Graphical abstract
Keywords: Strawberry, Targeted Metabolomics, GC–MS/MS, Flavor Compounds, Stable Isotope Dilution Analysis (SIDA), Ethyl vanillin
Chemical compounds studied in this article: Ethyl vanillin: (PubChem CID: 8467), Vanillin: (PubChem CID: 1183), Furaneol: (PubChem CID: 19309), Mesifurane: (PubChem CID: 61325)
Highlights
-
•
A targeted metabolomics strategy was developed based on LLE and GC–MS/MS.
-
•
The adopted strategy was used for screening flavor compounds in strawberry.
-
•
A total of 131 flavor compounds were accurately identified in this study.
-
•
Multiple techniques were applied to ensure the qualitative precision of the ethyl vanillin.
-
•
Ethyl vanillin was identified for the first time in natural food using the adopted strategy.
Abstract
Improving flavor can be an important goal of strawberry through breeding that is enhanced through the accurate identification and quantification of flavor compounds. Herein, a targeted metabolomics strategy was developed using liquid–liquid extraction, an in-house standard database, and GC–MS/MS analysis. The database consisted of key food odorants (KFOs), artificial flavor compounds (AFCs) and volatiles. A total of 131 flavor compounds were accurately identified in Medallion® ‘FL 16.30-128′ strawberry. Importantly, ethyl vanillin was identified for the first time in natural food. Multiple techniques, including GC–MS, GC–MS/MS and UPLC-MS/MS were applied to ensure the identification. The ethyl vanillin in the Medallion® samples were determined in a range of concentrations from 0.070 ± 0.0006 µg/kg to 0.1372 ± 0.0014 µg/kg by using stable isotope dilution analysis. The identification of ethyl vanillin in strawberry implys the future commercial use a natural flavor compound and the potential to identify genes and proteins associated with its biosynthesis.
1. Introduction
Strawberry (Fragaria × annanassa Duchesne ex Rozier) is one of the most popular fruits due to its attractive color, pleasing flavor and abundant nutritients (Du, Plotto, Baldwin, & Rouseff, 2011). Flavor is a key characteristic for strawberry quality and plays an important role in consumer acceptance (Bhat, Geppert, Funken, & Stamminger, 2015). Therefore, it is important to improve the flavor of strawberry through breeding while maintaining necessary agronomic traits (Oh et al., 2021, Porter et al., 2023). Multiple “omics” approaches, such as metabolomics, genomics, transcriptomics, proteomics, and phenomics have been applied as successful technologies for fruit improvement over the last few decades (Yang et al., 2021). Integration of genomics, transcriptomics, and metabolomics can reveal genomic loci genes and regulatory elements responsible for production of important flavor compounds. By utilizing high-quality of reference geneomes and advanced genomic tools, strawberry breeding programs can select for genotypes and elite breeding lines with higher production of favorable compounds (Hardigan et al., 2021, Han et al., 2022, Fan et al., 2022). Thus, the development of an efficient metabolomics strategy to elucidate flavor compounds has significant impact on the progress of flavor breeding.
Currently, gas chromatography-mass spectrometry (GC–MS) based untargeted metabolomics has been the commonly used approach to investigate volatile compounds in food, fruit, and other matrices (Jia et al., 2020, Xu et al., 2023, Zhu et al., 2020). This approach emphasizes the investigation of the observable volatile compounds in samples along with unknown chemicals (Cajka & Fiehn, 2016). The GC–MS based untargeted metabolomics strategy has the advantages of lack of bias and high coverage, but it suffers from the challenges of co-eluting compounds, poor reproducibility, low sensitivity, and low efficiency of identification (Cajka and Fiehn, 2016, Lai et al., 2018, Yuan et al., 2022). Targeted analysis provides accurate quantitation and annotation of a predefined group of metabolites. Yuan et al., developed a widely targeted volatilomics (WTV) method, to combine the strengths of untargeted and targeted metabolomics (Yuan, et al., 2022). WTV uses triple quadrapole GC–MS in multiple reaction monitoring mode to increase sensitivity. This strategy has higher sensitivity and efficiency of identification than the GC–MS based untargeted method. The adopted WTV method has been successfully applied to explore the volatile compounds of rice grains and fruits (Yuan, et al., 2022). Meanwhile, to develop a targeted metabolomics methods to explore important flavor compounds in fruits is of great importance.
Targeted identification of volatile compounds on a broad range in a GC–MS-based metabolomics study is quite challenging (Xue, Xu, Feng, Lu, & Zhou, 2022). Building a standards database would be very valuable for an analysis which can efficiently screen and identify flavor compounds. To meet this demand, the key food odorants (KFOs) database could be directly used as a library database. This was summarized by a literature survey based on the molecular sensory science results in more than 200 types of foods, such as fruits, baked goods, meat products, vegetables, fermented beverages etc. The results showed that only 226 aroma compounds out of more than 10,0000 volatile compounds, defined as KFOs, have been characterized as significantly contributing to the overall flavors of food matrices (Dunkel et al., 2014, Nicolotti et al., 2019). In addition, Wang et al., recently identified the oxime V using a metabolomics strategy, which was only known as a synthetic compound and had not been identified in nature (Wang, Gmitter, Grosser, & Wang, 2022). Ethyl vanillin is a very important artificial flavor in food production; however, its use is very limited due to its missing status and potential usability as a natural flavor compound (Jones et al., 2022, Qu et al., 2021). Therefore it is essential to cover the important artificial flavor compounds (AFCs) in an in-house database, which would be useful for screening natural products.
Currently, solid-phase microextraction (SPME) is the most used method to extract volatiles from the strawberry for metabolomics analysis due to the advantages of not requiring exhaustive sample preparation and being able to determine a broad range of compounds (Fan et al., 2022, Pontes et al., 2009, Tieman et al., 2017, Zhu et al., 2023). However, some important odorants with semi-volatile property remain poorly understood using the SPME method. For example, 2,5-dimethyl-4-hydroxy-3(2H)-furanone (furaneol) is one of the most important aroma compounds in strawberry, providing sweet and caramel-like aroma notes and present at high concentrations (Barbey et al., 2021). In order to accurately quantify furaneol and its methylated derivative 2,5-dimethyl-4-methoxy-3(2H)-furanone (mesifurane), an alternative to SPME is required (Du, Plotto, Baldwin, & Rouseff, 2011). Additionally, the efficiency of the SPME fiber will decrease with use which will affect the reproducibility of the method (Yuan, et al., 2022). Thus, there is an urgent need to develop a convenient extraction method and efficient targeted metabolomics strategy to comprehensively elucidate flavor compounds in strawberry with high accuracy and reproducibility.
The aim of this study was to (1) build a database based on the KFOs, AFCs and volatile compounds. (2) develop a convenient liquid–liquid extraction method to extract flavor compounds from strawberry and (3) elucidate the flavor compounds in strawberry.
2. Materials and methods
2.1. Plant material and fruit production
Medallion® ‘FL 16.30-128′ (hereafter referred to as Medallion®), is a new strawberry variety from University of Florida commercialized in 2022 primarily for its exceptional flavor. In previous field trials this variety had been described by breeding program personnel as exhibiting a faint vanilla flavor. Samples were harvested from the strawberry breeding trials at the Gulf Coast Research and Education Center (GCREC) of the University of Florida (UF) in 2022 and 2023 (Table 1). Fully ripe fruit with at least 90 % red surface coloration were harvested and stored at −20 °C freezer. The sample consisted of 6 fruits collected from a plot of 10 adjacent plants.
