Abstract
Introduction
Asthma is one of the common chronic polygenic inflammatory diseases. Genome wide association studies have identified ADAM33 as an asthma candidate gene. The present study investigated possible association of rs2280090 (T1), rs2280091 (T2) and rs3918396 (S1) single nucleotide polymorphisms (SNPs) of ADAM33 with aeroallergen induced asthma in West Bengal population, India. In addition, in-silico analysis was performed to find out changes in protein function.
Methods
Forced expiratory volume in 1 second (FEV1)/Forced vital capacity (FVC), peak expiratory flow rate (PEFR) were assessed using spirometry in 1039 participants. Allergic sensitivity of 619 spirometry positive asthma patients was assessed by skin prick test (SPT) against 22 aeroallergens. For genotyping of T1, T2, and S1 SNPs in 540 allergic asthma patient and 420 control subjects, polymerase chain reaction-based restriction fragment length polymorphism was performed. Total Immunoglobulin-E (IgE) level was measured in both patients and controls. ADAM333 haplotype blocks were constructed using Haploview software v.4.2. Structural model of transmembrane and cytoplasmic domains of ADAM33 was generated using RaptorX. Protein-protein interaction was analysed using the STRING server.
Results
Highest number of patient sensitivity was observed towards Cocos nusifera (n = 215) and Dermatophagoides farinae (n = 229). Significant difference in sensitivity was observed between child and late adult (P = 0.03), child and early adult (P = 0.02), adolescent and late adult (P = 0.02) and adolescent and early adult (P = 0.01). Genotypic frequencies differed significantly between patients and controls (P < 0.05). rs2280090 GG, rs2280091GG and AG genotype, and rs3918396 AA carried significant risk for asthma (P = 0.02, P = 0.008, P = 0.04, P = 0.01 respectively). ADAM33 T1, T2, and S1 polymorphisms were in high Linkage Disequilibrium (D = 0.98). Haplotype consisting of rs2280090G, rs2280091G and rs3918396A alleles were found significantly higher in patient population in comparison with controls (OR = 2.03). IgE level differed significantly among different genotypes for T1, T2, and S1 SNPs analysed in pair (P < 0.0001). FEV1/FVC ratio differed significantly among different genotypes for T1, T2 and S1 SNPs analysed in pair (P < 0.0001). Significant difference of FEV1/FVC was also found between GGA and AAG haplotype (P < 0.0001). In-silico analysis revealed T1 and T2 polymorphisms are located in cytoplasmic domain of ADAM33 may cause bronchial smooth muscle cell mobility and cellular hyperplasia as well as cytoskeletal remodelling by altered interaction with different cytoplasmic proteins found by string analysis.
Conclusion
Present study showed significant association of T1, T2, and S1 polymorphisms of ADAM33 with aeroallergen-induced asthma in West Bengal, India. These polymorphisms may be used as prognostic markers and possible targets for therapeutics in future.
Keywords: Asthma, Airway remodelling, SNPs, Pulmonary function test, In-silico modelling
Introduction
Atopic asthma is a very complicated disease, pigeonholed by the chronic tenderness and indisposition of airways. Multiple genetic and environmental factors are intricated in its etiology.1 It is characterized by variable and persistent symptoms which include chronic inflammation, reversible obstruction of the airways, and increased bronchial hyper-responsiveness. Extensive epidemiological studies from different parts of the world have shown that approximately around 300 million people suffer from asthma and with its rising trends it is expected to reach 400 million by 2025 worldwide.2 In India around 34.3 million people are the sufferers of asthma which accounts for 13.09% of the global burden according to Global burden of disease (GBD 1990–2019). In allergic asthma, aeroallergens are the major causative agent; hence identification of those offending aeroallergens becomes crucial so that avoidance may help to reduce the symptoms of asthma to some extent.3 Several case-control, family and twin studies had identified more than 100 genomic loci linked with asthma.4 Hence, to deliver with the preeminent probable diagnosis and management, early detection of genetically predisposed individuals to asthma becomes essential for better management of the disease. A disintegrin and metalloproteinase domain 33 (ADAM 33) is regarded as one of the asthma candidate genes because of its likely role in airway remodelling in asthma and higher expression in bronchial smooth muscle cells and fibroblasts.5,6 The airway remodelling process has a link to aberrant epithelial-mesenchymal signalling and proliferation of fibroblast and smooth muscle cells, smooth muscle cell contraction which ultimately leads to bronchial hyper-responsiveness that characterizes asthma pathophysiology.7 However, the role of ADAM33 polymorphisms in airway remodelling is yet to be discovered. ADAM33 is located on chromosome 20p13 and spreads over 14 kb region having 22 exons and 21 introns that encodes a zinc transmembrane metalloproteinase involved in diversified functions like cell-cell adhesion, cell fusion, signal transduction, cell-cell interaction, cell proliferation, airway remodelling, apoptosis, growth factor shedding, proteolysis and inflammatory responses.8,9 This gene is highly polymorphic and around 100 single nucleotide polymorphisms (SNP) have been identified so far.9 The present study is the first study investigating possible association of the ADAM33 SNPs rs2280090(T1), rs2280091(T2), and rs3918396 (S1) with aeroallergens (pollen, dust and dust mites) induced asthma among West Bengal population, India. In addition, in-silico analysis was performed on ADAM33 T1 (Met764Thr), T2 (Pro774Ser), and S1 (Val710Ile) missense polymorphisms to examine the changes in protein function. Our study also attempted to determine the signalling molecules of downstream pathway which are responsible for elevated asthmatic response in individuals carrying the risk allele.
Methods
Ethics approval and consent to participate
This study has been conducted with the approval of Institutional review board (CREC-AARC Ref:62/21). All the participants for this study have provided their written consent of participation.
Inclusion and exclusion criteria
One thousand and seventy participants were preliminarily selected for this case control study. Participants who were unwilling to participate (n = 21), pregnant and lactating women (n = 3), and having co-morbidities and another atopy diseases (n = 7) were excluded. Finally, 1039 participants underwent through spirometry test for the confirmation of asthma. The following parameters were measured viz. Forced Expiratory Volume in 1 second (FEV1), Forced Vital Capacity (FVC), FEV1/FVC, and Peak Expiratory Flow Rate (PEFR). Following the guideline of Global Initiative for Asthma (GINA) 2017,10 FEV1/FVC ratio less than 0.75–0.80 and reduction of PEFR and FEV1 value by 20% or more is considered as asthmatic. 619 participants were positive and 420 were negative in spirometry test. For the confirmation of atopic status of these 619 asthma patients, skin prick test (SPT) has been performed against a panel of 22 aeroallergen extracts which included 17 types of pollens (Cocos nusifera, Cynodon dactylon, Azadirachta indica, Caesalpinia pulcherima, Brassica nigra, Pinus sylvestris, Areca catechu, Carica papaya, Parthenium histerophorous, Peltophorum pterocarpum, Zea mays, Plantago lanceolata, Eucalyptus spp, Triticum aestivum, Chenopodium album, Lolium perenne, and Poa pratensis) and 5 types of dust and dust mite extracts (house dust, hay dust, paper dust, Dermatophagoides pteronyssinus, and D. farinae) supplied by Credisol, Mumbai, India. Histamine (10 mg/ml) acid phosphate and 0.9% normal saline were used as positive and negative controls respectively. Skin prick test was performed on the flexor side of the forearm of all the participants. After about 15 min wheal diameter on the skin as a consequence of allergic reaction were recorded. Wheal diameter of ≥3 mm was considered as positive.11 Five hundred forty out of 619 asthmatic patients were SPT positive. Finally, 540 spirometry and SPT positive individuals were considered as patients and 420 spirometry negative individuals were considered as control in our study. Participants were provided with a self-made questionnaire for obtaining important information such as age, sex, family history, residence, house condition, specific time or season when asthma symptoms were prominent, onset of disease, and clinical features (wheezing, shortness of breath, chest tightness etc.).
