., Garcia E., McCarter L., McFarland N. Nitrogen control in Salmonella: regulation by the glnR and glnF gene products. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4576–4580. doi: 10.1073/pnas.76.9.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. T., Shanmugam K. T. Regulation of hydrogen utilisation in Rhizobium japonicum by cyclic AMP. Biochim Biophys Acta. 1979 May 16;584(3):479–492. doi: 10.1016/0304-4165(79)90121-1. [DOI] [PubMed] [Google Scholar]
- Nelson L. M., Salminen S. O. Uptake hydrogenase activity and ATP formation in Rhizobium leguminosarum bacteroids. J Bacteriol. 1982 Aug;151(2):989–995. doi: 10.1128/jb.151.2.989-995.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Argüeso T., Maier R. J., Evans H. J. Hydrogen Evolution from Alfalfa and Clover Nodules and Hydrogen Uptake by Free-Living Rhizobium meliloti. Appl Environ Microbiol. 1979 Mar;37(3):582–587. doi: 10.1128/aem.37.3.582-587.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R., Evans H. J. Hydrogen evolution: A major factor affecting the efficiency of nitrogen fixation in nodulated symbionts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1207–1211. doi: 10.1073/pnas.73.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]