Table 1.
The transition ions, response factor, limits of quantitation, recovery rate, precision and concentration of ethyl vanillin in different strawberry samples.
| Sample | Harvest date | concentration (μg/kg) | Transition1a | Transition2 b | RF c | Recover rate (100 %) | precision RSD, % |
LOQ (μg/kg) |
|
|---|---|---|---|---|---|---|---|---|---|
| intra-day | inter-day | ||||||||
| Medallion®-1 | 01/21/2022 | 0.1372 ± 0.0014 | 166–137d | 171–138e | 1.05 | 95.6 | 4.52 | 4.76 | 0.009 |
| Medallion®-2 | 02/12/2022 | 0.1059 ± 0.0077 | |||||||
| Medallion®-3 | 02/22/2022 | 0.0733 ± 0.0011 | |||||||
| Medallion®-4 | 12/08/2022 | 0.070 ± 0.0006 | |||||||
| Medallion®-5 | 12/22/2022 | 0.0727 ± 0.0007 | |||||||
| Medallion®-6 | 03/01/2023 | 0.0978 ± 0.0005 | |||||||
Transition ions of ethyl vanillin for quantitation.
Transition ions of [5H2]-ethyl vanillin for quantitation.
Response factor.
The collision energy for transition ions 166–137 was 12.
The collision energy for transition ions 166–137 was 12.
2.2. Chemicals
Hexane, dichloromethane, 0.22 nylon filter and 1.0 mL syringe were purchased from Fisher Scientific Technology (www. thermofisher.com). The [5H2]-ethyl vanillin was used as the internal standard (IS), which was purchased from Santa Cruz Biotechnology Inc. Compared to ethylvanillin, the hydrogen on its methyl and ethyl groups is replaced by deuterium. The retention index is also an important parameter to identify the compounds, which were determined by the mixture of n-alkanes (C5-C30, Sigma-Aldrich).
2.3. Extraction of flavor compounds from strawberry using Liquid-Liquid extraction (LLE)
Five grams of homogenized strawberry and 25 μL of [5H2]-ethyl vanillin (IS, 200 μg/L) were put in 15 mL glass tube and then shaken vigorously for 15 s. Afterward, 5 mL of organic solvent (dichloromethane/hexane; 1:1 (V/V)) were added to the sample and shaken vigorously for 30 s. Thereafter, the samples in glass tubes were shaken in oscillator for 3 min in speed rate 2 and then ultrasound for 15 min in room temperature. The mixed sample was further separated into two phases of homogenized strawberry and solvent by centrifugation. The parameters of rotating speed, time and temperature for centrifuge were 4000 rpm, 15 min and 4 ℃, respectively. Finally, the organic solvent extract, which was at the upper of the tube, was removed out to the glass tube. The above operation was repeated for three times. The combined solvents were concentrated to approximately 0.5 mL by nitrogen gas. The collected prepared strawberry samples were stored at −80 °C prior of the GC–MS and GC–MS/MS analysis (Song et al., 2020).
2.4. Extraction of ethyl vanillin from strawberry using UPLC-MS/MS analysis.
100 Grams of homogenized strawberry, 20 mL of a saturated sodium chloride solution and 100 mL of dichloromethane were mixed in 1 L glass bottle and stirred by magnetic stirrer for 1 h. The mixed sample was ultrasound for 30 min in room temperature and then divided into two phases by centrifugation at 4000 rpm for 15 min at 4 ℃ using 15 mL glass tubes. The collected organic solvent was evaporated by a rotary evaporator. The concentrated components were dissolved in 0.5 mL of acetonitrile and then filtered by 0.22 nylon filter. The collected prepared strawberry samples were stored at −20 °C within 24 h prior of the UPLC-MS/MS analysis.
2.5. Qualification of flavor compounds using GC–MS/MS
The Thermo GC–MS/MS equipment including a 1610 gas chromatograph coupled with a TSQ 8000 triple quadrupole MS detector. The equipment consists of a TriPlus liquid autosampler and split/splitless injector. A FFAP column (30 m × 0.25 mm × 0.25 µm, Thermo Fisher Scientific, USA) was applied for separating the volatile compounds. In addition, a Thermo GC–MS/MS equipment including a 1600 gas chromatograph coupled with a TSQ 9610 triple quadrupole MS detector were also used in this study. A TG-5MS column (30 m × 0.25 mm × 0.25 µm, Thermo Fisher Scientific, USA) and a Rt-bDEXsm column (chrial column) (30 m × 0.25 mm × 0.25 µm, Restek Corporation, USA) were used for confirmation of ethyl vanillin in strawberry by using TSQ 9610 GC–MS/MS. Helium was used as the carrier gas with a flow of 1 mL/min. A volume of 1.0 μL of extracted sample was injected into the GC–MS/MS for analysis. The injector was adapted to splitless mode and kept at 250 °C. The oven temperature program was initially at 45 °C, and kept for 1 min. Afterward, the temperature was increased form 45 °C to 230 °C at the rate of 4 °C/min and then held for 2 mins. Electron ionization (EI) was used as the ion source and with the 70 eV ionization energy. The temperatures of ion source and transmission line were set as 230 °C. For the full scan mode, the ions from 35 m/z to 350 °C were determined. For multiple reaction monitoring (MRM) mode, argon was used as the collision gas. The collision energy (CE) for each precursor-product ion pair (Q1-Q3) was optimized to achieve maximal signal intensity. The database standards were dissolved in ethanol at a concentration of 1 μg/mL for GC–MS/MS optimization. Two transitions were chosen based on the optimization data, one for qualification and another one for quantitation. The MRM detection window was ± 0.3 min, and dwell times were adjusted automatically based on loop time (0.15 s) for the maximized data acquisition.
2.6. Method validation
The concentration of ethyl vanillin in different samples was determined by stable isotope dilution analysis using LLE combined with TSQ 9610 GC–MS/MS. Commercial strawberries (variety unknown) were obtained from Walmart in order to use strawberries without ethyl vanillin as the blank matrix. The known amounts of ethyl vanillin in different concentrations (5, 3, 1, 0.333 and 0.2 μg/kg) and the 1 μg/kg of [5H2]-ethyl vanillin were added to the blank matrix sample. The combinations of unlabeled and labeled compounds were prepared in five differnet ratios (5:1, 3:1, 1:1,1:3 and 1:5). After equilibration, the samples with [5H2]-ethyl vanillin and ethyl vanillin were processed the same as Liquid-Liquid Extraction (LLE). The linear response curve of [5H2]-ethyl vanillin was built by the peak area ratio of unlabeled standard to labeled standard and concentration ratio of unlabeled standard to labeled standard. The response factor was obtained from the linear response curve (Burdack-Freitag & Schieberle, 2012). To determine the ethyl vanillin in strawberry, the 25 μL of [5H2]-ethyl vanillin (IS, 200 μg/L) was added to the 5 g of strawberry. After equilibration, the samples with labeled ethyl vanillin were processed in the same way by Liquid-Liquid Extraction (LLE). The transition ions 166–137 of ethyl vanillin and transition ions 171–138 of [5H2]-ethyl vanillin were selected for quantitation. The concentrations were calculated from the relative abundances of the selected transition ions for the analyte and the internal standards, and the data were corrected by means of response factors. To determine the recovery rate, 0.1 μg/kg of ethyl vanillin was spiked into the Medallion®-1 sample. Then, the concentration of the ethyl vanillin in the strawberry sample was determined before and after the chemical spiking to calculate the recovery rate (Song et al., 2021). The measurements of the limit of detection (LOD) of ethyl vanillin were performed on this equipment in full scan and MRM mode respectively. The ethyl vanillin was dissolved in ethanol for analysis by the above two analytical modes. Repeated analyses of ethyl vanillin at a concentration of 1 mg/L (n = 6) were used to measure the intraday precision, while the interday precision was determined on three different days at the same concentration. The limit of quantitation (LOQ) was also determined by LLE combined with GC–MS/MS (MRM). The LOD and LOQ values of the ethyl vanillin were determined at its concentrations when their signal–noise ratio (S/N) were 3 and 10, respectively.
