Abstract
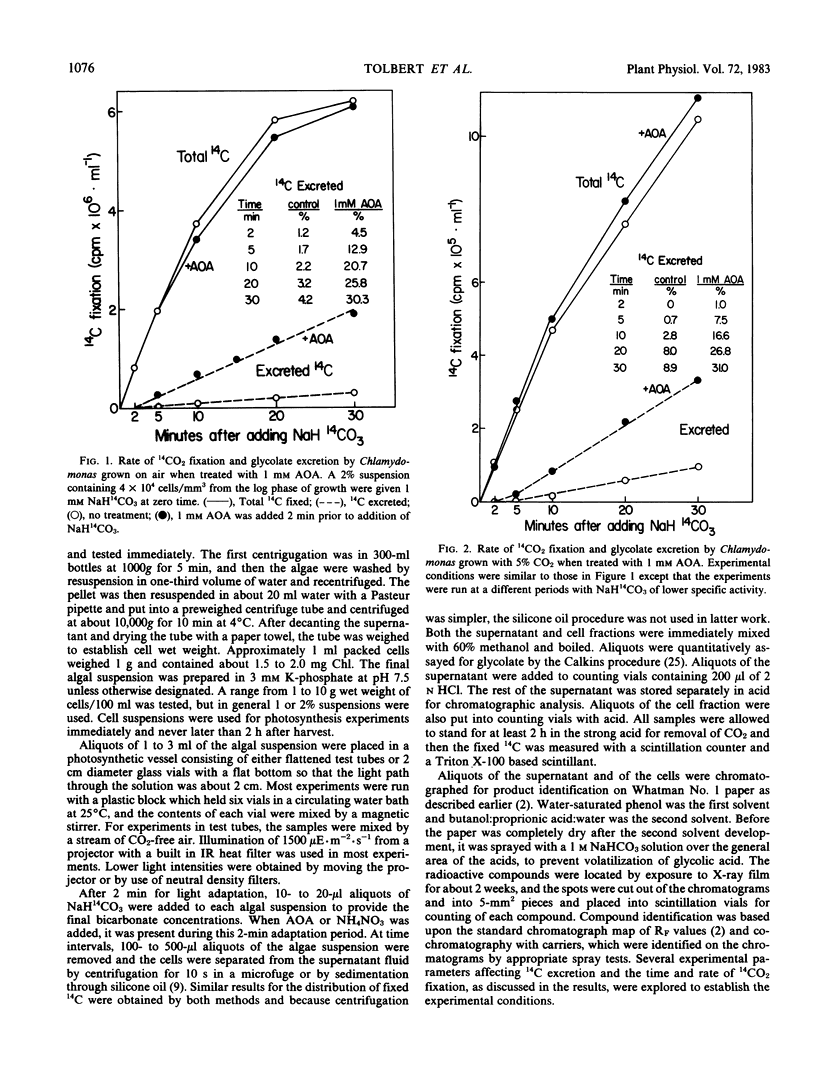
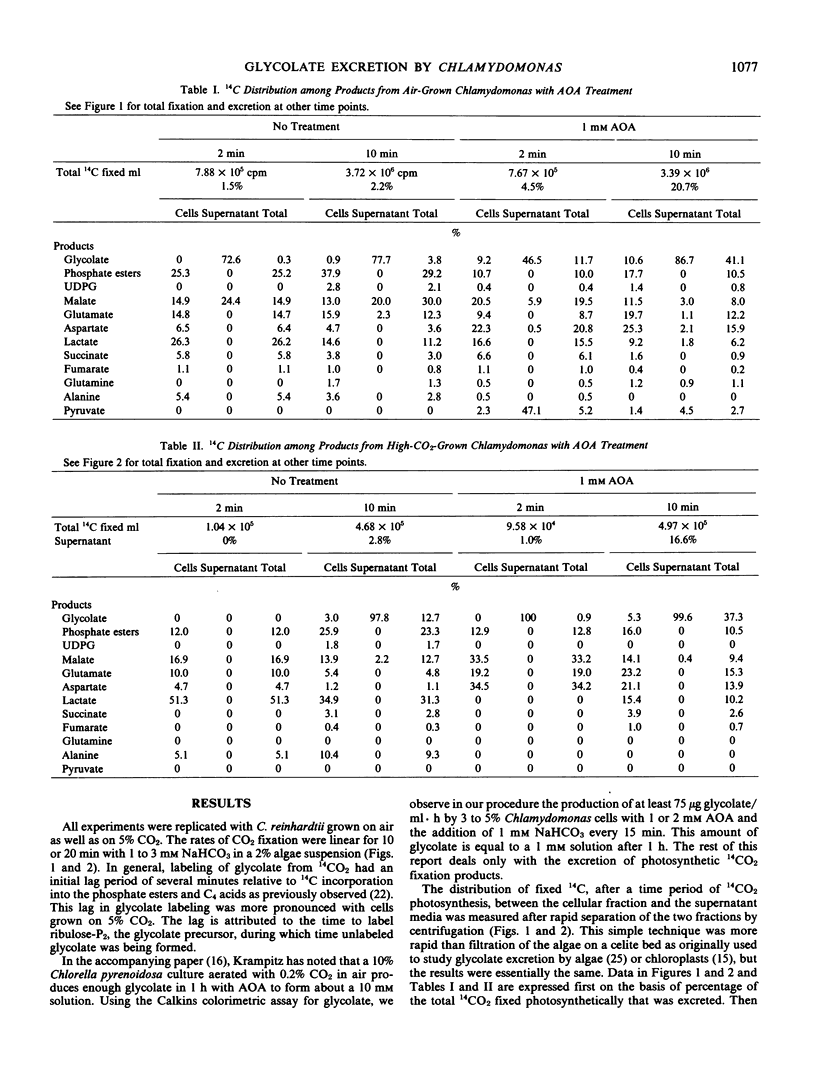
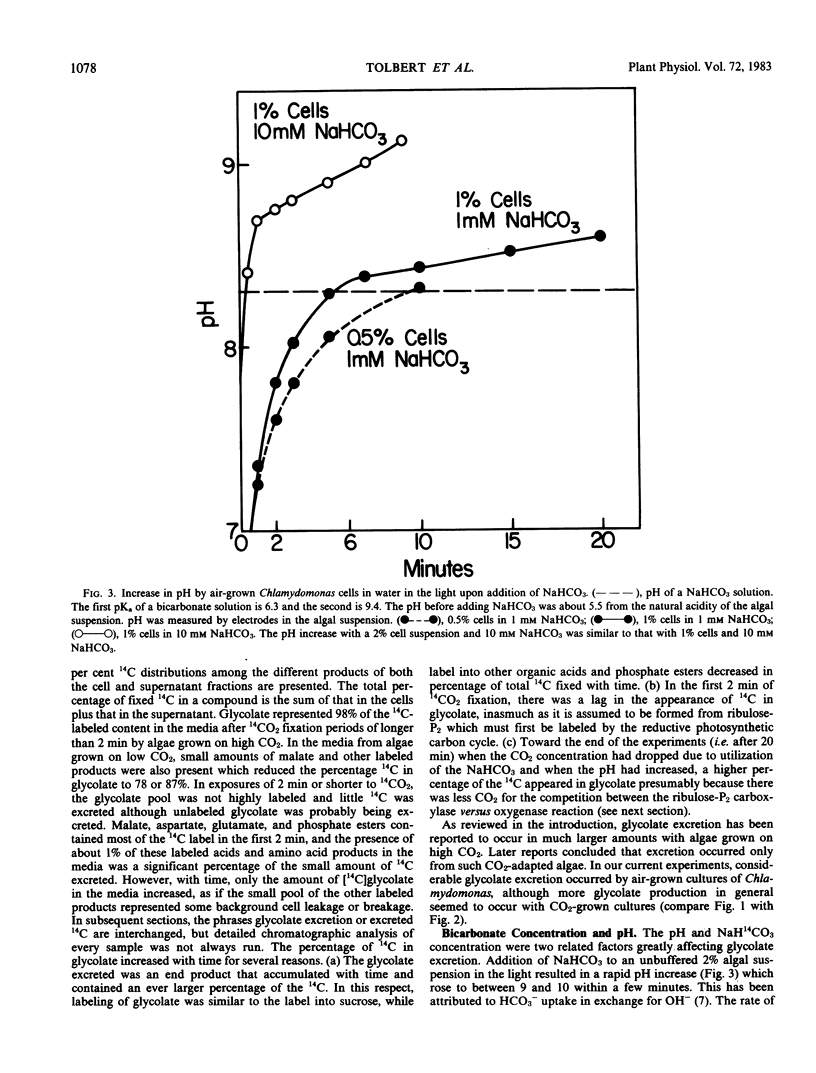
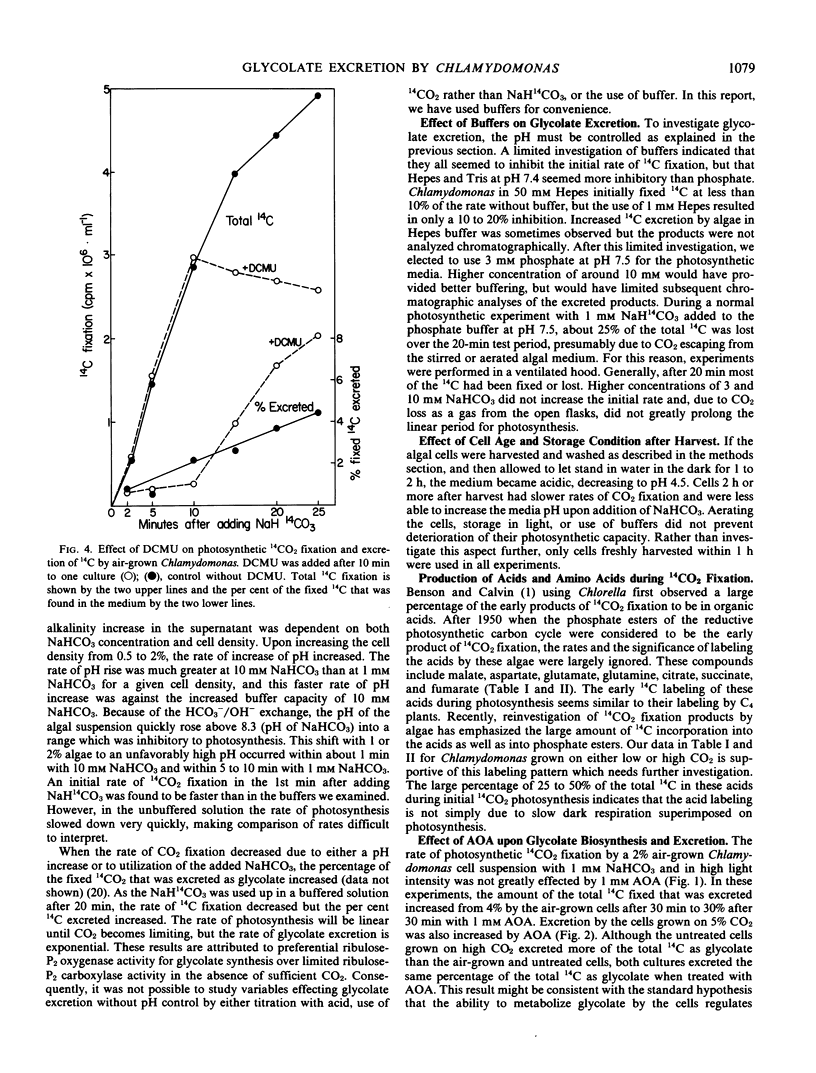
Aminooxyacetate (1 millimolar) did not inhibit photosynthetic 14CO2 fixation by Chlamydomonas reinhardtii Dangeard, (−) strain (N.90) but greatly stimulated the biosynthesis and excretion of glycolate. Similar results were obtained from cells grown with 5% CO2 or low CO2 (air). After 2 minutes with air-grown cells, [14C]glycolate increased from 0.3% of the total 14C fixed by the control to 11.7% in the presence of aminooxyacetate and after 10 minutes from 3.8% to 41.1%. Ammonium nitrate (0.2 millimolar) in the media blocked the aminooxyacetate stimulation of glycolate excretion. Chromatographic analyses of the labeled products in the cells and supernatant media indicated that aminooxyacetate also completely inhibited the labeling of alanine while some pyruvate accumulated and was excreted. A high percentage (35%) of initial 14CO2 fixation was into C4 acids. Initial products of 14CO2 fixation included phosphate esters as well as malate, aspartate, and glutamate in treated or untreated cells. Lactate was also a major early product of photosynthesis, and its labeling was