Abstract
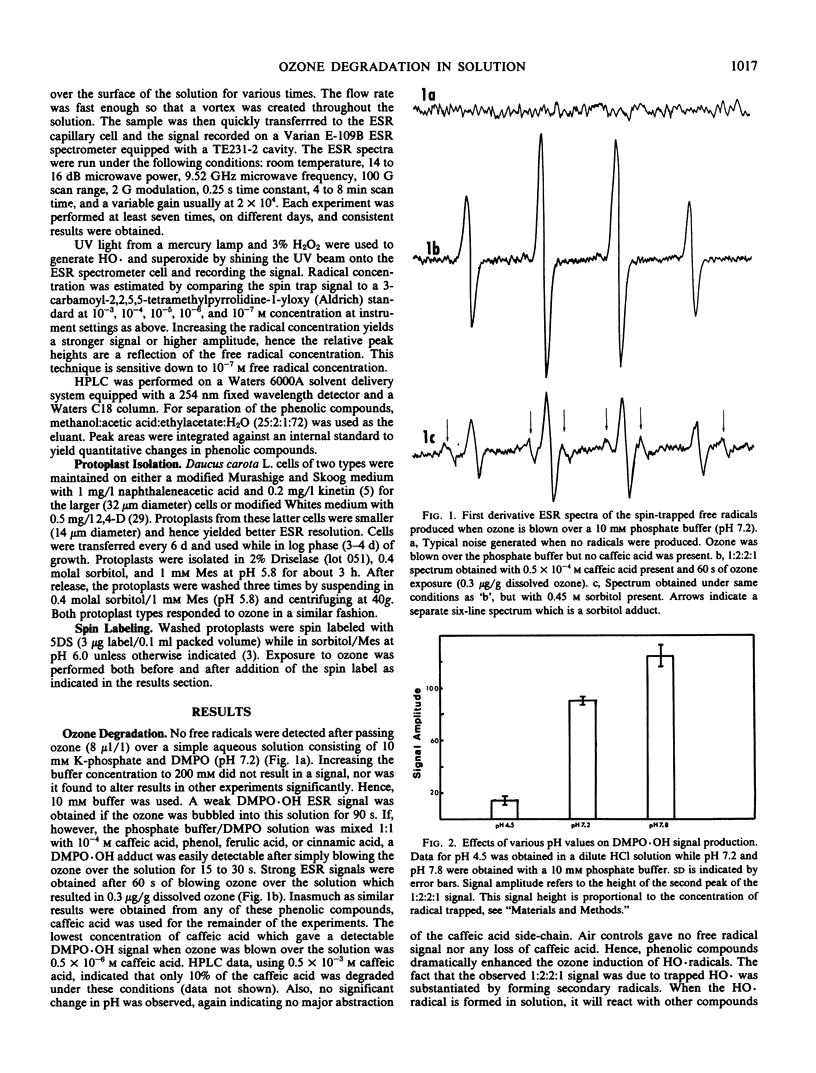
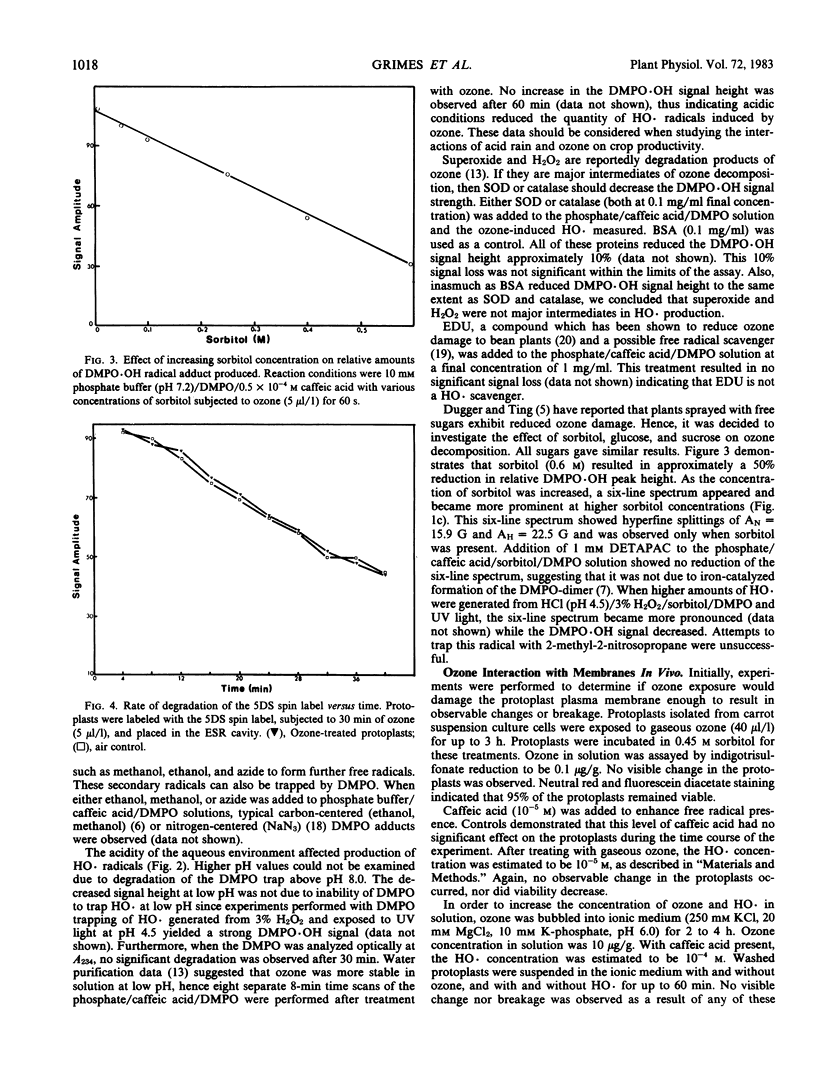
Defining the reactants is a critical step towards elucidating the mechanism of ozone toxicity to biomembranes. To document ozone-induced HO·radicals, the spin trap 5,5-dimethyl-1-pyrroline-N-oxide was used and the resulting spin adduct was monitored with electron spin resonance spectroscopy. Chelexed potassium phosphate buffer (10 millimolar and 0.2 molar) at pH 7.2 and 7.8 was exposed to ozone (1-40 microliters per liter) by directing a stream of ozone over the surface for 60 seconds. Under these conditions, no HO· was detected. Using 0.5 × 10−4 molar caffeic acid in phosphate buffer, strong DMPO·OH electron spin resonance signals were obtained, indicating HO· production. Air controls yielded no signal. High pH (7.8) enhanced signal strength. Furthermore, with sorbitol (0.4 osmolal final concentration), a net HO· signal loss of 28% was observed, while a carbon-centered sorbitol radical adduct appeared. Although HO· radicals were produced, no breakage of Daucus carota protoplast plasma membranes was observed nor were differences in membrane fluidity observed as determined by 5-doxyl stearic acid.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boss W. F., Mott R. L. Effects of divalent cations and polyethylene glycol on the membrane fluidity of protoplast. Plant Physiol. 1980 Nov;66(5):835–837. doi: 10.1104/pp.66.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein E., Rosen G. M., Rauckman E. J. Spin trapping of superoxide and hydroxyl radical: practical aspects. Arch Biochem Biophys. 1980 Mar;200(1):1–16. doi: 10.1016/0003-9861(80)90323-9. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Visualization of catalase on acrylamide gels. Anal Biochem. 1974 Mar;58(1):57–62. doi: 10.1016/0003-2697(74)90440-0. [DOI] [PubMed] [Google Scholar]