Study population
This case-control based study incorporated 540 clinically diagnosed allergic asthma patients, seeking treatment from Allergy and Asthma Research Center, West Bengal, India from the month of November 2021 to December 2022 and 420 control subjects from the same geographic area as that of the patients with no past or present history of allergy. Patient and control population were categorized in 4 groups viz child (5–12 years), adolescent (13–17 years), early adult (18–45 years), and late adult (46–67 years) according to age, 2 groups (male and female) according to sex, and 3 groups (urban, semi-urban and rural) as per the type of residence following the existing reports (Bureau of Applied Economics and Statistics, Department of Statistic and Programmed Implementation 2015)12 regarding the density of population, development, amenities, employment opportunities, education level, nature of vegetation, and human settlement.
Blood collection
1 ml of venous blood was collected in an EDTA vial from each participant. After centrifugation for about 10 min at 13 000 rpm, serum has been separated and kept in separate vial. Both the blood and serum samples were stored in −20 °C for further analysis.
DNA isolation and genotyping
From the peripheral blood samples of both patients and controls, genomic DNA has been isolated by using QIAmp DNA blood mini kit (Qiagen, Hilden, Germany). For the determination of genotype, polymerase chain reaction based restriction fragment length polymorphism (PCR-RFLP) has been performed. Each PCR reaction mixture of 20 μl volume comprised of 50 ng of genomic DNA, 200 nM of forward and reverse primer (Sigma), 200 mM of each deoxyribonucleotide triphosphate (Thermo Fisher Scientific), and 2.5 units of Taq polymerase. Polymerase. For the amplification of the rs2280090, rs2280091and rs3918396 snp site of ADAM33 gene TTCTCAGGGTCTGGGAGAAA, GAGGGCATGAGGCTCACTTG, TGTGCAGGCTGAAAGTATGC forward primers and GCCAACCTCCTGGACTCTTA, ACTCAAGGTGACTGGGTGCT, AGAGCTCTGAGGAGGGGAAC reverse primers were used consecutively. Cycling conditions were as follows: 95 °C for 5 min, followed by 30 cycles at 95 °C for 30s, annealing temperature for 30 s, 72 °C for 7 min. PCR products for rs2280090, rs2280091, and rs3918396 were of length 312 bp, 401 bp and 306 bp. PCR products were digested overnight at 37 °C using hinfI restriction enzyme for the identification of rs3918396 polymorphism, NcoI for rs2280091 polymorphism and HpyCH4III restriction enzyme for RS2280090 polymorphism of ADAM33. Pcr products were digested overnight at 37 °C using hinfI restriction enzyme for the identification ofrs3918396 polymorphism, NcoI for rs2280091 polymorphism and HpyCH4III restriction enzyme for RS2280090 polymorphism of ADAM33. After the completion of digestion process, digested products were separated through 2.5% agarose gel electrophoresis and visualized through gel documentation system (Bio-Rad GS800). Length digestion of 312 bp for rs2280090 yielded band 212 bp, 100 bp in common homozygotes (AA), 312 bp in rare homozygotes (GG), 312 bp, 212 bp, 100 bp for heterozygotes (AG). Length digestion of 401 bp for rs2280091 yielded band 257 bp, 143 bp in common homozygotes (AA), 401 bp in rare homozygotes (GG), 401 bp, 257 bp, 143 bp for heterozygotes (AG). Length digestion of 306 bp for rs3918396 yielded band 67 bp, 115 bp and 122 bp in common homozygotes (GG), 183, 122 bp in rare homozygotes (AA), 183 bp, 122 bp, 115 bp, 67 bp for heterozygotes (GA).
Total Immunoglobulin E estimation
Total Immunoglobulin (IgE) of both the patient and control population was estimated by Enzyme Immuno Assay (EIA) using pathozyme immunoglobulin (Glaxo Smithkline Pharmaceuticals Ltd., Mumbai, India). Calibration has been performed as per World Health Organization (WHO) standard for table 5IgE in different ages, i.e., 1–5 years<60 IU/ml, 6–9 years<90 IU/ml, 10–16 years < 200 IU/ml and 16+ years<100 IU/ml.
SNP selection and haplotype formation
SNP selection was based on information gathered from SNPpedia database regarding SNP validation, putative function, known association with allergy, asthma and related phenotypes. Furthermore, selected SNPs should not significantly deviate from Hardy Weinberg equilibrium (HWE) in control groups. As per the above criteria, three SNPs were selected for the present study: rs2280090 (T1), rs2280091 (T2) and rs3918396 (S1) of ADAM33 gene. ADAM333 haplotype blocks were constructed according to Gabriel et al. (2002)13 using Haploview software v.4.2.
In-silico analysis
Motif scan analysis was performed using Expasy Prosite server. Structural model of transmembrane and cytoplasmic domains of ADAM33 was generated using RaptorX.14 Figures were prepared using PyMol.15 Protein-protein interaction was analysed using the STRING server.
Statistical analysis
Continuous data was expressed by mean ± standard deviation and categorical data by number with percentage. For the comparison of continuous variables between patient and control population, independent t-test has been performed and for categorical variables Pearson chi-square test has been done. Contingency chi square or Fisher's exact test has been used for analysing allele and genotype distribution in patient and control population. Association between polymorphisms and allergic asthma risk have been estimated by odds ratio (OR) and 95% confidence interval (CI) under additive, recessive and dominant model. HWE has been checked for the genotypes by goodness-of-fit chi-square test with one degree of freedom (df). Benjamini-Hochberg (BH) corrections (considering a 5% false discovery rate) were performed to find the adjusted P values. One-way-ANOVA test followed by Tukey's Multiple Comparison test was used to analyse SPT sensitivity for pollen and dust among different age groups. One Way ANOVA test has been performed to estimate any significant difference of total IgE among polymorphic genotypes for each of the 3 studied SNPs in patient population. Besides, One Way ANOVA test has also been performed to find out any significant difference of FEV1/FVC among different inhabitants in both the patient and control population. One-way-ANOVA test followed by Tukey's Multiple Comparison test had been performed to find out if there is any significant difference of FEV1/FVC among different polymorphic genotypes for each of the 3 SNPs within the patient population. Independent t-test has been performed to find out any significant difference of FEV1/FVC between different haplotypes in patient population of our study. All the statistical analysis were performed using GraphPad Prism ver. 7 (San Diego, CA). Significance level was taken as p < 0.05.
Results
Characteristics of the patients and controls
The demographic details such as age, sex, residence, family history of the disease, and the values of all the clinical parameters such as total IgE, FEV1/FVC ratio, and PEFR are depicted for both the patient and control population in Table 1. Majority of the studied population were from urban area (55% for patient and 60% for control population). Significant difference of total IgE level (P < 0.0001), FEV1/FVC ratio (P < 0.0001) and PEFR (P < 0.0001) were observed between patient and control population (Table 1). One Way ANOVA test confirmed that there was a significant difference of FEV1/FVC ratio among different inhabitants (urban, semi urban, and rural) of the patient population (F = 196.7, p < 0.0001), although the difference was insignificant in the case of control population (F = 1.07, p = 0.3) (Supplementary Figure 1).
Table 1.