reduced by aminooxyacetate. Inasmuch as lactate was not excreted, glycolate excretion seemed to be specific. When photosynthesis was inhibited by 3-(3,4-dichlorophenyl)-1,1-dimethylurea, labeled organic and amino acids but not phosphate esters were lost from the cells. Aminooxyacetate did not inhibit the enzymes associated with glycolate synthesis from ribulose bisphosphate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang W. H., Tolbert N. E. Excretion of Glycolate, Mesotartrate and Isocitrate Lactone by Synchronized Cultures of Ankistrodesmus braunii. Plant Physiol. 1970 Sep;46(3):377–385. doi: 10.1104/pp.46.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd G. A., Smith B. M. Glycollate formation and excretion by the purple photosynthetic bacterium Rhodospirillum rubrum. FEBS Lett. 1974 Nov 1;48(1):105–108. doi: 10.1016/0014-5793(74)81073-2. [DOI] [PubMed] [Google Scholar]
- Colman B., Miller A. G., Grodzinski B. A study of the control of glycolate excretion in chlorella. Plant Physiol. 1974 Mar;53(3):395–397. doi: 10.1104/pp.53.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber P. J., Frederick S. E., Tolbert N. E. Enzymes related to lactate metabolism in green algae and lower land plants. Plant Physiol. 