Abstract
Non-alcoholic fatty liver disease (NAFLD) has become one of the most common causes of liver diseases globally, with a projected exponential rise. In contrast to the exponential rise in disease burden, there are limited options in the pharmacotherapeutic armamentarium against NAFLD. Saroglitazar belongs to the class of drugs known as peroxisome proliferator-activated receptor (PPAR) agonists, initially introduced for managing diabetic dyslipidemia. However, based on translational and clinical studies, it has been shown to be efficacious in NAFLD. It has been shown to modify key parameters in NAFLD, including reduction of transaminase levels, improvement in overall metabolic health, reduction of liver fat content, and improvement of liver stiffness and histology. Given the promising results, it has been made a part of society's guidelines in the therapeutic management of NAFLD. However, there remains a dearth of detailed reviews encompassing both pre-clinical and clinical data on the effectiveness of saroglitazar in NAFLD. In this review, we comprehensively review the pharmacology, pre-clinical data, and clinical studies on saroglitazar usage in NAFLD and conduct a subgroup meta-analysis of studies focussing on the impact of saroglitazar on liver stiffness changes.
Keywords: metabolic health, liver stiffness, diabetic dyslipedemia, nafld, saroglitazar
Introduction and background
Non-alcoholic fatty liver disease (NAFLD) refers to the presence of ≥5% steatosis in the liver in the absence of known causes of steatosis [1]. The entity encompasses diverse phenotypes, ranging from bland steatosis to steatohepatitis, advanced fibrosis, and cirrhosis [2]. The burden of NAFLD has seen an exponential increase globally, with current literature showing 25% of the global population being affected by NAFLD and a projected rise of 63% between 2015 and 2030 [1,3]. Contrasting to the meteoric rise in disease burden, the therapeutic armamentarium against NAFLD has, however, seen limited development [1]. While patients with non-alcoholic steatohepatitis (NASH) with stage 2 or higher fibrosis are candidates for liver-directed therapy, current treatment strategies are limited to risk factor mitigation, lifestyle and dietary adjustments, and, in specific cases, use of vitamin E and pioglitazone [4].
Saroglitazar belongs to the class of drugs known as peroxisome proliferator-activated receptors (PPARs) agonists, which was given marketing authorization in India in 2013 as an agent for the management of atherogenic diabetic dyslipidemia [5]. Based upon its unique mechanism of action, the drug showed efficacy in NAFLD and, based upon subsequent trials, was granted approval as an agent for NAFLD in India [6,7].
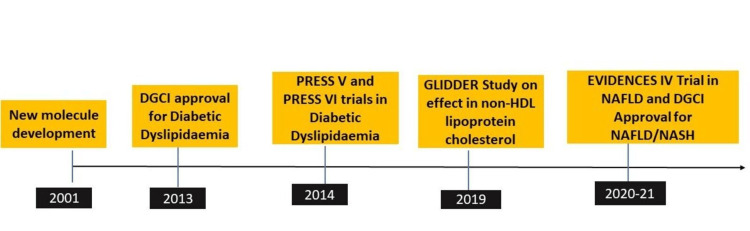
A schematic representation of the development history of saroglitazar is shown in Figure 1.
Figure 1. Showing timeline of saroglitazar development in NAFLD.
DCGI, Drug Controller General of India; NAFLD, Non-alcoholic fatty liver disease; NASH, Non-alcoholic steatohepatitis; HDL, High-density lipoprotein
However, there remains a paucity of literature that summarizes in a comprehensive manner the evidence for the use of this drug especially in the backdrop of recent landmark trials [5-7]. In this review, we aimed to review the available literature supporting the use of saroglitazar in patients with NAFLD with an attempt to incorporate both pre-clinical and clinical data and understand its usage in current-day practice. Additionally, we perform a subgroup meta-analysis on studies reporting changes in liver stiffness measurement (LSM) with saroglitazar.