Demographic and clinical characteristics of patient and control subjects
| FEATURES | PATIENTS | CONTROL | P value |
|---|---|---|---|
| AGE | 0.06 | ||
| Children (5–12) | 95 (17.5%) | 48(11.36%) | |
| Adolescents (13–17) | 156(28.8%) | 102(24.24%) | |
| Early Adult (18–45) | 148(27.40%) | 143(34%) | |
| Late adult (46–67) | 141(26.11%) | 127(30.3%) | |
| GENDER | |||
| Male | (58.14%) | (57.5%) | |
| Children | 55 | 26 | |
| Adolescents | 92 | 57 | 0.90 |
| Early Adult | 72 | 79 | |
| Late adult | 95 | 80 | |
| Female | (41.85%) | (42.42%) | |
| Children | 40 | 22 | |
| Adolescents | 64 | 45 | |
| Early Adult | 76 | 64 | |
| Late Adult | 46 | 47 | |
| FAMILY HISTORY | |||
| Paternal | (15%) | ||
| Children | 12 | Nil | |
| Adolescents | 18 | ||
| Early Adult | 28 | – | |
| Late Adult | 23 | ||
| Maternal | (26%) | ||
| Children | 31 | ||
| Adolescents | 35 | ||
| Early Adult | 42 | ||
| Late Adult | 32 | ||
| Paternal + Maternal | (11%) | ||
| Children | 20 | ||
| Adolescents | 12 | ||
| Early Adult | 15 | ||
| Late Adult | 12 | ||
| Absent | (48%) | ||
| Children | 32 | ||
| Adolescents | 91 | ||
| Early Adult | 63 | ||
| Late Adult | 74 | ||
| Height(cm) | 149.89 ± 0.82 (148.21–150.52) | 145.55 ± 0.83(144.12–146.21) | 0.3 |
| Weight(kg) | 45.58 ± 1.12(43.3–47.5) | 44.65 ± 1.65(43.10–45.21) | 0.6 |
| Residence | 0.4 | ||
| URBAN | (55%) | (60%) | |
| Children | 54 | 28 | |
| Adolescent | 90 | 74 | |
| Early Adult | 80 | 83 | |
| Late adult | 73 | 67 | |
| Semi urban | (28%) | (29%) | |
| Children | 28 | 13 | |
| Adolescent | 43 | 22 | |
| Early Adult | 35 | 42 | |
| Late Adult | 45 | 45 | |
| Rural | (17%) | (11%) | |
| Children | 13 | 7 | |
| Adolescent | 23 | 6 | |
| Early Adult | 33 | 18 | |
| Late Adult | 23 | 15 | |
| Total IgE(IU/mL) | 1056.92 ± 23.94 (1009.88–1103.96) | 59.9 ± 1.8(56.2–63.6) | <0.0001∗ |
| FEV1/FVC | 0.60 ± 0.002 (0.59–0.61) | 0.85 ± 0.002(0.84–0.86) | <0.0001∗ |
| PredictedPEFRmalea | 462.36 ± 2.37 (458.09–467.43) | 530.021 ± 6.79(516.48–543.56) | <0.0001∗ |
| ActualPEFRmale | 344.92 ± 1.87 (341.23–348.61) | 456.21 ± 6.92(442.38–470.04) | <0.0001∗ |
| PredictedPEFRfemaleb | 309.97 ± 1.87 (306.28–313.66) | 310.78 ± 3.96(302.84–318.72) | <0.0001∗ |
| ActualPEFRfemale | 230.75 ± 1.48 (227.83–233.68) | 272.23 ± 3.55(265.11–179.35) | <0.0001∗ |
| Positive SPT | |||
| Monosensitization | 351(65%) | Nil | – |
| Disensitization | 112(21%) | ||
| Polysensitization | 77(14%) |
∗P value significant.
(FEV1/FVC- Forced Expiratory Volume in 1 second/Forced Vital Capacity (FVC); IgE- Immunoglobulin E; PEFR- Peak Expiratory Flow Rate (PEFR); SPT- Skin Prick Test).
Predicted PEFR for male (Litre/min) = −1.807∗age + 3.206∗height in cm.16
Predicted PEFR for female (Litre/min) = −1.454∗age + 2.368∗height in cm.16
Skin reactions of patients against different aero-allergens (pollens, dust and dust mites)
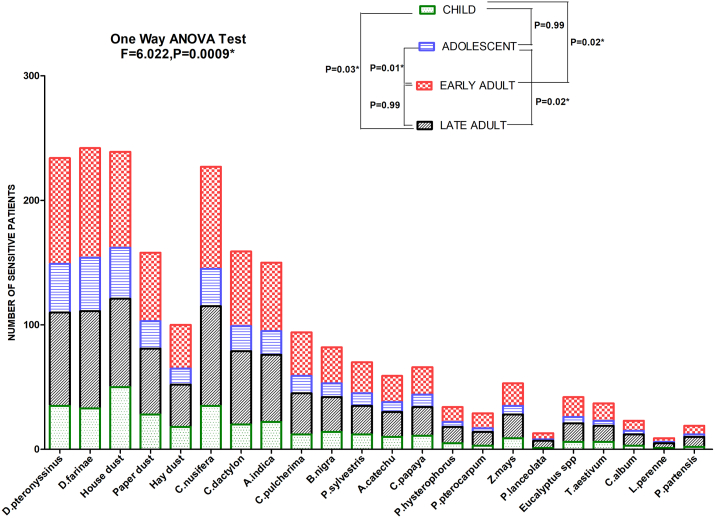
Maximum number of patients were found to be sensitized to C. nusifera (215 no. of patients) followed by C. dactylon (155 no. of patients), A. indica (150 no. of patients) etc, among pollens and D. farinae (229 no. of patients) followed by D. pteronyssyinus (220 no. of patients), house dust (225 no. of patients), paper dust (152 no. of patients) and hay dust (92 no. of patients) among dust and dust mites (Fig. 1). Significant difference of sensitivity against aeroallergens among different age groups was observed by One-way ANOVA test (F = 6.002, P = 0.0009). Pairwise post-hoc Tukey's Multiple Comparison Test showed significant difference in sensitivity between child and late adult (P = 0.03), child and early adult (P = 0.02), adolescent and late adult (P = 0.02) and adolescent and early adult (P = 0.01).
Fig. 1.