1974 Feb;53(2):167–170. doi: 10.1104/pp.53.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz K. T., McCarty R. E. pH Dependence and Kinetics of Glycolate Uptake by Intact Pea Chloroplasts. Plant Physiol. 1982 Oct;70(4):949–952. doi: 10.1104/pp.70.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEARNEY P. C., TOLBERT N. E. Appearance of glycolate and related products of photosynthesis outside of chloroplasts. Arch Biochem Biophys. 1962 Jul;98:164–171. doi: 10.1016/0003-9861(62)90162-5. [DOI] [PubMed] [Google Scholar]
- Kaplan A., Berry J. A. Glycolate Excretion and the Oxygen to Carbon Dioxide Net Exchange Ratio during Photosynthesis in Chlamydomonas reinhardtii. Plant Physiol. 1981 Feb;67(2):229–232. doi: 10.1104/pp.67.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krampitz L. O., Yarris C. E. Glycolate Formation and Excretion by Chlorella pyrenoidosa and Netrium digitus. Plant Physiol. 1983 Aug;72(4):1084–1087. doi: 10.1104/pp.72.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawyer A. L., Cornwell K. L., Larsen P. O., Bassham J. A. Effects of carbon dioxide and oxygen on the regulation of photosynthetic carbon metabolism by ammonia in spinach mesophyll cells. Plant Physiol. 1981 Dec;68(6):1231–1236. doi: 10.1104/pp.68.