Review
Methodology
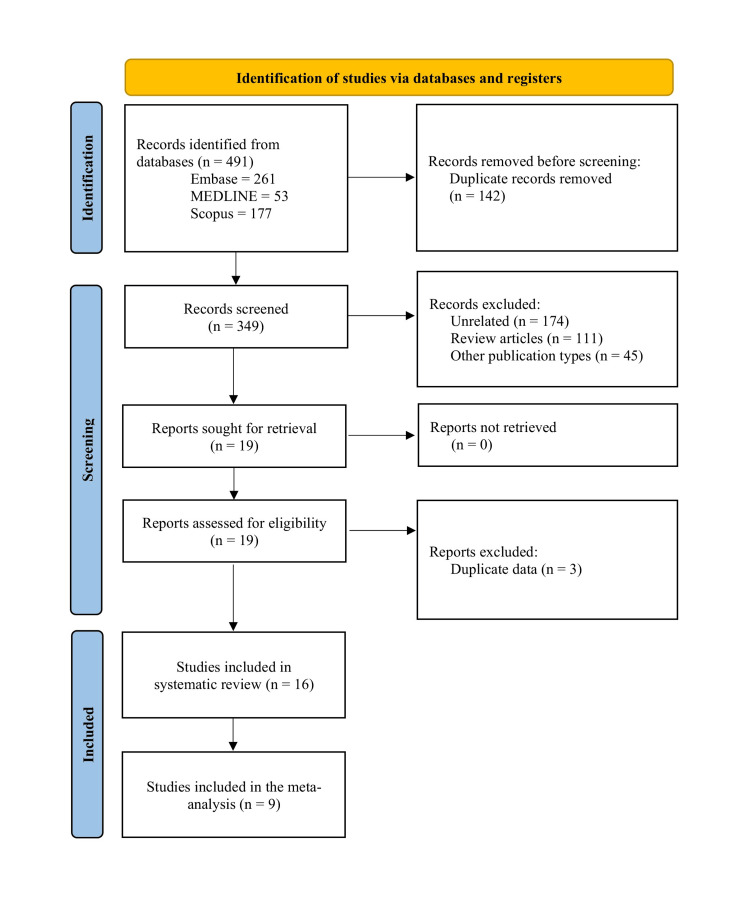
We conducted this narrative review according to the guidelines and checklist provided by Green et al. [8]. Literature for this review was identified using the specific search term “Saroglitazar” in MEDLINE and EMBASE. All studies from the inception of the particular database to September 1, 2023, were searched. We reviewed all designs of articles (cohort studies, case-control studies, case series, case reports). Cohort studies, case-control studies, and case series were included, while case reports were excluded. The articles' language was restricted to English. After summarising the available literature, we performed a random-effects meta-analysis on studies reporting changes in LSM, a key component of efficacy acting as a surrogate for histological fibrosis reduction. We identified 491 papers (MEDLINE 53, EMBASE 261, and SCOPUS 177). Four pre-clinical studies and 12 clinical studies were identified for detailed review. Sub-group meta-analysis was carried out with nine studies for which relevant data were available. The PRISMA flow diagram is shown in Figure 2.
Figure 2. PRISMA flow diagram for the selection of studies on saroglitazar.
What are PPARs?
Prior to embarking on a detailed discussion on saroglitazar, it is imperative to understand the key receptors that form the crux of drug efficacy. PPARs are a group of nuclear receptors associated with the proliferation of peroxisomes. They are primarily involved in lipid and glucose metabolism with potential favorable effects on hepatic inflammation and fibrogenesis processes [9]. These receptors exist in three different isoforms (α, β/δ, and γ(two sub-isotypes γ-1 and γ-2)), which have variable tissue distributions and primary functions, while, specifically in the liver, these lead to a reduction of hepatic steatosis and improvement in inflammation and fibrosis [10]. Specifically, PPARα has a mechanistic basis for improving lipid metabolism by regulating lipid influx, fatty acid transport, and β-oxidation [9]. Additionally, it has been shown to reduce splanchnic inflammation and intestinal permeability. PPARβ/δ has anti-inflammatory properties exerted at the level of macrophages. PPARγ acts with potential regulatory roles in insulin sensitivity within the adipose tissue. Furthermore, PPARγ prevents hepatocyte stellate cell (HSC) activation, playing a key role in hepatic fibrosis pathways. Thus, working at various axes, the three PPAR isotypes act in different cells and organs, influencing different pathways and mechanisms involved in NASH and fibrosis progression [9].