- Kong S., Davison A. J. The relative effectiveness of .OH, H2O2, O2-, and reducing free radicals in causing damage to biomembranes. A study of radiation damage to erythrocyte ghosts using selective free radical scavengers. Biochim Biophys Acta. 1981 Jan 8;640(1):313–325. doi: 10.1016/0005-2736(81)90555-1. [DOI] [PubMed] [Google Scholar]
- Lee E. H., Bennett J. H., Heggestad H. E. Retardation of Senescence in Red Clover Leaf Discs by a New Antiozonant, N-[2-(2-Oxo-1-imidazolidinyl)ethyl]-N'-phenylurea. Plant Physiol. 1981 Feb;67(2):347–350. doi: 10.1104/pp.67.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. H., Bennett J. H. Superoxide Dismutase: A POSSIBLE PROTECTIVE ENZYME AGAINST OZONE INJURY IN SNAP BEANS (PHASEOLUS VULGARIS L.). Plant Physiol. 1982 Jun;69(6):1444–1449. doi: 10.1104/pp.69.6.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R. E., Mudd J. B. Inhibition by ozone of the acylation of glycerol 3-phosphate in mitochondria and microsomes from rat lung. Arch Biochem Biophys. 1982 Jun;216(1):34–41. doi: 10.1016/0003-9861(82)90185-0. [DOI] [PubMed] [Google Scholar]
- Saprin A. N., Piette L. H. Spin trapping and its application in the study of lipid peroxidation and free radical production with liver microsomes. Arch Biochem Biophys. 1977 Apr 30;180(2):480–492. doi: 10.1016/0003-9861(77)90063-7. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., Hawkins R. E., Brian M., Carrell R. W. The estimation of red cell superoxide dismutase activity. J Lab Clin Med. 1975 Feb;85(2):337–341. [PubMed] [Google Scholar]