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Osmond C. B., Akazawa T., Asami S. On the mechanism of glycolate synthesis by Chromatium and Chlorella. Arch Biochem Biophys. 1978 Jan 15;185(1):49–56. doi: 10.1016/0003-9861(78)90142-x. [DOI] [PubMed] [Google Scholar]
- Nelson E. B., Tolbert N. E. The regulation of glycolate metabolism in Chlamydomonas reinhardtii. Biochim Biophys Acta. 1969 Jul 30;184(2):263–270. doi: 10.1016/0304-4165(69)90028-2. [DOI] [PubMed] [Google Scholar]
- Orth G. M., Tolbert N. E., Jimenez E. Rate of Glycolate Formation During Photosynthesis at High pH. Plant Physiol. 1966 Jan;41(1):143–147. doi: 10.1104/pp.41.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICKLI E. E., GHAZANFAR S. A., GIBBONS B. H., EDSALL J. T. CARBONIC ANHYDRASES FROM HUMAN ERYTHROCYTES. PREPARATION AND PROPERTIES OF TWO ENZYMES. J Biol Chem. 1964 Apr;239:1065–1078. [PubMed] [Google Scholar]
- TOLBERT N. E., ZILL L. P. Excretion of glycolic acid by algae during photosynthesis. J Biol Chem. 1956 Oct;222(2):895–906. [PubMed] [Google Scholar]
- Takabe T., Akazawa T. Mechanism of glycolate transport in spinach leaf chloroplasts. Plant Physiol. 1981 Nov;68(5):1093–1097. doi: 10.1104/pp.68.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]