2.7. Confirmation of ethyl vanillin in strawberry using UHPLC/Q-Orbitrap MS and UHPLC/QQQ MS
The UHPLC/Q-Orbitrap MS (Thermo Fisher Scientifc) equipment including Vanquish Flex Binary UHPLC connected to a Q Exactive Plus UHPLC/mass spectrometer. The separation of compounds was performed on an Agilent Poroshell HPH-C18 column (150 × 2.1 mm, 1.9 μm; Agilent, Santa Clara, CA). The temperature of column was set as 30 °C. The mobile phase A used water with 0.1 % formic acid, while mobile phase B was acetonitrile with 0.1 % formic acid. The gradient elution program was as follows: 0–6.5 min, 20–65 % B; 6.5–11.5 min, 65–70 % B; 11.5–15 min, 70–100 % B; 15–30 min, 100–20 % B. Prior to the analysis, the equilibration time for the column was 5 min at the initial mobile phase ratio. The flow rate was set as 0.3 mL/min. A volume of 2 μL was injected for the analysis. The ion mode of the mass spectrometer was set as positive. The parameters for heated electrospray ionization (HESI) were as follows: capillary temperature, 325 °C; heater temperature, 350 °C; spray voltage, 3.5 kV; sheath gas, 50 arb; auxiliary gas, 10 arb; and sweep gas, 0 arb; purge gas flow, 0 arb, and S-lens RF level, 55 %. The Target-SIM (selected ion monitoring)/ddMS2 scan mode was applied in the mass spectrometer and operated in positive ion mode. The inclusion list includes m/z 167.0702 (C9H10O3) to acquire the quasi-molecular ion of ethyl vanillin in the SIM mode. The SIM parameters were configured as follows: a resolution of 70,000 was established, the automatic gain control (AGC) was set to 5 × 104, and the maximum injection time (IT) was set at 100 ms and the isolation window was set as 4.0 m/z. To obtain second-stage fragmentation of m/z 167.0702, the following ddMS2 parameters were applied: a resolution of 35,000 was established, the automatic gain control (AGC) was set to 2 × 105, and the maximum injection time (IT) was set at 100 ms and the isolation window was set as 0.8 m/z.
The UPLC-MS/MS (Thermo Fisher Scientifc) equipment including Ultimate 3000 ultra-performance liquid chromatography (UPLC) connected to a TSQ Quantiva Triple Quadrupole mass spectrometer. The separation of compounds was performed on an Agilent Poroshell HPH-C18 column (150 × 2.1 mm, 1.9 μm; Agilent, Santa Clara, CA). The temperature of column was set as 30 °C. The mobile phase A used water with 0.1 % formic acid, while mobile phase B was acetonitrile with 0.1 % formic acid. The gradient elution program was as follows: 0–6.5 min, 20–65 % B; 6.5–11.5 min, 65–70 % B; 11.5–15 min, 70–100 % B; 15–30 min, 100–20 % B. Prior to the analysis, the equilibration time for the column was 5 min at the initial mobile phase ratio. The flow rate was set as 0.4 mL/min. A volume of 2 μL was injected for the analysis. The ion mode of the mass spectrometer was set as positive. The parameter for heated electrospray ionization (HESI) was as follows: ion transfer tube temperature, 325 °C; vaporizer temperature, 350 °C; spray voltage, 3.5 kV; sheath gas, 35 arb; auxiliary gas, 10 arb; and sweep gas, 2 arb. The MRM mode was used for the analysis of the strawberry sample. Argon was used as the collision gas and with a pressure of 1.5 mTorr. The optimization of ethyl vanillin in MS/MS was performed by flow injection analysis.
2.8. Statistical analysis
The GC–MS raw data was process by MS-DIAL to deconvolute the co-eluting compounds and then matched to the NIST library. GC–MS/MS and UPLC-MS/MS data were obtained by the Xcalibur and then processed by TraceFinder 4.1 software (Thermo scientific). The UHPLC/Q-Orbitrap MS data was processed by Compound Discoverer v3.1 (Thermo Fisher Scientifc, Waltham, MA).
3. Results and discussion
3.1. Development of a targeted flavor standards database in GC–MS/MS
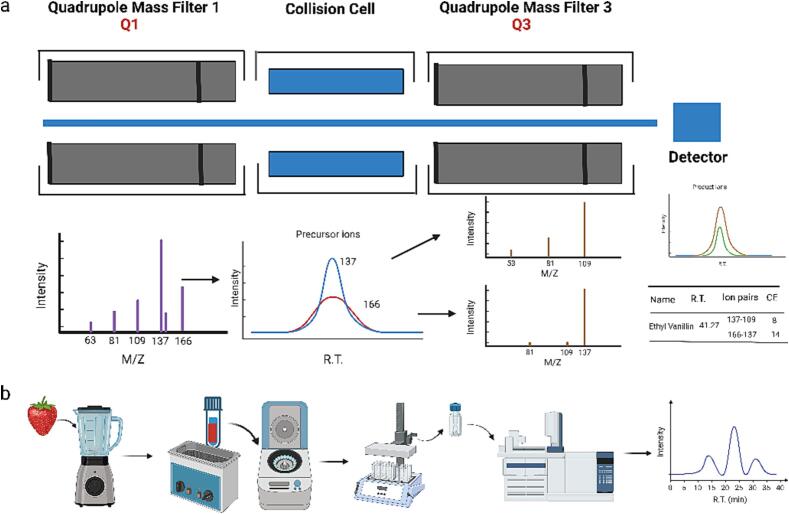
The in-house database assembled 346 flavor compounds including 100 key food odorants (KFOs), 2 artificial flavor compounds (AFCs) and other volatile compounds. For example, ethyl vanillin yielded two transitions, 166–137 and 137–109 (Fig. 1a). Two pairs of percursor and product ions were chosen for qualification and the pair with higher intensity was selected for quantitation. According to our MRM-based database, one of the primary benefits is its high efficiency in annotating key food odorants, attributable to the fact that it contains the KFO standard compounds, which were based on a literature survey of molecular sensory science. The MRM-based database also has the advantage of including important standards of artificially synthesized flavor compounds, which could facilitate the screening of natural products. In future studies, this library including KFOs, AFCs and volatiles can be used to efficiently screen and identify flavor compounds.