SPT sensitization profile of asthma patients against different aeroallergen sources. Each bar represents number of sensitive patients stratified according to age. One Way ANOVA revealed a significant difference of sensitivity among different ages. Tukey's Multiple Comparison test was applied for pairwise comparison between different ages (∗P value significant)
Genotypic distribution analysis of selected SNPs in patient and control population
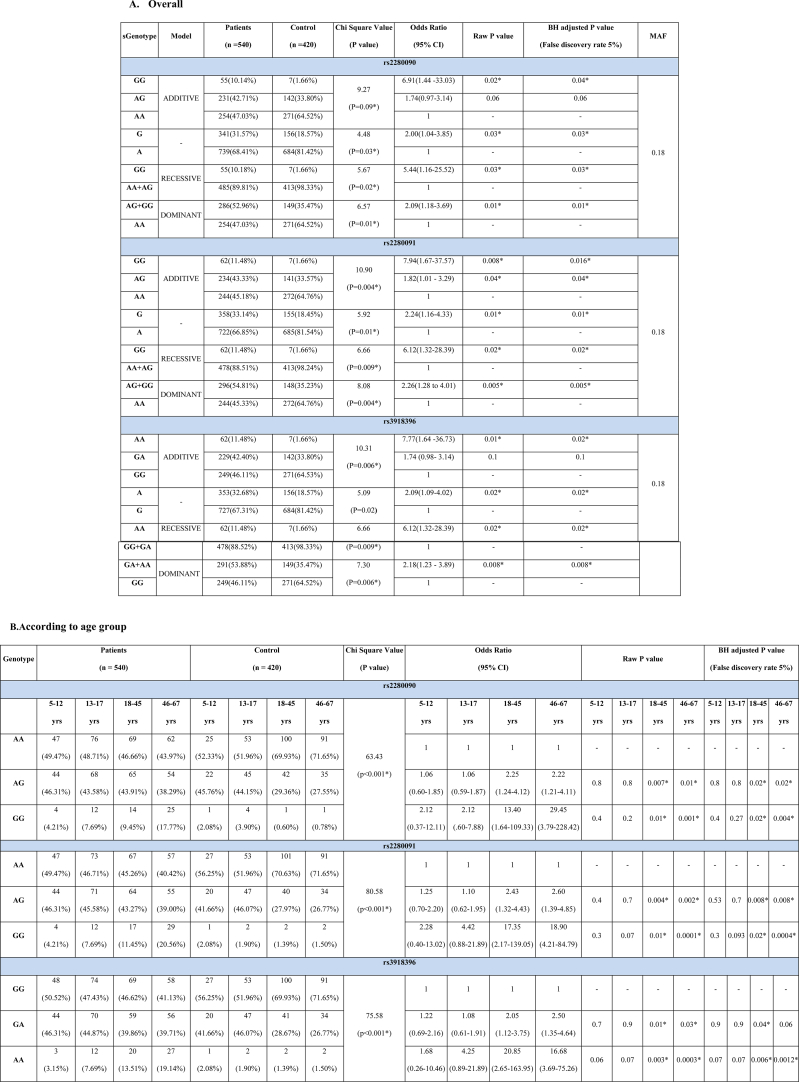
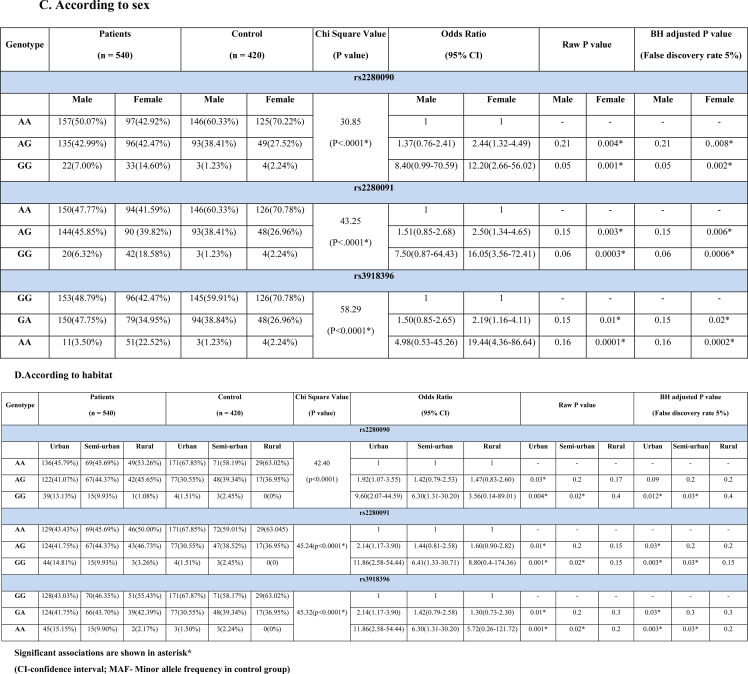
Genotype frequencies differed significantly between patient and control groups in rs2280090, rs2280091 and rs3918396 SNPs under additive, recessive and dominant models (Table 2A). Selected SNPs did not significantly deviate from HWE in control groups (χ2 = 5.83, 5.60, 5.83 and P = 0.06, 0.07, 0.06 respectively). rs2280090 GG genotype (OR = 6.91, BH adjusted P = 0.04), rs2280091GG (OR = 7.94, BH adjusted P = 0.016) and AG genotype (OR = 1.82, BH adjusted P = 0.04) and rs3918396 AA (OR = 7.77, BH adjusted P = 0.02) genotype carried significant risk for asthma. We found significant difference of genotype frequencies among all the age groups between patient and control population (χ2 = 63.43, 80.58, 75.58 and P < 0.0001 for rs2280090, rs2280091 and rs3918396 respectively). In case of rs2280090 and rs2280091, the GG and AG genotype bear significant risk for allergic asthma in early and late adult group. For rs3918396, AA and GA genotypes showed significantly higher risk in early (BH adjusted P = 0.006 and 0.04 respectively) and only AA genotype showed risk in late adult patients (BH adjusted P = 0.0012) (Table 2B). Significant difference of genotypic frequencies was observed in both the sexes between patient and control population (χ2 = 30.85, 43.25, 58.29; P < 0.0001, 0.0001, 0.0001 for rs2280090, rs2280091 and rs3918396 respectively). In rs22800900 and rs2280091, AG and GG genotype showed significant risk of allergic asthma in female patients. Also, in rs3918396, AA and GA genotypic frequencies are significantly higher in female patients (BH adjusted P = 0.0002 and 0.02 respectively) (Table 2C). Genotypic frequencies differ significantly among all the inhabitants between patient and control group (Table 2D). In rs2280090 GG, rs2280091GG and rs2280091AG genotypes showed significant odds ratio in urban population but in semi-urban group, only GG genotype of both the SNPs rs2280090 and rs2280091 showed significant risk in allergic asthma patients. In case of rs3918396, AA and GA genotypes carried significant risk of allergic asthma in urban population (OR = 11.86 and 2.14 and BH adjusted P = 0.003 and 0.03 respectively) while AA genotype (OR = 6.30, P = 0.02) carried risk for allergic asthma in semi urban population (Table 2D). P values against each of the odds ratio could not guarantee a prudent result by predicting the false discovery rate. Therefore, in addition, the BH approach for adjusting P values has been taken here. Assuming a false discovery rate of 5 %, most of the adjusted P values were found to be significant similar to raw P values (Table 2A–D).
Table 2.
Genotypic frequency distribution of ADAM33 rs2280090, rs2280091, rs3918396 polymorphisms in cases and control population.
Linkage disequilibrium (LD) and haplotype analysis of ADAM33 polymorphisms
ADAM33 rs2280090, rs2280091, and rs3918396 polymorphisms were in high LD (D = 0.98) forming a well-defined haplotype block which spanned 1 kb region (Fig. 2). Haplotype frequencies (Table 3) differed significantly between allergic asthma and control groups (χ2 = 4.63, P = 0.03). Haplotype consisting of rs2280090G, rs2280091G and rs3918396A alleles were found significantly higher in patient population in comparison with controls (OR = 2.03). All the other haplotypes were not shown here as their frequencies were below 0.01.
Fig. 2.
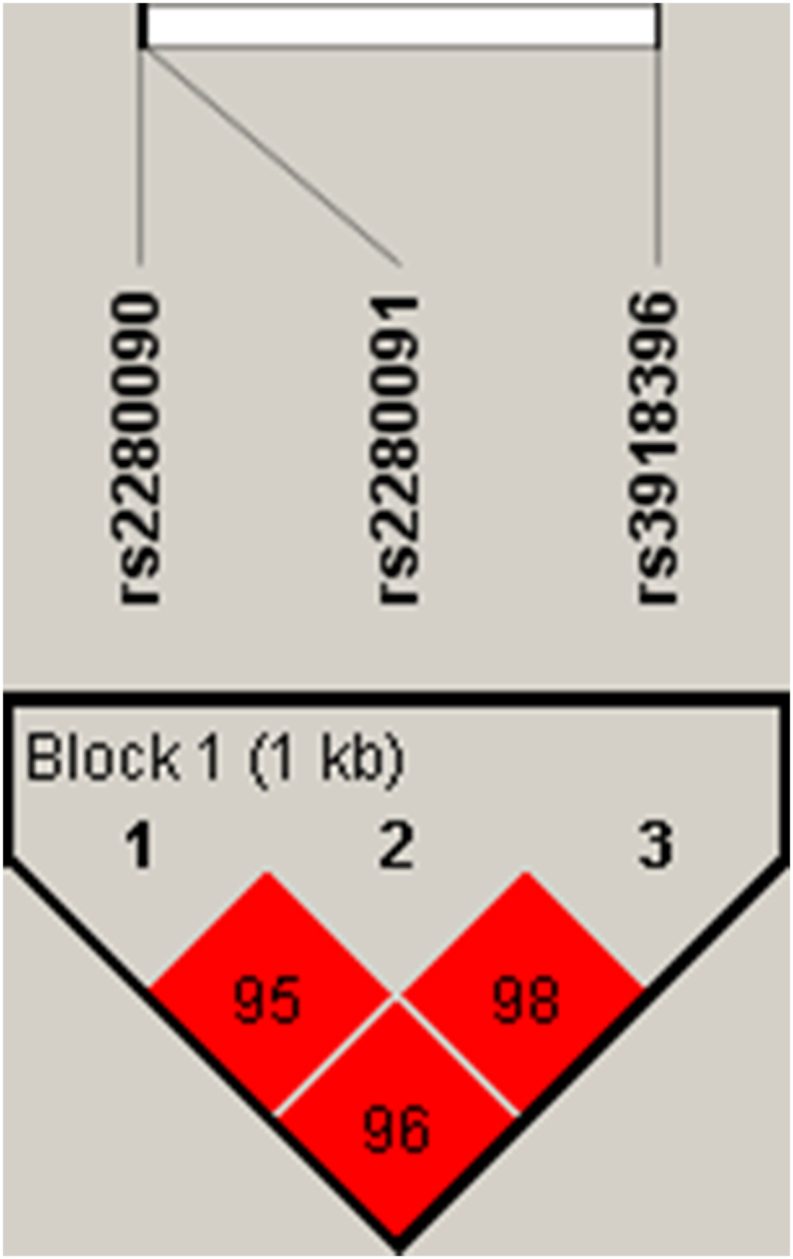
Haplotype block structure spanning 1 kb region consisting of rs2280090, rs2280091 and rs3918396 polymorphisms. The number within the block represents the D′ value
Table 3.