Pharmacology of saroglitazar
As outlined before, saroglitazar is a PPAR agonist with predominant PPAR α and moderate PPAR γ activity. Hence, the molecule is essentially designed to induce the benefits of both fibrates and glitazone drugs. Previous molecules based upon similar mechanisms included muraglitazar, tesaglitazar, aleglitazar, and naveglitazar, all of which had safety concerns and, hence, were withdrawn from further studies [11]. The primary indication of the molecule was for dyslipidemia with favorable effects on glycaemic control, which led to its approval for “diabetic dyslipidemia.” This can be mechanistically explained by the reduction of TG mediated by the PPAR α agonism and improvement in insulin resistance and glycaemic control by PPAR γ agonism. Data from pharmacokinetic studies showed saroglitazar having good oral absorption (largely unaffected by food intake), with a median time to the peak plasma concentration of less than one hour (range 0.63-1 hour) and an average terminal half-life of 5.6 hours [12].
Evidence in diabetic dyslipidemia
In an extensive review, Kaul et al. provided insights into the potential benefits of saroglitazar [13]. The review of 18 studies spanning 5,824 patients (mean age 49.6-59.1 years, 22%-42% females) showed saroglitazar to consistently reduce triglyceride levels (45%-62%), total cholesterol levels (17%-26%), non-high-density lipoprotein cholesterol levels (21%-36%), low-density lipoprotein cholesterol levels (11%-27%), and glycosylated hemoglobin levels ( 0.7%-1.6%), leading to an increase in mean high-density lipoprotein cholesterol levels (up to 9%). The drug was extremely well-tolerated, with minor side effects reported as knee joint pain, chest discomfort, burning soles, and hypoglycemia [13].
Literature from pre-clinical studies in NAFLD
Multiple studies in pre-clinical mouse models have established the proof of concept for the efficacy of saroglitazar in NAFLD [14-17]. Saroglitazar at doses of 3 mg or 4 mg was shown to positively impact histology with improvement in hepatic steatosis, lobular inflammation, and ballooning. The study by Kumar et al. also showed significant improvements in fibrosis and NASH resolution in all cases [16]. Table 1 summarises the literature available from pre-clinical studies of saroglitazar in NAFLD/NASH.
Table 1. Preclinical animal model studies on saroglitazar in NAFLD.
CDAHFD, Choline-deficient L-amino acid defined high-fat diet; WDSW, Western diet sugar water, HOMA-IR, Homeostatic model assessment for insulin resistance, TG, Triglycerides; ALT, Alanine transaminase, NASH, Non-alcoholic steatohepatitis; HFHF, High fat, high fructose; SAR, Saroglitazar
| Authors, Year | Model | Arms/Groups | Effects of Saroglitazar | Histological Effects |
| Akbari et al. (2021) [14] | Male Wistar rats fed with a high-fat emulsion | Saroglitazar (3 mg/kg), pioglitazone (30 m/kg), fenofibrate (100 mg/kg)and vehicle | Improvement in body weight, transaminases, leptin, and adiponectin decreased pro-inflammatory cytokines | Improvement in fatty appearance, lobular inflammation, hepatocellular ballooning decreased fibrosis |