Fig. 1.
a, The structure of GC–MS/MS and the workflow for optimizing the collision energy. b, The workflow of the liquid–liquid extraction method.
3.2. Optimization of the liquid–liquid extraction method
In the present study, the performance of extraction solvents was compared based on their extraction efficiency and density. Specifically, dichloromethane, hexane, and a mixed solution of dichloromethane and hexane (1:1, V/V) were evaluated for extraction under identical conditions. Dichloromethane, hexane, and the mixed solvent identified 165, 141, and 107 metabolites, respectively, with S/N > 10. Notably, dichloromethane has a higher density and tends to settle at the bottom of the strawberry mixture after centrifugation, which can make separation more challenging. On the other hand, the mixed solvent stays at the top of the strawberry mixture, allowing for easy and rapid separation of the extraction solution. Additionally, due to the simplicity and repeatability of the operation, mixed solvents are recommended for extracting aroma components in strawberries. The supernatant obtained from the mixed solvent extraction was almost colorless and transparent, and can be further concentrated and filtered for GC–MS analysis. This is in contrast to SPME which can suffer from poor reproducibility over time due to loss of fiber efficiency and batch effects. Liquid-Liquid Extraction (LLE) is fast, simple and uses microquantities of solvent, which enables its application in large-scale studies, such as metabolite-based genome-wide association studies (GWAS).
3.3. Validation of the targeted metabolomics method
To verify the developed extraction method, Medallion® strawberry samples were used for extraction by LLE and then analyzed via GC–MS/MS based on a targeted metabolomics database (The workflow is shown in Fig. 1b). A total of 131 flavor compounds were accurately identified, including 41 ester compounds, 11 aldehydes, 9 lactones, 7 ketone compounds, 21 alcohol compounds, 8 terpene, 2 furanones, 9 phenol compounds, 9 acid compounds, 7 sulfur-containing compounds, 4 nitrogen compounds, and 3 aromatic compounds (Supplementary Table 1). Beta-hydroxy-benzenepropanoic acid ethyl ester, (2E)-2-hexenyl-pentanoicacid, ethyl nicotinoate, ethyl vanillin, and p-methoxybenzyl acetate were identified for the first time in strawberry. The expanded flavor compounds annotation repertoire obtained using the developed method allowed us to discover new compounds in strawberry.
Among the determined flavor compounds, furaneol and mesifurane have been characterized as the most important aroma compounds in many food matrices (Gao et al., 2021), espically in strawberry (Yan et al., 2018). Previous studies have shown that mesifurane content in strawberry is modulated by the gene FaOMT, which may be related to the conversion of furaneol into mesifurane. An expression study showed that the FaOMT could could be involved in precursors for achene lignification at early stages of fruit development (Zorrilla-Fontanesi et al., 2012). Due to its semi-volatile property, furaneol is remains poorly understood in strawberry since it is not well-quantified with the SPME method. In this study, the LLE combined with GC–MS/MS was successfully apply to accurately quantify furaneol and its methylated derivative mesifurane. Comparison to the GC–MS full scan mode, the signal-to-noise of furaneol has been improved from 625 to 98,001 in MRM mode, which showed higher sensitivity. The limit of quantitation (LOQ) of furaneol is 0.02 ug/kg, which indicated that the optimized approach was able to determine the furaneol with extremely low concentration.
Lactones are described as having fruity, milky, and coconut-like odors, which play a significant role in the overall flavor profile of strawberry. In this study, 9 lactones were identified, including γ-butyrolactone, γ-hexanolide, γ-heptanolide, γ-octalactone, δ-octanolide, γ-decalactone, δ-decanolide, γ-dodecalactone and δ-dodecalactone. It is worth noting that Medallion® has a high concentration of γ-decalactone, which confers peach-like notes (Chambers, Pillet, Plotto, Bai, Whitaker & Folta, 2014). The occurrence of γ-decalactone in strawberry is largely controlled by a single locus, FaFAD1. In previous studies the genomic structure of FaFAD1 has been explored and the allele dosage effects quantified, showing that increasing allele dosage is an effective breeding strategy for increasing this compound (Oh et al., 2021). While γ-decalactone and γ-dodecalactone have been shown to be important flavor compounds in strawberry, little is known about the contribution of other lactones. Future work can explore the role of other lactones in strawberry flavor. The identification transitions of ethyl vanillin, vanillin, furaneol, mesifurane and lcatones are shown in the Supplementary Table 2.
3.4. Identification of ethyl vanillin in strawberry by GC–MS/MS
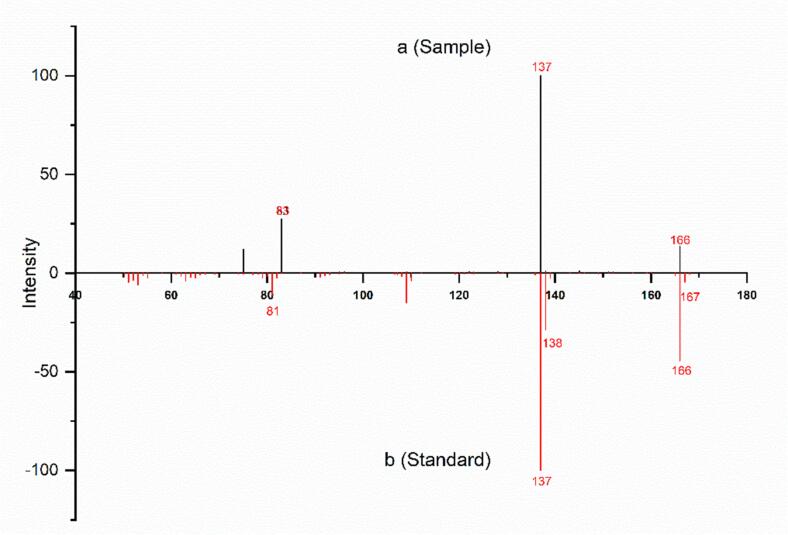
Notably, two flavor compounds, vanillin, and ethyl vanillin were identified in our study, which were correlated to their representative vanilla-like odor. Multiple techniques, including GC–MS, GC–MS/MS, UHPLC/Q-Orbitrap MS and UPLC-MS/MS were applied to ensure the qualitative precision of the ethyl vanillin. The total ion chromatogram of Medallion® strawberry did not produce a response peak at the expected retention time of ethyl vanillin (41.27 min) in GC–MS analysis (Supplementary Fig. 1). However, the extracted ion chromatogram showed the specific ions 166, 137, 109 and 81 of ethyl vanillin but all were low in intensity, presumably due to its low intensity and the presence of contaminants. (Supplementary Fig. 2). The MS-DIAL software was used to deconvolute coeluting signals in order to solve the convolution and to enhance the precision of the identification. An extracted ion chromatogram was obtained for the low response at the retention time of 41.27 min (Fig. 2a) which produced a match for ethyl vanillin using the NIST library (Fig. 2b). However, there is no confidence to identify the ethyl vanillin based on the GC–MS result due to the extremely low concentration.