ADAM33 haplotype frequencies in patient and control subjects.
| ADAM33 Haplotypea | Frequency | Chi square (P value) | Adjusted ORb (95% CI) | P value | |
|---|---|---|---|---|---|
| PATIENT | CONTROL | 4.63 (P = 0.03∗) | |||
| AAG | 0.669 | 0.814 | 1 | – | |
| GGA | 0.316 | 0.185 | 2.03 (1.05–3.91) | 0.03∗ | |
(∗P value significant).
(CI- confidence interval, OR- Odds ratio).
The haplotype consists of rs2280090, rs2280091 and rs3918396 polymorphisms respectively.
Adjusted for age and sex.
Association of total IgE level with ADAM33 polymorphisms
In this study the total IgE level of different polymorphic genotypes (rs2280090, rs2280091, and rs3918396) were analysed. Risk genotypes showed significant higher IgE level than both heterozygous and homozygous genotypes for all studied SNPs (Supplementary Figure 2).
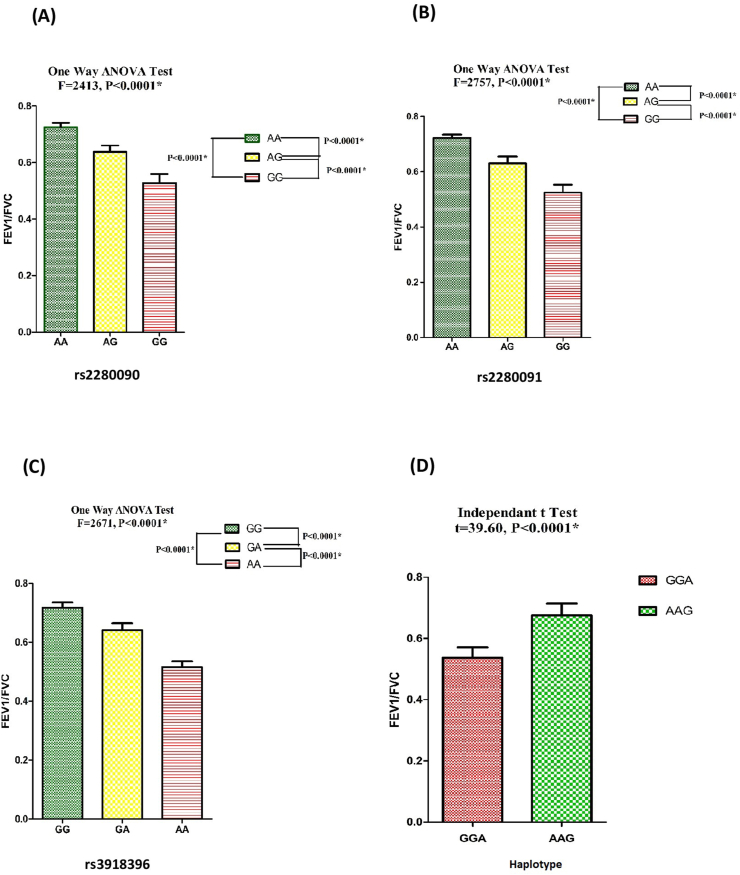
Comparison of FEV1/FVC ratio in different polymorphic genotypes/haplotype in patient population
FEV1/FVC ratio in polymorphic genotypes for each SNPs (rs2280090, rs2280091, and rs3918396) was analysed in patient population using One Way ANOVA test followed by post hock Tukey test. FEV1/FVC ratio differed significantly among different genotypes analysed in pair (P < 0.0001) (Fig. 3A–C). Significant difference of FEV1/FVC was also found between GGA and AAG haplotype (t = 39.60, P < 0.0001) (Fig. 3D).
Fig. 3.
Level of FEV1/FVC ratio in patients bearing genotypes for ADAM33 (A) rs2280090 (B) rs2280091 (C) rs3918396 polymorphisms and (D) their haplotypes. One Way ANOVA test was performed for genotypes and independent t-test for haplotypes (∗P value significant)
Structural and functional characterization of the SNPs
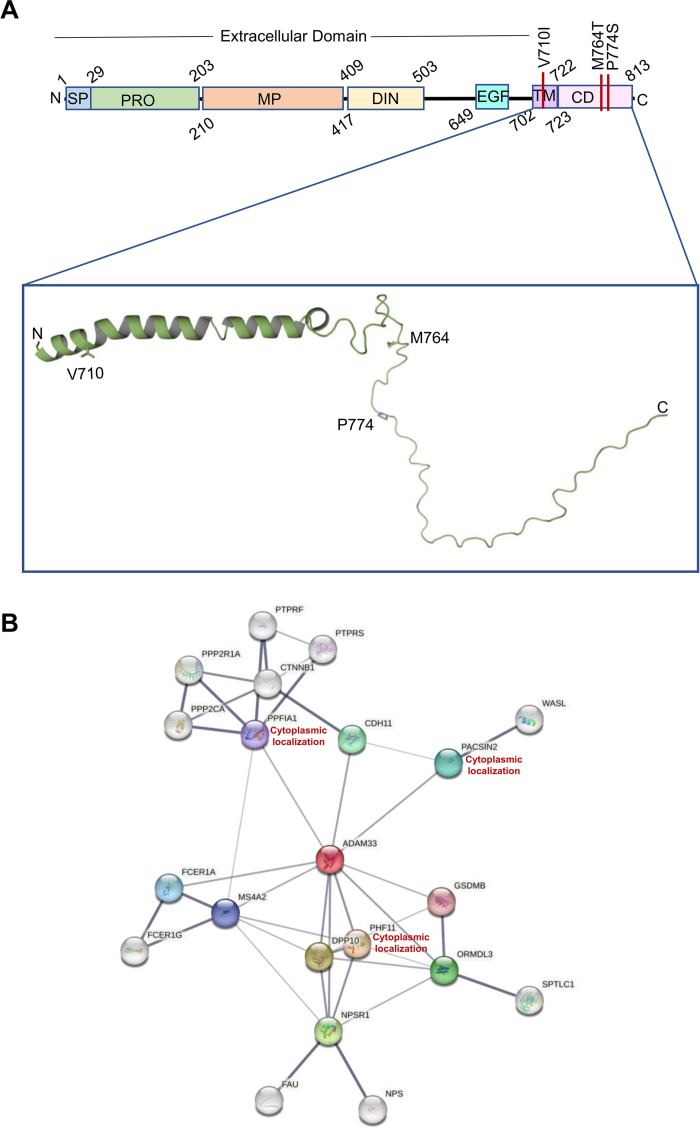
Expasy Prosite analysis demonstrated that similar to other ADAM family of proteins ADAM33 is comprised of several domains including a signal peptide (SP), a pro-domain (PRO), a disintegrin domain (DIN), an EGF domain (EGF), a transmembrane domain (TM), and a cytoplasmic domain (CD). Lack of structural information about the transmembrane and cytoplasmic domains encouraged us for structural analysis. Secondary structural analysis using Predict Protein server denoted there is a transmembrane helix followed by loop. We have prepared a three-dimensional structural model of ADAM33 using the artificial intelligence (AI) based tool AlphaFold217 and deep learning-based protein folding framework RaptorX.14 Both the analysis provided similar result. The structure of ADAM33 transmembrane and cytoplasmic domains is shown in the inset view (Fig. 4). According to the structural analysis, S1 (Val710Ile) is a transmembrane helix SNP. Both Val and Ile amino acids are hydrophobic in nature, hence drastic changes in the protein stability cannot be expected. Nevertheless, Val has a smaller, more compact side chain than Ile, which may affect the ADAM33 interaction with membranes. The other two SNPs T1 (Met764Thr) and T2 (Pro774Ser) are located in the cytoplasmic domain (Fig. 4A). Met is a sulfur-containing non-polar amino acid whereas Thr is a polar amino acid. On the other hand, Pro is a non-polar hydrophobic amino acid whereas Ser is a polar amino acid. Moreover, the cytoplasmic domain of ADAM family of proteins is often rich in proline amino acid and performs critical regulatory activities through interactions with intracellular signalling proteins containing SH3 motifs.18 Hence alteration of proline to serine and non-polar Met to polar amino acid Thr can lead to alteration in the interaction with cytoplasmic proteins. Protein–protein interaction (PPI) network analysis using STRING database demonstrated proteins that may be able to interact with ADAM33 (Fig. 4 B). The results demonstrated that among all of the proteins viz. PACSIN-2, PPFIA1 and PHF11 localized in cytoplasm (Supplementary Table 1). PACSIN-2 is SH3 motif containing cytoskeletal associated proteins. PACSIN-2 protein interacts with WASL protein which acts as actin nucleation stimulating factor, regulates actin polymerization through boosting the Arp2/3 complex's actin-nucleating activity.19 PPFIA1 is another cytoplasmic protein associated with connecting internal actin bundles (focal adhesion).20 Hence, it can be predicted that SNPs at the cytoplasmic domain of ADAM33 may alter its interaction with actin cytoskeleton. Further, NetPhos 3.1 analysis predicted potentiality of phosphorylation to the SNPs present at the cytoplasmic domain. The findings indicated that the amino acid Pro774Ser alteration significantly increases the likelihood of phosphorylation (Supplementary Figure 3). Phosphorylation can alter a variety of signal transduction pathways.21
Fig. 4.