| Kumar et al. (2020) [16] | DIAMOND mice fed with Western Diet Sugar Water (WDSW) | WDSW alone, WDSW plus pioglitazone(30mg/kg), WDSW plus saroglitazar (4mg/kg), vehicle control group | Improvement in weight, HOMA-IR, TG, total cholesterol, and ALT | Saroglitazar improved steatosis, lobular inflammation, hepatocellular ballooning, and fibrosis stage. NASH resolved in all mice receiving saroglitazar |
| Hasan et al. (2019) [15] | Female Wistar rats fed with standard chow diet and water ad libitum | Rats in group 1 (the control group) received saline (10 ml/kg/daily, oral gavage), while, in rats in group 2 (the HFE/LPS model group) and group 3 (the SAR-treated group), steatohepatitis was induced by the administration of HFE (10 ml/kg/day, oral gavage) and LPS (0.5 mg/kg/week) | Counteracted body weight gain and normalized liver function, glucose, (HOMA-IR) score, and lipid profile levels | Decrease in inflammation |
| Sarkar et al. (2021) [17] | C57BL/6 male mice on HFHF diet for four weeks | Saroglitazar (3 mg/kg/po), and Hepano - a formulation of five herbs (200 mg/kg/po) | Saroglitazar improved IR, obesity, reduced TG, and modulated phospholipids | None |
| Jain et al. (2018) [18] | HepG2 cells treated with palmitic acid (PA; 0.75 mM) | Saroglitazar (3 mg/kg), pioglitazone (25 mg/kg), and fenofibrate (100 mg/kg) | Significantly higher reduction of NAFLD activity score by saroglitazar | Antifibrotic effect of saroglitazar (4 mg/kg) observed in carbon tetrachloride-induced fibrosis model |
| Giri et al. (2023) [19] | Hepatocellular carcinoma induction in C57BL/6 mice by intraperitoneal injection of 25 mg/kg diethylnitrosamine (DEN) a | Saroglitazar (1 and 3 mg/kg) treatment for 27 weeks | All disease control animals showed hepatic tumors, which were absent in saroglitazar (3 mg/kg), indicating 100% prevention of tumorigenesis | None |
Clinical studies of saroglitazar in NAFLD
Multiple clinical studies have subsequently emerged in patients with NAFLD analyzing the effects of saroglitazar [6,7,20-29]. The majority of the studies are from India and are single-center retrospective/prospective single-arm studies with variable follow-up, ranging from 12-52 weeks. Most studies from India have looked at biochemical improvements in transaminase levels and improvement in lipid profile parameters, while few studies have also looked at improvement in liver stiffness measurements and controlled attenuation parameter values [20,23-26]. Moreover, these data come from multicentric biopsy-proven studies and pooled individual data analysis of three multicentric cohorts [6,7,27]. Interestingly, one study also looked at the impact of saroglitazar on post-transplant NAFLD, reporting positive outcomes, and another abstract-only study reported similar results [30]. Two studies also included patients with compensated cirrhosis and reported no significant side effect concerns [25,26]. A detailed summary of the available evidence on saroglitazar based on different clinical studies is shown in Table 2.
Table 2. Clinical studies on saroglitazar.
NAFLD, Non-alcoholic fatty liver disease; NASH, Non-alcoholic steatohepatitis; ALT, Alanine transaminase; AST, aspartate transaminase; TG, Triglycerides; TC, Total cholesterol; HDL, High-density lipoprotein; LDL, Low-density lipoprotein; LSM, Liver stiffness measurement; SWE, Sheer wave elastography, NA, Not available, BMI, Body mass index, DM, diabetes mellitus, MRI-PDFF, Magnetic resonance imaging proton density fat fraction; CAP, Controlled attenuation parameter; ALP, Alkaline phosphatase; VLDL, Very low-density lipoprotein, Hba1c: Glycosylated hemoglobin; HOMA-IR, Homeostatic model assessment for insulin resistance