Fig. 2.
a, The deconvoluted mass chromatogram at a retention time of 41.27 min; b, The mass spectrum of ethyl vanillin from the NIST library.
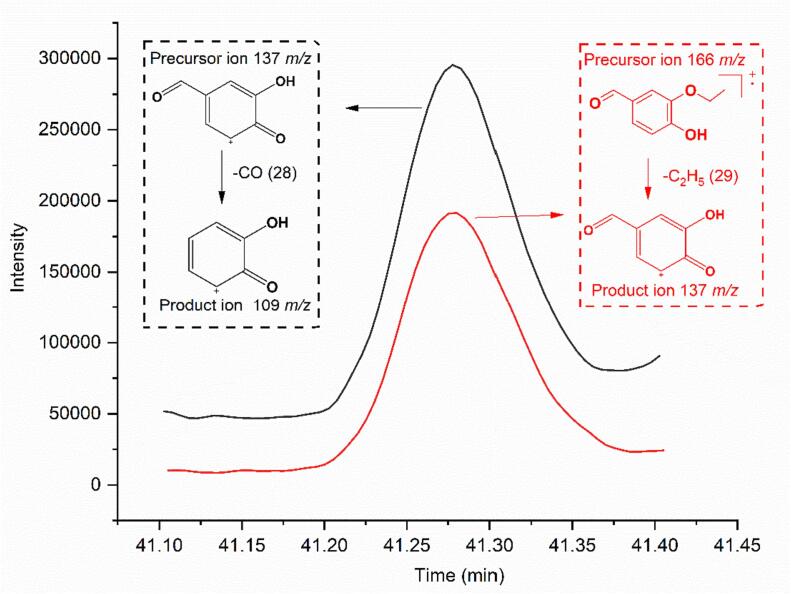
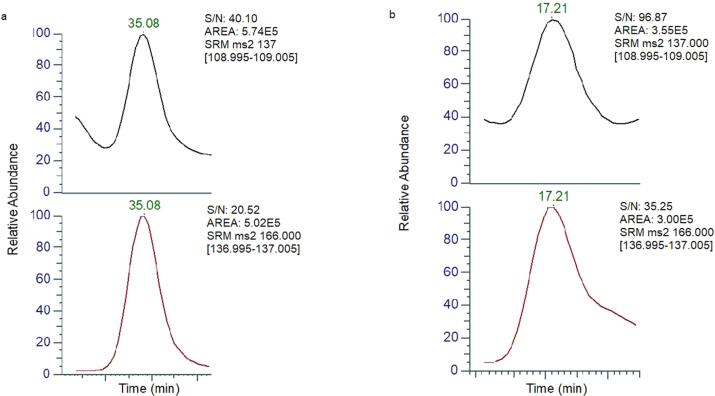
Subsequently, it was successfully identified by the MRM mode in GC–MS/MS. Based on the optimization results, the MRM transitions 166–137 and 137–109 were used for identification. The GC–MS/MS chromatograph of ethyl vanillin was clearly obtained, as shown in Fig. 3. Additionally, the ratio of the areas of two transitions (137–109/166–137) in optimization was 1.25:1. For the Medallion® sample, the ratio of the areas of these two transitions was 1.35: 1. The slight difference can be attributed to the matrix effect. To verify the matrix effect, 50 μg/kg of ethyl vanillin was added to the blank strawberry and then extracted using LLE. The GC–MS/MS results revealed that the ratio of the areas of two transitions in the spiked blank strawberry was 1.36:1, confirming the identification of ethyl vanillin. The intensity data is shown in the Supplementary Table 3. To enhance the confirmation of qualitative precision, a non-polar TG-5MS column and a chiral Rt-bDEXsm column were used to conduct the identification analysis of ethyl vanillin utilizing the MRM mode in GC–MS/MS. As shown in Fig. 4, the GC–MS/MS chromatograph of ethyl vanillin were clearly obtained in these two different columns. Overall to identify ethyl vanillin successfully in the strawberry fruit a combination of GC–MS/MS (MRM) and three different capillary columns, including nonpolar, polar, and chiral polar stationary phases, were used.
Fig. 3.
The chromatogram of ethyl vanillin detected in Medallion®-1 sample by GC–MS/MS in MRM and proposed fragmentation pattern with tentative structures for the product ions of ethyl vanillin.
Fig. 4.
A, the chromatogram of ethyl vanillin detected in medallion®-4 sample by tg-5 ms column utilizing the mrm mode in gc–MS/MS; b, The chromatogram of ethyl vanillin detected by Rt-bDEXsm column (chrial column) in Medallion®-4 strawberry utilizing the MRM mode in GC–MS/MS.
Furthermore, the instrument sensitivity of GC–MS (full scan) and GC–MS/MS (MRM) was compared in light of the LOD value of ethyl vanillin. The LODs of GC–MS and GC–MS/MS were 50 μg/L and 2 μg/L, respectively. It is clearly demonstrated that GC–MS/MS possesses a 25 times higher sensitivity (lower LOD) than GC–MS for ethyl vanillin. Triple quadrupole GC–MS/MS is more selective and sensitive than single quadrupole GC–MS due to its ability to monitor specific ion transitions which reduces background noise. The integration of the flavor database and GC–MS/MS not increases sensitivity and extends the annotation coverage of flavor compounds, demonstrating the effectiveness and feasibility of the targeted metabolomics strategy for analysis of flavor compounds in strawberry.