Structural analysis of ADAM33. (A) Domain architecture. The inset view is showing the structural model of the transmembrane and cytoplasmic domain of ADAM33 protein. (B) Protein-protein interaction network. Coloured nodes depict the first shell of interactors and white coloured nodes depict the second shell of interactors.
Discussion
Bronchial asthma, which is considered as a heterogenous chronic airway disease, has become a twenty-first century epidemic. By various degrees of severity, it is not only deteriorating quality of life but also imposing life threat. Though the potential role of gene and environment for influencing asthma has long been identified, there is a great necessity for improvement of our knowledge regarding the biological mechanism of allergic asthma by expanding genomic approaches. ADAM33 was first positionally cloned as asthma susceptible gene by Van Eerdewegh et al,22 who found ADAM33 SNPs to be associated with asthma and bronchial hyper-responsiveness in Caucasian populations of United States and United Kingdom. Hirota et al23 included 14 SNPs of ADAM33 in their study on the basis of more than 15% frequency measured by pairwise Linkage Disequilibrium (LD).22 However, they established that among these 14 SNPs, only 4 SNPs viz T1, T2, V3, and S2 of ADAM33 gene, were associated with asthma in Japanese population and also in linkage disequilibrium. So, in the present study we have included 4 SNPs along with a non-synonymous SNP, S1. The criteria for SNP selection in this study is that selected SNPs should be in Hardy Weinberg equilibrium (HWE) in control population. We found that in case of S2 and V3 SNPs, the control population was not in HWE (Chi square value = 7.30 and 6.23, P = 0.02 and 0.04 respectively). Hence, we excluded S2 and V3 SNPs from further analysis. T1, T2 and S1 single nucleotide polymorphisms are non-synonymous and located in the exon region of ADAM33 gene. Non-synonymous SNPs are more likely to have functional consequences. Genetic association of T1, T2, and S1 SNPs was reported with asthma in different Asian populations by Lee et al,24 Hirota et al,23 Li et al,9 etc. As these associations may vary country and ethnicity wise due to environmental changes and different genetic makeup, so the present study was designed to investigate the possible association of rs2280090 (T2), rs2280091 (T1), and rs3918396 (S1) SNPs of ADAM33 gene with 22 different aeroallergen induced asthma followed by in-silico analysis in West Bengal population, India. Repetitive exposure of aeroallergens plays a significant role in developing asthma in sensitized and genetically predisposed patients.25 We have investigated allergenic sensitization of patient population against 17 different pollens and 5 different types of dust and dust mites commonly available in West Bengal. Throughout India more than 83 types of pollen have been identified so far26 and among those Cocos and Phoenix are predominant in eastern part of India. In our study, maximum patients were sensitized to Cocos nusifera followed by C. dactylon, Azaridacta indica and so on. Among different dust and dust mites, patients showed highest sensitization to D. pteronyssinus and D. farinae followed by house dust. In our study the adult age group (both early and late adult) showed much more sensitization than the other two age groups (child and adolescent) towards pollen, dust, and dust mites. Our findings are in accordance with the observation of Dey et al.27
In our study we found significant association of rs2280090 GG, rs2280091 GG, GA, and rs3918396 AA genotypes of ADAM33 with aeroallergens (pollen, dust and dust mites) induced asthma in our population. Van Eerdewegh et al22 found that S1 polymorphism to be associated in Caucasian populations of the United States and United Kingdom. Both T1 and T2 SNPs were found to be associated in Korean and Japanese populations worldwide.24,28 Although another study from Mexican and Puerto Rican populations of the United States and Australian population reported that none of the 3 SNPs were associated with asthma.29,30 Our study has investigated for the first time the association of T1, T2, and S1 polymorphism of ADAM33 gene with aeroallergen induced asthma in West Bengal population.
Genotypic distribution of T1, T2, and S1 SNPs according to age showed that the adult age group (both early and late adult) bearing heterozygous and risk genotypes for T1, T2, and S1 SNP had higher risk for asthma than adolescent and child. In a Japanese population study Hirota et al found T1 and T2 SNPs to be significantly associated with adult asthma which is in line with our result. Adults are more exposed to heterogeneous environmental condition than child and adolescent which might trigger asthma.
Gender wise stratification of genotypic frequency showed females carrying risk and heterozygous genotypes had higher risk for asthma than males. This gender wise difference of asthma susceptibility might be due to expression of female sex hormone receptors on mast cells.31
We found that patients of urban habitats carrying risk and heterozygote genotypes for T1, T2, and S1 SNPs and semi-urban inhabitants carrying only risk genotypes had higher asthma risk than rural population. Difference in asthma susceptibility may be due to environmental variation between urban and rural environment which was first reported in Germany by Andrejevic et al.32
We have found in our study that these 3 SNPs (T1, T2, and S1) are in strong linkage disequilibrium and the GGA haplotypes are significantly more frequent in asthmatics, thus supporting the association of T1 G-allele, T2 G-allele and S1 A-allele with aeroallergen induced asthma in West Bengal population. Khayyat et al found that T1 and T2 SNPs were in strong linkage disequilibrium (D = 0.95; p < 0.001) in the Saudi Arabian population and associated within childhood asthma in Saudi Arabian population.33 To the best of our knowledge, no other study has been performed on association of ADAM33 SNPs with allergic asthma on West Bengal population till date. This is the first case-control study reporting that T1, T2, and S1 polymorphisms are associated with allergic asthma in West Bengal population.