| Authors, Year | Design | Arms | Population | Number of Patients | Follow-up | Key Demographics | Biochemical Changes | Safety |
| Padole et al. 2021 (India) [20] | Prospective | Single arm | NAFLD (No specifications) | 91 | 12 weeks | Mean age=45 (18–66), 81% males, BMI 29.3 (23.6–42.2), ALT:48 (13–164), LSM:6.7 (3.6–13.1) 308 (249–400) | Outcomes divided into those with/without weight loss (5%). Weight Loss Group: Decrease in ALT, AST, CAP, and LSM (P<0.05) for all no weightloss group: Significant decrease in ALT, AST but not in LSM or CAP | NA |
| Jaiswal et al. 2021 (India) [21] | Retrospective | Single arm | Non-diabetic NAFLD | 45 | 24 weeks | Mean age=46±8.20, 55% males, ALT 85.52±17.12, LSM:8.11±2.18, CAP365.84±56.22 | Does not account for weight loss decrease in ALT, AST, CAP, and LSM (P<0.05) for all | NA |
| Roy et al. 2021 (India) [22] | Retrospective | Single arm | NAFLD with DM and dyslipidemia | 10 | 36 weeks | Mean age=59.3 years, 70% males, BMI 25.21± 3.07, HbA1c 7.8±0.343, TG 298.2±35.75, ALT 64.7±15.56, SWE 1.837±0.0691 | Significant decrease in all parameters (p<0.05 for all) | NA |
| Rajesh et al. 2021 (India) [23] | Prospective | Single | NAFLD with DM | 85 | 12 weeks | Mean age 56.81 ±4.06 BMI 25.94 ±2.20 HBA1c 10.29 ±0.64 Triglycerides 359.89 ±5.46 HDL 49.20 ±3.08 SGPT 49.62 ±.31 LSM 9.68 ±0.30 | Significant decrease in FBS, HBA1c, TC, TG, and SGPT, Mean decrease in LSM 3.61±3.98 | No ADR |
| Goyal et al. 2020 (India) [24] | Prospective | Single | NAFLD with DD | 107 | 24 weeks | Mean age 50.4 ± 12.3 BMI 28.8 ± 4.2 HBA1c 7.2 ± 0.65 TC 209.8 ± 62.4 Triglycerides 326.4 ± 98.5 HDL 38.2 ± 8.1 SGPT 94 (47–122) LSM 8.4 (7.1–9.3) CAP 335 (281–392) | Significant decrease in FBS, HBA1c, TC, TG, SGPT, SGOT, CAP, and LSM | Minor adverse events reported were fatigue in 2.8% (n=3), nausea in 1.9% (n=2), and dyspepsia 1.9% (n=2) |
| Siddiqui et al. 2020 (Multicentric, USA) [7] | Prospective | Double-blind placebo-controlled | Biopsy-proven NASH with NAS>4 | 16 paients Saro 2 mg:n=6 Saro 4 mg:n=7 Placebo n=3 | 24 weeks | Mean age 52±14; 85% of males rest not provided | Change in NAS was not statistically different with 4 mg (-1.9±1.57, p=0.60) when compared with saroglitazar 2 mg group (-1.5±0.84, p=0.77) and placebo (-1.3±0.58). Significant improvement in ballooning from 1.2±0.41 to 0.3±0.52 at week 24 with saroglitazar 2 mg and from 1.3±0.49 to 0.4±0.53 with saroglitazar 4 mg. Significant reductions in TG, TC, sd-LDL-C, and LDL-C | N=2 Not related to drug |
| Gawrieh et al. 2021 (Multicentric, USA) [6] | Prospective | Double-blind randomized | NAFLD established either by imaging (ultrasound, CT, or MRI) or liver biopsy showing NASH or simple steatosis and ALT ≥ 50 U/L | Saroglitazar 1 mg, n=26 group, saroglitazar 2 mg, n=25 group, saroglitazar 4 mg, n=27 group, and placebo n=28 | 16 weeks | The mean % ↓ ALT at week 16 was -45.8% (5.7) with saroglitazar 4 mg versus 3.4% with placebo. Significant ↓ in LFC [4.1%), HOMA-IR (-1.3), TG (-5.3 mg/dL) (p<0.05 for all). A mean weight gain of 1.5 kg was observed with saroglitazar 4 mg versus 0.3 kg with placebo (p>0.05). | Diarrhea n=3 cough n=3 Abdominal pain n=2 Bronchitis n=2 | |