3.5. Confirmation of ethyl vanillin in strawberry by UPLC-MS/MS and UHPLC/Q-Orbitrap MS
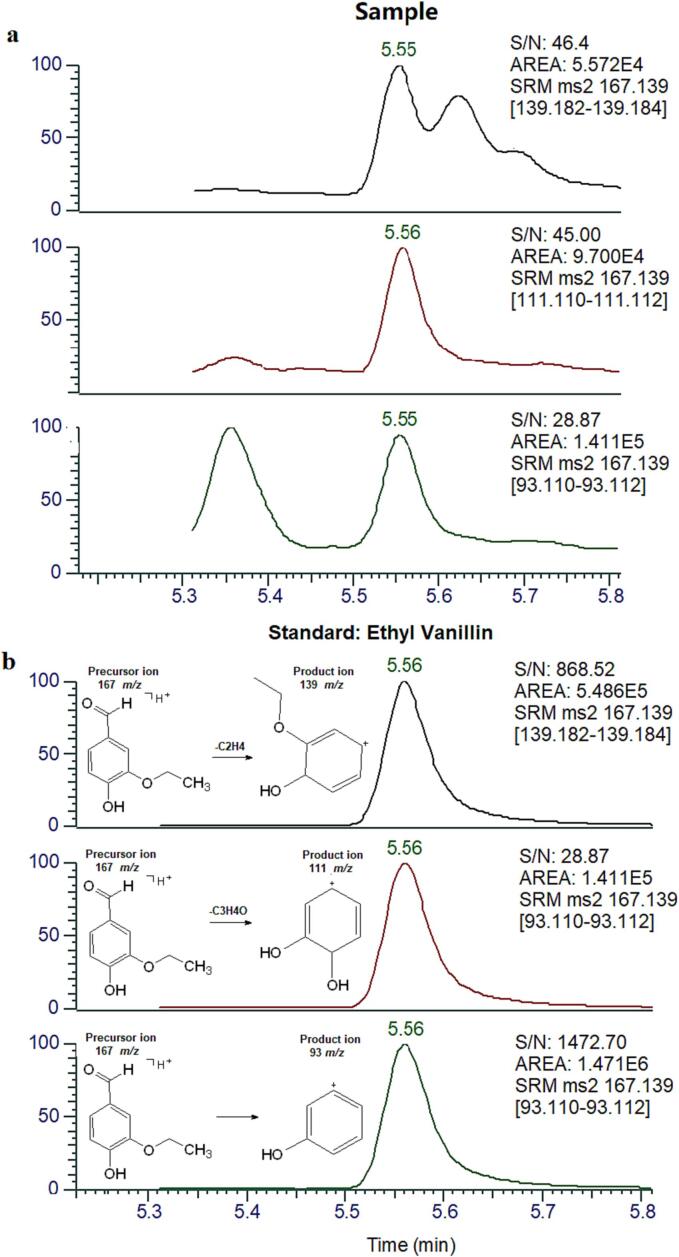
Because it is crucial to carefully confirm the presence of ethyl vanillin in strawberries, UPLC-MS/MS was also used to verify its identification. The optimization results indicated that MRM transitions 167–139, 167–93, and 167–111 could be used for qualification. A clear chromatograph of ethyl vanillin was also obtained using UPLC-MS/MS (Fig. 5a). The chromatogram of the standard compound of ethyl vanillin detected by LC-MS/MS in SRM was shown in the Fig. 5b. In addition, the ratios of the areas of 167–93 to 167–111 and 167–111 to 167–139 in optimization were 1.28:1 and 2.10:1, respectively. However, the ratios of the areas of 167–93 to 167–111 and 167–111 to 167–139 were 1.46:1 and 1.74:1 in the Medallion® sample, respectively. The slight difference can also be attributed to the matrix effect. To verify the matrix effect, 20 μg/kg of ethyl vanillin was added to the blank strawberry and then analyzed by UPLC-MS/MS. The UPLC-MS/MS results showed that the ratios of the areas of 167–93 to 167–111 and 167–111 to 167–139 in the spiked blank sample were 1.50:1 and 1.87:1, respectively. The intensity data was shown in the Supplementary Table 4.
Fig. 5.
a, The chromatogram of ethyl vanillin detected in Medallion®-4 sample by LC-MS/MS in SRM; b, The chromatogram of the standard compound of ethyl vanillin detected by LC-MS/MS in SRM and proposed fragmentation pattern with tentative structures for the product ions of ethyl vanillin.
It's essential to use high-resolution mass spectrometry for precise mass-based qualitative analysis. The fragment mass spectrometry (MS/MS) data reveal the fragmentation patterns of molecules and providing information about molecular structure. This helps in gaining a more comprehensive understanding of the structure and composition of compounds. Thus, UHPLC/Q-Orbitrap MS was applied to identify the ethyl vanillin in strawberry. It’s unable to obtain the MS/MS spectra of the quasi-molecular ion using the Full-MS/ddMS scan mode, due to the low concentration of ethyl vanillin in strawberry. Subsequently, the Target-SIM/ddMS2 scan mode was applied to scan the quasi-molecular ion (m/z 167.0699 [M + H]+) and then selected it as target precursor ions for further MS/MS fragmentation. A clear chromatographic peak at same retention times to those of the standard compound was obtained. Furthermore, the identification of ethyl vanillin in strawberry was performed by comparing to the MS/MS fragmentation of authentic standard. This approach yields a substantial sensitivity improvement for targeted compound qualitative analysis. The annotated MS/MS spectra of sample and standard were presented in Supplementary Figs. 3 and 4. There is a difference in the ratio of m/z 167.0699 to 111.0438 between sample and standard, which could be attributed to the extremely low concentration of ethyl vanillin in the sample. It may have been influenced by matrix effects during the acquisition of the MS/MS spectra. Furthermore, as shown in Fig. 5a, one pair ion of 167–139 was observed at the retention time of 5.62 min. The exceptionally close retention times result in both ions still undergoing simultaneous fragmentation in the secondary mass spectrum. It generates unknown fragments that significantly impact to secondary mass spectrometry of m/z 167.0699.
It is important to note that the crucial diagnostic ion fragments were detected in the sample. As shown in Supplementary Fig. 2, Its protonated quasi-molecular ion was m/z 167.0699 [M + H]+. The most characteristic base peak fragment ion of m/z 111.0438, which could be produced form the decarbonylation (losing one CO) of m/z 139.0388, followed by a dehydration (losing one H2O) leading to the significant fragment of m/z 93.0333. Another fragment with m/z 139.0751 originated from m/z 167.0699 was also through decarbonylation. Although present in low abundance, this fragment proved diagnostically valuable, followed by a dehydration leading to the significant fragment of m/z 121.0645. The fragment m/z 149.0594 was derived from the dehydration of m/z 167.0699, and the subsequent decarbonylation led to a fragment of m/z 121.0645. Based on these results, it was concluded that that the ethyl vanillin was accurately identified in strawberry.
3.6. The concentrations of ethyl vanillin in Medallion® strawberry samples.
The concentrations of ethyl vanillin in different Medallion® strawberry samples were quantified by stable isotope dilution analysis using LLE in combination with GC–MS/MS. The response factor, LOQ, recovery rate, and repeatability were conducted for measuring the validation of the method. The linear response curve was established through the analysis of combinations of labeled and unlabeled compounds in five distinct ratios (5:1, 3:1, 1:1, 1:3, 1:5), which produced a response factor of 1.05. To investigate the efficiency of the extraction method, the recovery rate of ethyl vanillin was measured, which showed relative high ratio of 94.5 %. The method repeatability was expressed by the relative standard deviation (RSD), which were 4.52 % and 4.76 % for intra-day and inter-day, respectively. In addition, the LOQ of ethyl vanillin is 0.009 μg/kg, which indicated the adopted strategy has high sensitivity. The ethyl vanillin in the Medallion® samples were determined in a range of concentrations from 0.070 ± 0.0006 µg/kg to 0.1372 ± 0.0014 µg/kg (Table 1). Medallion®-1 had the highest concentrations of ethyl vanillin in 0.1372 ± 0.0014 μg/kg, and Medallion®-4 had the lowest concentration of 0.070 ± 0.0006 μg/kg. The content of ethyl vanillin in the fruits collected at the end of January and the middle of February was at a higher level. Its organoleptic role needs to be investigated in future studies.
Vanillin and ethyl vanillin exhibit a characteristic vanilla-like odor. Vanillin has been characterized as a key odorant in many food matrixes such as Chinese Baijiu, white bread (Pu et al., 2019, Song et al., 2019). At present there are three common routes to obtain vanillin, including: (1): Isolated from the vanilla pod extracts (Sinha, Sharma, & Sharma, 2008); (2): Chemical synthesized using the eugenol, guaiacol and lignin as substrates (Gallage et al., 2014, Gallage and Møller, 2015); (3) Biotechnology-derived production with the use of ferulic acid, eugenol, and glucose as substrates and bacteria, fungi, and yeasts as microbial production hosts. Additionally, the de novo biosynthetic pathway of vanillin in the vanilla orchid has been demonstrated, which is catalyzed by a single enzyme, vanillin synthase (VpVAN). This new knowledge could be applied in the biotechnology-derived production of vanillin. VpVAN catalyzes the two-carbon cleavage of ferulic acid and its glucoside into vanillin and vanillin glucoside, respectively (Gallage, et al., 2014). The organoleptic role of vanillin is not explored in strawberry. Based on the developed targeted metabolomics strategy, it will be possible to gain insight into the flavor contribution of vanillin in a broad range of strawberry varieties based on the odor activity values.