Significant difference of total IgE level was found between patient and control population in our study. The risk genotype bearing patients had significantly higher IgE level than those with wild and heterozygous genotype. Similar result was also found in Jordanian population.34
We found that FEV1/FVC ratio of different polymorphic genotypes differed significantly in each of the 3 studied SNPs among patient population. We also analysed for the first time FEV1/FVC ratio between GGA and AAG haplotype carrying patients and found that GGA haplotype bearing patients have significantly lower FEV1/FVC ratio than AAG bearers. In some of the previous studies, significant association was found between pulmonary function and various SNPs of ADAM33 gene.35
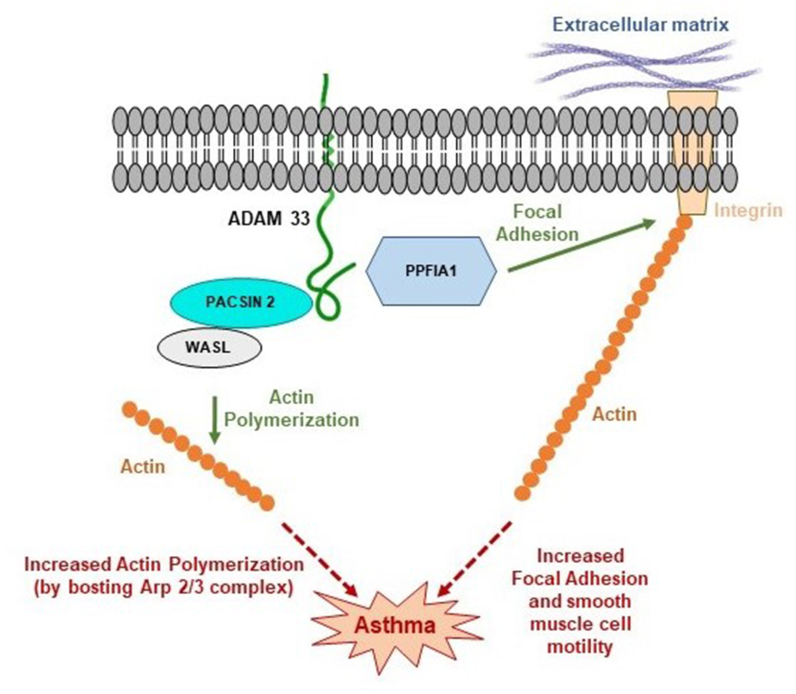
In-silico analysis revealed that transmembrane ADAM33 protein located in smooth muscle cell membrane interacts with cytoplasmic PPFIA1, PACSIN2 proteins via its cytoplasmic domain. PPFIA1 regulates focal adhesion signalling and disassembly20 and thus regulates cell motility.36 T1 and T2 polymorphisms in the cytoplasmic domain of ADAM33 may cause alteration of interaction with PPFIA1 protein which may exert its effect on smooth muscle cell mobility. Increased bronchial smooth muscle cell mobility leads to cellular hyperplasia and airway remodelling which may cause asthma37
On the other hand, PACSIN2 is another cytoplasmic protein which further interact with another cytoplasmic protein WASL which is involved in actin nucleation stimulation activity.19 Nucleation of actin leads to formation of F-actin from G-actin.38 T1, T2 polymorphisms in the cytoplasmic domain may exert its effect on interaction of ADAM33 with cytoskeletal associated cytoplasmic protein PACSIN2 and WASL protein. This alteration of interaction with PACSIN2 and WASL may increase the nucleation of actin activity by boosting Arp2/3 complex.19 Increased proportion of F-actin is related with generation of mechanical tension in smooth muscle cells39 which can ultimately lead to prolonged bronchial contraction and asthma.
Thus T1, T2 polymorphism in the cytoplasmic domain of ADAM33 protein can potentially cause asthma by higher smooth muscle cell mobility, cellular hyperplasia and smooth muscle cell contraction of bronchus via altered interaction with these cytoplasmic proteins ie, PPFIA1, PACSIN2, WASL (Fig. 5).
Fig. 5.
Model for ADAM33 mediated asthma. Asthma may result from an alteration in the interaction between ADAM33 cytoplasmic domain and various cytoplasmic proteins as represented by the red dot arrow. Green arrow depicted usual function in cell. Normal cellular activity was shown by the green arrow
Conclusion
The current study has provided numerous lines of evidence to support the observation that T1, T2, and S1 polymorphisms of ADAM33 gene act as risk factor for allergic asthma in West Bengal population, India. The present in-silico study paves a new way for target gene-based therapeutics for personalized asthma treatment. In order to confirm the proposed mechanistic pathway, cross talk between different key proteins of ADAM33 pathway can be investigated for better understanding the effect on the increased incidence of atopic asthma in near future.
Abbreviations
ADAM33, A disintegrin and metalloproteinase domain 33; FEV1/FVC, Forced Expiratory Volume in 1 s (FEV1)/Forced Vital Capacity; GINA, Global Initiative for Asthma; IgE, Immunoglobulin E; LD, Linkage disequilibrium; PCR, Polymerase chain reaction; PEFR, Peak Expiratory Flow Rate; RFLP, Restriction fragment length polymorphism; SNP, Single nucleotide polymorphisms; SPT, Skin Prick Test.
Funding
Funding was provided by Science and Engineering Research Board, Govt. of India (Sanction no. EEQ/2021/000042 dated February 24, 2022) and Council of Scientific and Industrial Research, Govt. of India (Sanction no: 09/0025(13,528)/2022-EMR-I dated February 28, 2022).
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to consent requirements to protect subjects’ privacy but are available from the corresponding author on reasonable request.
Author contributions
All the authors have made substantial contributions. Saheen Sultana, Indranil Ganai, Nasima Sultana and Himani Biswas contributed to data collection. Saheen Sultana wrote first draft of the manuscript. Priyajit Banerjee did in-silico analysis. Saibal Moitra diagnosed the disease and provided the sample. Nimai Chandra Saha and Arghya Laha helped in statistical analysis. Sanjoy Podder conceived the study design, supervised the work, critically revised the manuscript and made the final draft. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study has been conducted with the approval of Institutional review board (CREC-AARC Ref:62/21). All the participants for this study have provided their written consent of participation.
Authors’ consent for publication
All the authors approved final version of the manuscript and provided their consent for publication.
Potential conflict of interests
The authors report no conflict of interests.
Confirmation of unpublished work
Our manuscript is original, has not been published before, is not currently being considered for publication elsewhere, and has not been posted to a preprint server.
Acknowledgements
The authors convey thanks to the patients who voluntarily participated in our study. We are also grateful to the lab professionals of the Allergy and Asthma Research Center, Kolkata, for their technical help.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2023.100834.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Varricchi G., Ferri S., Pepys J., et al. Biologics and airway remodeling in severe asthma. Allergy. 2022 Dec;77(12):3538–3552. doi: 10.1111/all.15473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganai I, Saha I, Banerjee P, Laha A, Sultana S, Sultana N, et al. In silico analysis of single nucleotide polymorphism (rs34377097) of TBXA2R gene and pollen induced bronchial asthma susceptibility in West Bengal population, India. Front Immunol. 2023 Mar 1;14:1089514. 10.3389/fimmu.2023.1089514. [DOI] [PMC free article] [PubMed]
- 3.Laha A, Moitra S, Biswas H, Saha NC, Podder S. Assessment of Co-Sensitization between Pollen and Food Allergen Sources among Bengali Population, West Bengal, India. Int Arch Allergy Immunol. 2023;184(2):161–170. doi: 10.1159/000526707. [DOI] [PubMed] [Google Scholar]
- 4.Bogari N.M., Amin A.A., Rayes H.H., et al. Next generation exome sequencing of pediatric asthma identifies rare and novel variants in candidate genes. Dis Markers. 2021 Feb 8;2021 doi: 10.1155/2021/8884229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S.H., Pei Q.M., Jiang P., Yang M., Qian X.J., Liu J.B. Effect of active vitamin D3 on VEGF-induced ADAM33 expression and proliferation in human airway smooth muscle cells: implications for asthma treatment. Respir Res. 2017 Jan 5;18(1):7. doi: 10.1186/s12931-016-0490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hur G.Y., Broide D.H. Genes and pathways regulating decline in lung function and airway remodeling in asthma. Allergy Asthma Immunol Res. 2019 Sep;11(5):604–621. doi: 10.4168/aair.2019.11.5.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camoretti-Mercado B., Lockey R.F. Airway smooth muscle pathophysiology in asthma. J Allergy Clin Immunol. 2021 Jun;147(6):1983–1989. doi: 10.1016/j.jaci.2021.03.035. [DOI] [PubMed] [Google Scholar]