| Mitra et al. 2020 (India) [25] | Prospective | Single | T2DM and NAFLD documented by ultrasonography of the abdomen | N=30 | 24 weeks | 11 patients had fibrosis F3 grade (9.5-12.4 kPa), and 19 patients had a fibrosis F4 grade (≥12.5 kPa) | At the six-month changes were noted as (HbA1c) ↓ (8.14 ± 0.52% to 7.74 ± 0.53%) TG ↓ (179.4 ± 38.33 mg/dL to 112.33 ± 26.82 mg/dL) LSM↓ (13.933 ± 2.87 kPa to 8.503 ± 1.86 kPa ) P<0.05 for all | None |
| Chaudhuri et al. 2023 (India) [26] | Prospective | Single | Patients with NAFLD with elevated ALT levels along with liver stiffness value ≥6 kPa and/or liver steatosis CAP >290 dB/m | N=63 | 2-point follow-up analysis at 24 and 52 weeks | Mean age=49.1(±11.09), Mean BMI 27.2(±4,1), 46% DM, 27% dyslipedemic, mean LSM 8.5±3.9, mean CAP 320(±46) 11 patients had compensated cirrhosis | Significant ↓in LSM baseline: 11.03±7.19 kPa 24-week (9.29±6.39 kPa), 52-week 8.59±6.35 kPa. Significant ↓ in CAP, ALT, AST, HbA1c, LDL, TC, and TG levels | Pruritis in 1 Increase stool frequency in 1 |
| Siddiqui et al. 2023 (Multicentric) [27] | Prospective | Pooled data analysis from multicentric phase II/III trials (USA, India, and Mexico) | Histologically proven NASH NAFLD or confirmed on the basis of imaging (ultrasound, computed tomography scan, or magnetic resonance imaging) | N=221 Saroglitazar 130 Placebo 91 | 16-24 weeks | Mean age=47.9±10.6 years, 56.1% females, mean BMI 30.9 ±5.3kg/m2 (36), 2% hypertensive, 32.6% DM, 21.7% on statins | Significant improvement in lipid parameters TC (–17 mg/dL, 95% CI, –24 to 9), TG (–45 mg/dL, 95% CI, –60 to 31), LDL (–8 mg/dL, 95% CI, –15 to –1), VLDL-C(–8 mg/dL, –14 to –3), and c sdLDL-C (–10 mg/dL, –17 to –2) | NA |
| Siddiqui et al. 2023 (USA) [28] | Prospective | Phase 2, single-arm study | Post liver transplant NAFLD NAFLD defined as CAP ≥264 dB/m. Primary endpoint: Liver fat reduction on MRI-PDFF | N=15 | 24 weeks | Mean age=58±12 years, mean BMI 37.4±7.4 kg/m2, DM: 26%, dyslipedemia 26%, hypertension 93% | Significant ↓ in MRI-PDFF (10.3±10.5% at baseline to 8.1±7.6%). A relative 30% reduction from the baseline MRI-PDFF value was noted in 47% of patients. Reduction ALP emerged as a predictor of PDFF response | Fluctuations in eGFR in two patients, one splanchnic vein thrombosis not related to drug |
| Hajare et al. 2019 (India) [29] | Prospective | Single arm | NAFLD and dyslipidemia with or without type 2 diabetes mellitus | N=52 | 52 weeks | Mean age=45.88±11.66 years, 71.15% males, 28.8% DM, 17.3% hypertensive, mean BMI 27.84±5.97 kg/m2 | Significant ↓ALT (p<0.001), AST, (p<0.001), TG (p<0.001), LSM ↓12.33±9.99 to 9.62±4.53 9, p=0.01) | NA |
Subgroup meta-analysis
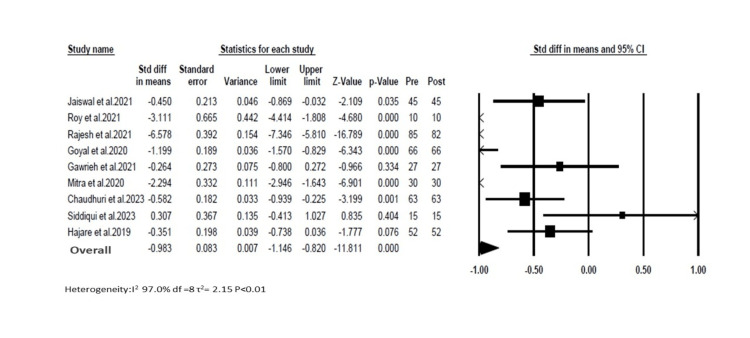
We conducted a subgroup meta-analysis (random effect) on studies that reported changes in LSM pre- and post-therapy with saroglitazar. Ten studies reported changes in LSM. We excluded the study by Padole et al. [20] as it did not provide overall LSM changes and subdivided based on weight changes [18]. We observed high heterogeneity among the studies (I2=97%). The overall pooled estimate for standard differences in means of LSM reduction was -0.98 (95% CI=1.1 to -0.8; Figure 3).
Figure 3. Showing the forest plot for random effects meta-analysis for liver stiffness measurement changes with saroglitazar in clinical studies.