Ethyl vanillin was identified for the first time in strawberry fruit, i.e. in a natural food product. It is a fine powder with three times more aromatic potency than vanillin and offers a more concentrated flavor profile in order to provide sweet, pure, and rich creamy notes of vanilla. In addition, it’s one of the most widely used flavors in the world and is applied extensively in the food, beverage, perfumery, and pharmaceutical industries. However, it is prohibited from being added to certain foods as it is an artificial synthetic flavor compound. Presently, consumers are inclined to seek more natural flavors. Up to now, there are two routes to obtain ethyl vanillin: (1) Chemical synthesized using the glyoxylic acid and o-ethoxyphenol as substrates (Gallage & Møller, 2015); (2) biotechnology-derived production with the use of 3-ethoxy-4-hydroxymandelic acid, and 3-ethoxy-4-hydroxyacetophenone acid as substrates and pseudomonas putida, and pseudomonas aeruginosa as microbial production hosts. The biosynthesis pathway of ethyl vanillin in plants is not understood. Future studies will integrate the developed targeted metabolomics strategy and GWAS with a range of varieties and breeding selections to better understand the natural variation ethyl vanillin in strawberry and genes underlying its biosynthesis. Controlled crosses have been initiated with Medallion® in the UF breeding program to determine the possibility of increasing the concentration of both vanillin and ethyl vanillin in strawberries.
4. Conclusion
In this study, a targeted metabolomics strategy based on LLE combined with GC–MS/MS (MRM) was developed to accurately identify 131 flavor compounds in strawberry. The method has the advantages of high sensitivity, high coverage and being more targeted toward flavor compounds. Notably, ethyl vanillin was identified for the first time in a natural food. The successful identification of ethyl of vanillin in strawberry holds implications for its future commercial use a natural flavor compound and the potential to identify genes and proteins associated with its production. The adopted targeted metabolomics strategy was suitable for uncovering the flavor compounds in large-scale sampling and will be integrated with genomic data to explore the genetic architecture of ethyl vanillin biosynthesis.
Funding Sources
This work was supported by Florida Strawberry Research and Education Foundation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
Thanks to Dr. Zhixin Wang (CREC) and Dr. Jinpyo An (CREC), members of our lab, for assisting us in verifying the identification of ethyl vanillin. Special thanks to Mr. Tony Trama (Florida Department of Citrus) for his help in editing the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100944.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Barbey C.R., Hogshead M.H., Harrison B., Schwartz A.E., Verma S., Oh Y.…Whitaker V.M. Genetic analysis of methyl anthranilate, mesifurane, linalool, and other flavor compounds in cultivated strawberry (Fragaria x ananassa) Frontiers in Plant Science. 2021;12 doi: 10.3389/fpls.2021.615749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R., Geppert J., Funken E., Stamminger R. Consumers perceptions and preference for strawberries-A case study from Germany. International Journal of Fruit Science. 2015;15(4):405–424. [Google Scholar]
- Burdack-Freitag A., Schieberle P. Characterization of the key odorants in raw Italian hazelnuts(Corylus avellanaL. var. Tonda Romana) and roasted hazelnut pasteby means of molecular sensory science. Journal of Agricultural and Food Chemistry. 2012;60:5057–5064. doi: 10.1021/jf300908d. [DOI] [PubMed] [Google Scholar]
- Cajka T., Fiehn O. Toward merging untargeted and targeted methods in mass spectrometry-based metabolomics and lipidomics. Analytical Chemistry. 2016;88(1):524–545. doi: 10.1021/acs.analchem.5b04491. [DOI] [PubMed] [Google Scholar]
- Chambers A., Pillet J., Plotto A., Bai J., Whitaker V., Folta K. Identification of a strawberry flavor gene candidate using an integrated genetic-genomic-analytical chemistry approach. BMC Genomics. 2014;15(217):1471–2164. doi: 10.1186/1471-2164-15-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Plotto A., Baldwin E., Rouseff R. Evaluation of volatiles from two subtropical strawberry cultivars using GC-olfactometry, GC-MS odor activity values, and sensory analysis. Journal of Agricultural and Food Chemistry. 2011;59(23):12569–12577. doi: 10.1021/jf2030924. [DOI] [PubMed] [Google Scholar]
- Dunkel A., Steinhaus M., Kotthoff M., Nowak B., Krautwurst D., Schieberle P., Hofmann T. Nature's chemical signatures in human olfaction: A foodborne perspective for future biotechnology. Angewandte Chemie International Edition. 2014;53(28):7124–7143. doi: 10.1002/anie.201309508. [DOI] [PubMed] [Google Scholar]
- Fan Z., Tieman D.M., Knapp S.J., Zerbe P., Famula R., Barbey C.R.…Whitaker V.M. A multi-omics framework reveals strawberry flavor genes and their regulatory elements. New Phytologist. 2022;236(3):1089–1107. doi: 10.1111/nph.18416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallage N.J., Hansen E.H., Kannangara R., Olsen C.E., Motawia M.S., Jørgensen K.…Møller B.L. Vanillin formation from ferulic acid in Vanilla planifolia is catalysed by a single enzyme. Nature Communications. 2014;5(1):4037. doi: 10.1038/ncomms5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallage N.J., Møller B.L. Vanillin-Bioconversion and bioengineering of the most popular plant flavor and its De novo biosynthesis in the vanilla orchid. Molecular Plant. 2015;8(1):40–57. doi: 10.1016/j.molp.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Gao X., Feng T., Sheng M., Wang B., Wang Z., Shan P.…Ma H. Characterization of the aroma-active compounds in black soybean sauce, a distinctive soy sauce. Food Chemistry. 2021;364 doi: 10.1016/j.foodchem.2021.130334. [DOI] [PubMed] [Google Scholar]
- Han, H., Barbey, C. R., Fan, Z., Verma, S., Whitaker, V., & Lee, S. (2022). Telomere-to-Telomere and haplotype-phased genome assemblies of the heterozygous octoploid′ florida brilliance′ strawberry (Fragaria× ananassa). BioRxiv, 2022-10.
- Hardigan, M. A., Feldmann, M. J., Pincot, D. D., Famula, R. A., Vachev, M. V., Madera, M. A., & Knapp, S. J. (2021). Blueprint for phasing and assembling the genomes of heterozygous polyploids: application to the octoploid genome of strawberry. BioRxiv, 2021-11.