- 8.Zhou J., Bai W., Liu Q., Cui J., Zhang W. Silencing of ADAM33 restrains proliferation and induces apoptosis of airway smooth muscle cells in ovalbumin-induced asthma model. J Cell Biochem. 2019 Feb;120(2):1435–1443. doi: 10.1002/jcb.27263. [DOI] [PubMed] [Google Scholar]
- 9.Li H.F., Yan L.P., Wang K., Li X.T., Liu H.X., Tan W. Association between ADAM33 polymorphisms and asthma risk: a systematic review and meta-analysis. Respir Res. 2019 Feb 21;20(1):38. doi: 10.1186/s12931-019-1006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallucci M., Carbonara P., Pacilli A.M.G., di Palmo E., Ricci G., Nava S. Use of symptoms scores, spirometry, and other pulmonary function testing for asthma monitoring. Front Pediatr. 2019;7:54. doi: 10.3389/fped.2019.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bousquet J., Heinzerling L., Bachert C., et al. Global allergy and asthma European network; allergic rhinitis and its impact on asthma. Practical guide to skin prick tests in allergy to aeroallergens. Allergy. 2012 Jan;67(1):18–24. doi: 10.1111/j.1398-9995.2011.02728.x. [DOI] [PubMed] [Google Scholar]
- 12.Bureau of Applied Economics and Statistics . State Statistical Handbook. 2015. Department of Statistics and Programme Implementation, Government of West Bengal; pp. 79–138. West Bengal, India. [Google Scholar]
- 13.Gabriel S.B., Schafner S.F., Nguyen H., et al. The structure of haplotype blocks in the human genome. Science. 2002;296(5576):2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 14.Xu J., Mcpartlon M., Li J. Improved protein structure prediction by deep learning irrespective of co-evolution information. Nat Mach Intell. 2021 Jul;3:601–609. doi: 10.1038/s42256-021-00348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeLano W.L. An open-source molecular graphics tool CCP4 Newsletter on protein 442 crystallography. Pymol. 2002;40:82–92. [Google Scholar]
- 16.Kodgule R.R., Singh V., Dhar R., et al. Reference values for peak expiratory flow in Indian adult population using a European Union scale peak flow meter. J Postgrad Med. 2014 Apr-Jun;60(2):123–129. doi: 10.4103/0022-3859.132311. [DOI] [PubMed] [Google Scholar]
- 17.Jumper J., Evans R., Pritzel A., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021 Aug;596(7873):583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cakebread J.A., Haitchi H.M., Holloway J.W., et al. The role of ADAM33 in the pathogenesis of asthma. Springer Semin Immunopathol. 2004 Feb;25(3-4):361–375. doi: 10.1007/s00281-003-0153-z. [DOI] [PubMed] [Google Scholar]
- 19.May R.C. The Arp2/3 complex: a central regulator of the actin cytoskeleton. Cell Mol Life Sci. 2001 Oct;58(11):1607–1626. doi: 10.1007/PL00000800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serra-Pagès C., Kedersha N.L., Fazikas L., Medley Q., Debant A., Streuli M. The LAR transmembrane protein tyrosine phosphatase and a coiled-coil LAR-interacting protein co-localize at focal adhesions. EMBO J. 1995 Jun 15;14(12):2827–2838. doi: 10.1002/j.1460-2075.1995.tb07282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen P. The regulation of protein function by multisite phosphorylation--a 25 year update. Trends Biochem Sci. 2000 Dec;25(12):596–601. doi: 10.1016/s0968-0004(00)01712-6. [DOI] [PubMed] [Google Scholar]
- 22.Van Eerdewegh P., Little R.D., Dupuis J., et al. Association of the ADAM33 gene with asthma and bronchial hyperresponsiveness. Nature. 2002 Jul 25;418(6896):426–430. doi: 10.1038/nature00878. [DOI] [PubMed] [Google Scholar]
- 23.Hirota T., Hasegawa K., Obara K., et al. Association between ADAM33 polymorphisms and adult asthma in the Japanese population. Clin Exp Allergy. 2006 Jul;36(7):884–891. doi: 10.1111/j.1365-2222.2006.02522.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee J.H., Park H.S., Park S.W., et al. ADAM33 polymorphism: association with bronchial hyper-responsiveness in Korean asthmatics. Clin Exp Allergy. 2004 Jun;34(6):860–865. doi: 10.1111/j.1365-2222.2004.01977.x. [DOI] [PubMed] [Google Scholar]
- 25.Baxi S.N., Phipatanakul W. The role of allergen exposure and avoidance in asthma. Adolesc Med State Art Rev. 2010 Apr;21(1):57–71. [PMC free article] [PubMed] [Google Scholar]
- 26.Singh A.B., Shahi S. Aeroallergens in clinical practice of allergy in India- ARIA Asia Pacific Workshop report. Asian Pac J Allergy Immunol. 2008 Dec;26(4):245–256. [PubMed] [Google Scholar]
- 27.Dey D, Mondal P, Laha A, et al. Sensitization to Common Aeroallergens in the Atopic Population of West Bengal, India: An Investigation by Skin Prick Test. Int Arch Allergy Immunol. 2019;178(1):60–65. doi: 10.1159/000492584. [DOI] [PubMed] [Google Scholar]
- 28.Sakagami T., Jinnai N., Nakajima T., et al. ADAM33 polymorphisms are associated with aspirin-intolerant asthma in the Japanese population. J Hum Genet. 2007;52(1):66–72. doi: 10.1007/s10038-006-0081-6. [DOI] [PubMed] [Google Scholar]
- 29.Lind D.L., Choudhry S., Ung N., et al. ADAM33 is not associated with asthma in Puerto Rican or Mexican populations. Am J Respir Crit Care Med. 2003 Dec 1;168(11):1312–1316. doi: 10.1164/rccm.200306-877OC. [DOI] [PubMed] [Google Scholar]
- 30.Kedda M.A., Duffy D.L., Bradley B., O'Hehir R.E., Thompson P.J. ADAM33 haplotypes are associated with asthma in a large Australian population. Eur J Hum Genet. 2006 Sep;14(9):1027–1036. doi: 10.1038/sj.ejhg.5201662. [DOI] [PubMed] [Google Scholar]
- 31.Pignataro F.S., Bonini M., Forgione A., Melandri S., Usmani O.S. Asthma and gender: the female lung. Pharmacol Res. 2017 May;119:384–390. doi: 10.1016/j.phrs.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 32.Andrejević M. On qualitative differences in the bronchial asthma of the urban and rural population. Allergic asthma (Lepiz) 1965;11(4):156–161. [PubMed] [Google Scholar]
- 33.Al-Khayyat A.I., Al-Anazi M., Warsy A., et al. T1 and T2 ADAM33 single nucleotide polymorphisms and the risk of childhood asthma in a Saudi Arabian population: a pilot study. Ann Saudi Med. 2012 Sep-Oct;32(5):479–486. doi: 10.5144/0256-4947.2012.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zihlif M., Mahafza T., Obeidat N.M., et al. Frequency of genetic polymorphisms of ADAM33 and their association with allergic rhinitis among Jordanians. Gene. 2013 Dec 1;531(2):462–466. doi: 10.1016/j.gene.2013.08.085. [DOI] [PubMed] [Google Scholar]
- 35.Jongepier H., Boezen H.M., Dijkstra A., et al. Polymorphisms of the ADAM33 gene are associated with accelerated lung function decline in asthma. Clin Exp Allergy. 2004 May;34(5):757–760. doi: 10.1111/j.1365-2222.2004.1938.x. [DOI] [PubMed] [Google Scholar]
- 36.Alfarsi L.H., El Ansari R., Craze M.L., et al. PPFIA1 expression associates with poor response to endocrine treatment in luminal breast cancer. BMC Cancer. 2020 May 14;20(1):425. doi: 10.1186/s12885-020-06939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salter B., Pray C., Radford K., Martin J.G., Nair P. Regulation of human airway smooth muscle cell migration and relevance to asthma. Respir Res. 2017 Aug 16;18(1):156. doi: 10.1186/s12931-017-0640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Firat-Karalar E.N., Welch M.D. New mechanisms and functions of actin nucleation. Curr Opin Cell Biol. 2011 Feb;23(1):4–13. doi: 10.1016/j.ceb.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gunst S.J., Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol. 2008 Sep;295(3):WEC576–W587. doi: 10.1152/ajpcell.00253.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to consent requirements to protect subjects’ privacy but are available from the corresponding author on reasonable request.