Discussion
Despite the epidemic proportions of NAFLD and consequent health implications, there remains a paucity of clinically and histologically effective drugs in the management of NAFLD [3]. Saroglitazar, based on its mechanism of action, is an effective molecule targeting key pathophysiological pathways and has a concomitant effect on atherogenic dyslipidemia. Our current review, transitioning from pre-clinical studies to real-world studies and randomized trials, indicates the effectiveness of saroglitazar on biochemical parameters, fat fraction reduction, and improvements in liver stiffness and histology. However, there remains a paucity of large placebo-controlled trials based on histology or its predicted surrogate markers such as MRi-PDFF fat fraction reduction [31].
A recent systematic review of saroglitazar, including 10 studies, showed overall pooled reductions in ALT, AST, glycated hemoglobin, total cholesterol, and triglyceride [32]. LSM changes in eight studies reported similar results, as shown by our analysis corroborating reductions in LSM, albeit with significant heterogeneity (99%). However, it needs to be borne in mind that most of the studies are single-center, single-arm studies originating out of India, and, hence, it mandates more extensive randomized trials from multiple centers.
The beneficial effects of saroglitazar potentially transcend beyond hepatic parameters as the drug has been used for a substantial period in atherogenic dyslipidemia. This is further reflected in the pooled analysis of NAFLD patients from three multicentric studies showing significant beneficial effects on lipid biomarkers and indicating cardiovascular benefits [27]. Safety has been an important point of concern for any drugs in the pipeline for NAFLD. Based on data from pre-clinical and clinical studies, the molecule appears to be safe and extremely well-tolerated. Of interest is the limited proportion of patients with compensated cirrhosis in two studies wherein no safety concerns were reported [25-26].
Our review has key strengths, the most important of which is for the first time we have collated evidence from both pre-clinical and clinical studies. We assessed, in a subgroup meta-analysis, the effect of the dug on LSM, which is one of the key factors that determine drug efficacy and serves as an excellent surrogate of fibrosis. The review has certain limitations, of which an important aspect is we did not analyze other markers such as ALT, fat fraction reduction, or change in metabolic parameters.
Conclusions
In this review, we summarize for the first time the overall evidence of saroglitazar in NAFLD spanning across pharmacology, clinical, and pre-clinical studies. Saroglitazar, as a molecule, is safe in patients with NAFLD and, based on currently available literature, shows improvements in transaminase levels, metabolic parameters, liver fat content, and liver stiffness. However, larger multicentric biopsy-proven or adequate surrogates of biopsy-based studies are needed to promote global acceptance and approval.
The authors have declared that no competing interests exist.
Author Contributions
Concept and design: Akash Roy, Suprabhat Giri, Bikram Tewari, Mahesh Goenka
Acquisition, analysis, or interpretation of data: Akash Roy, Suprabhat Giri, Bikram Tewari, Mahesh Goenka
Drafting of the manuscript: Akash Roy, Suprabhat Giri, Bikram Tewari, Mahesh Goenka
Critical review of the manuscript for important intellectual content: Akash Roy, Suprabhat Giri, Bikram Tewari, Mahesh Goenka
Supervision: Mahesh Goenka
References
- 1.Preparing for the NASH epidemic: a call to action. Kanwal F, Shubrook JH, Younossi Z, et al. Metabolism. 2021;122:154822. doi: 10.1016/j.metabol.2021.154822. [DOI] [PubMed] [Google Scholar]
- 2.The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Chalasani N, Younossi Z, Lavine JE, et al. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 3.Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Younossi Z, Tacke F, Arrese M, et al. Hepatology. 2019;69:2672–2682. doi: 10.1002/hep.30251. [DOI] [PubMed] [Google Scholar]