- Jia X., Wang L., Zheng C., Yang Y., Wang X., Hui J., Zhou Q. Key odorant differences in fragrant brassica napus and brassica juncea oils revealed by gas chromatography-olfactometry, odor activity values, and aroma recombination. Journal of Agricultural and Food Chemistry. 2020;68(50):14950–14960. doi: 10.1021/acs.jafc.0c05944. [DOI] [PubMed] [Google Scholar]
- Jones, S. K., McCarthy, D. M., Vied, C., Stanwood, G. D., Schatschneider, C., & Bhide, P. G. (2022). Transgenerational transmission of aspartame-induced anxiety and changes in glutamate-GABA signaling and gene expression in the amygdala. Proceedings of the National Academy of Sciences, 119(49), e2213120119. [DOI] [PMC free article] [PubMed]
- Lai Z., Tsugawa H., Wohlgemuth G., Mehta S., Mueller M., Zheng Y.…Fiehn O. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nature Methods. 2018;15(1):53–56. doi: 10.1038/nmeth.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolotti L., Mall V., Schieberle P. Characterization of key aroma compounds in a commercial rum and an australian red wine by means of a new sensomics-based expert system (SEBES)-An approach to use artificial intelligence in determining food odor codes. Journal of Agricultural and Food Chemistry. 2019;67(14):4011–4022. doi: 10.1021/acs.jafc.9b00708. [DOI] [PubMed] [Google Scholar]
- Oh Y., Barbey C.R., Chandra S., Bai J., Fan Z., Plotto A.…Lee S. Genomic characterization of the fruity aroma gene, FaFAD1, reveals a gene dosage effect on gamma-decalactone production in strawberry (Fragaria x ananassa) Frontiers in Plant Science. 2021;12 doi: 10.3389/fpls.2021.639345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontes M., Marques J.C., Câmara J.S. Headspace solid-phase microextraction-gas chromatography-quadrupole mass spectrometric methodology for the establishment of the volatile composition of Passiflora fruit species. Microchemical Journal. 2009;93(1):1–11. [Google Scholar]
- Porter M., Fan Z., Lee S., Whitaker V.M. Strawberry breeding for improved flavor. Crop Science. 2023;00:1–15. [Google Scholar]
- Pu D., Zhang H., Zhang Y., Sun B., Ren F., Chen H. Characterization of the key aroma compounds in white bread by aroma extract dilution analysis, quantitation, and sensory evaluation experiments. Journal of Food Processing and Preservation. 2019;43(5):e13933. [Google Scholar]
- Qu B., Jiang J., Mao X., Dong G., Liu Y., Li L., Zhao H. Simultaneous determination of vanillin, ethyl vanillin and methyl vanillin in Chinese infant food and other dairy products by LC-MS/MS. Food Additives and Contaminants Part A-Chemistry Analysis Control Exposure & Risk Assessment. 2021;38(7):1096–1104. doi: 10.1080/19440049.2021.1902573. [DOI] [PubMed] [Google Scholar]
- Sinha A.K., Sharma U.K., Sharma N. A comprehensive review on vanilla flavor: Extraction, isolation and quantification of vanillin and others constituents. International Journal of Food Sciences and Nutrition. 2008;59(4):299–326. doi: 10.1080/09687630701539350. [DOI] [PubMed] [Google Scholar]
- Song X., Jing S., Zhu L., Ma C., Song T., Wu J.…Chen F. Untargeted and targeted metabolomics strategy for the classification of strong aroma-type baijiu (liquor) according to geographical origin using comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. Food Chemistry. 2020;314 doi: 10.1016/j.foodchem.2019.126098. [DOI] [PubMed] [Google Scholar]
- Song X., Wang G., Zhu L., Zheng F., Ji J., Sun J.…Sun B. Comparison of two cooked vegetable aroma compounds, dimethyl disulfide and methional, in Chinese Baijiu by a sensory-guided approach and chemometrics. LWT-Food Science and Technology. 2021;146 [Google Scholar]
- Song X., Zhu L., Wang X., Zheng F., Zhao M., Liu Y.…Chen F. Characterization of key aroma-active sulfur-containing compounds in Chinese Laobaigan Baijiu by gas chromatography-olfactometry and comprehensive two-dimensional gas chromatography coupled with sulfur chemiluminescence detection. Food Chemistry. 2019;297 doi: 10.1016/j.foodchem.2019.124959. [DOI] [PubMed] [Google Scholar]
- Tieman D., Zhu G., Resende M.F.R., Lin T., Nguyen C., Bies D.…Klee H. A chemical genetic roadmap to improved tomato flavor. Science. 2017;355(6323):391–394. doi: 10.1126/science.aal1556. [DOI] [PubMed] [Google Scholar]
- Wang Z., Gmitter F.G., Jr., Grosser J.W., Wang Y. Natural sweeteners and sweetness-enhancing compounds identified in citrus using an efficient metabolomics-based screening strategy. Journal of Agricultural and Food Chemistry. 2022;70(34):10593–10603. doi: 10.1021/acs.jafc.2c03515. [DOI] [PubMed] [Google Scholar]
- Xu L., Wang S., Tian A., Liu T., Benjakul S., Xiao G.…Ma L. Characteristic volatile compounds, fatty acids and minor bioactive components in oils from green plum seed by HS-GC-IMS, GC–MS and HPLC. Food Chemistry: X. 2023;17 doi: 10.1016/j.fochx.2022.100530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L., Xu J., Feng C., Lu D., Zhou Z. Optimal normalization method for GC-MS/MS-based large-scale targeted metabolomics. Journal of Analytical Chemistry. 2022;77(3):361–368. [Google Scholar]
- Yan J.-W., Ban Z.-J., Lu H.-Y., Li D., Poverenov E., Luo Z.-S., Li L. The aroma volatile repertoire in strawberry fruit: A review. Journal of the Science of Food and Agriculture. 2018;98(12):4395–4402. doi: 10.1002/jsfa.9039. [DOI] [PubMed] [Google Scholar]
- Yang Y., Saand M.A., Huang L., Abdelaal W.B., Zhang J., Wu Y.…Wang F. Applications of multi-omics technologies for crop improvement. Frontiers in Plant Science. 2021;12 doi: 10.3389/fpls.2021.563953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H., Cao G., Hou X., Huang M., Du P., Tan T.…Luo J. Development of a widely targeted volatilomics method for profiling volatilomes in plants. Molecular Plant. 2022;15(1):189–202. doi: 10.1016/j.molp.2021.09.003. [DOI] [PubMed] [Google Scholar]
- Zhu L., Song X., Li X., Geng X., Zheng F., Li H.…Sun B. Interactions between kafirin and pickle-like odorants in soy sauce flavor Baijiu: Aroma profile change and binding mechanism. Food Chemistry. 2023;400 doi: 10.1016/j.foodchem.2022.133854. [DOI] [PubMed] [Google Scholar]
- Zhu L., Wang X., Song X., Zheng F., Li H., Chen F.…Zhang F. Evolution of the key odorants and aroma profiles in traditional Laowuzeng baijiu during its one-year ageing. Food Chemistry. 2020;310 doi: 10.1016/j.foodchem.2019.125898. [DOI] [PubMed] [Google Scholar]
- Zorrilla-Fontanesi Y., Rambla J.L., Cabeza A., Medina J.J., Sanchez-Sevilla J.F., Valpuesta V.…Amaya I. Genetic analysis of strawberry fruit aroma and identification of O-methyltransferase FaOMT as the locus controlling natural variation in mesifurane content. Plant Physiology. 2012;159(2):851–870. doi: 10.1104/pp.111.188318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.