- 4.Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. Sanyal AJ, Chalasani N, Kowdley KV, et al. https://doi.org/10.1056/NEJMOA0907929/SUPPL_FILE/NEJMOA0907929_DISCLOSURES.PDF. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The first approved agent in the Glitazar’s class: saroglitazar. Agrawal R. Curr Drug Target. 2014;15:151–155. doi: 10.2174/13894501113149990199. [DOI] [PubMed] [Google Scholar]
- 6.Saroglitazar, a PPAR-α/γ agonist, for treatment of NAFLD: a randomized controlled double-blind Phase 2 trial. Gawrieh S, Noureddin M, Loo N, et al. Hepatology. 2021;74:1809–1824. doi: 10.1002/hep.31843. [DOI] [PubMed] [Google Scholar]
- 7.A phase 2 double blinded, randomized controlled trial of Saroglitazar in patients with nonalcoholic steatohepatitis. Siddiqui MS, Idowu MO, Parmar D, et al. Clin Gastroenterol Hepatol. 2021;19:2670–2672. doi: 10.1016/j.cgh.2020.10.051. [DOI] [PubMed] [Google Scholar]
- 8.Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. Green BN, Johnson CD, Adams A. J Chiropr Med. 2006;5:101–117. doi: 10.1016/S0899-3467(07)60142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nonalcoholic steatohepatitis: the role of peroxisome proliferator-activated receptors. Francque S, Szabo G, Abdelmalek MF, et al. Nat Rev Gastroenterol Hepatol. 2021;18:24–39. doi: 10.1038/s41575-020-00366-5. [DOI] [PubMed] [Google Scholar]
- 10.PPAR-targeted therapies in the treatment of non-alcoholic fatty liver disease in diabetic patients. Lange NF, Graf V, Caussy C, Dufour JF. Int J Mol Sci. 2022;23:4305. doi: 10.3390/ijms23084305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Differential responses of PPARalpha, PPARdelta, and PPARgamma reporter cell lines to selective PPAR synthetic ligands. Seimandi M, Lemaire G, Pillon A, et al. Anal Biochem. 2005;344:8–15. doi: 10.1016/j.ab.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Effect of food on the pharmacokinetics of saroglitazar magnesium, a novel dual pparαγ agonist, in healthy adult subjects. Patel MR, Kansagra KA, Parikh DP, et al. Clin Drug Investig. 2018;38:57–65. doi: 10.1007/s40261-017-0584-2. [DOI] [PubMed] [Google Scholar]
- 13.New dual peroxisome proliferator activated receptor agonist-Saroglitazar in diabetic dyslipidemia and non-alcoholic fatty liver disease: integrated analysis of the real world evidence. Kaul U, Parmar D, Manjunath K, Shah M, Parmar K, Patil KP, Jaiswal A. Cardiovasc Diabetol. 2019;18:80. doi: 10.1186/s12933-019-0884-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saroglitazar improved hepatic steatosis and fibrosis by modulating inflammatory cytokines and adiponectin in an animal model of non-alcoholic steatohepatitis. Akbari R, Behdarvand T, Afarin R, Yaghooti H, Jalali MT, Mohammadtaghvaei N. BMC Pharmacol Toxicol. 2021;22:53. doi: 10.1186/s40360-021-00524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saroglitazar deactivates the hepatic LPS/TLR4 signaling pathway and ameliorates adipocyte dysfunction in rats with high-fat emulsion/LPs model-induced non-alcoholic steatohepatitis. Hassan NF, Nada SA, Hassan A, El-Ansary MR, Al-Shorbagy MY, Abdelsalam RM. Inflammation. 2019;42:1056–1070. doi: 10.1007/s10753-019-00967-6. [DOI] [PubMed] [Google Scholar]
- 16.The PPAR α/γ agonist saroglitazar improves insulin Resistance and steatohepatitis in a diet induced animal model of nonalcoholic fatty liver disease. Kumar DP, Caffrey R, Marioneaux J, et al. Sci Rep. 2020;10:9330. doi: 10.1038/s41598-020-66458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saroglitazar and Hepano treatment offers protection against high fat high fructose diet induced obesity, insulin resistance and steatosis by modulating various class of hepatic and circulating lipids. Sarkar S, Kumari D, Gupta SK, et al. Biomed Pharmacother. 2021;144:112357. doi: 10.1016/j.biopha.2021.112357. [DOI] [PubMed] [Google Scholar]
- 18.Dual PPARα/γ agonist saroglitazar improves liver histopathology and biochemistry in experimental NASH models. Jain MR, Giri SR, Bhoi B, et al. Liver Int. 2018;38:1084–1094. doi: 10.1111/liv.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saroglitazar suppresses the hepatocellular carcinoma induced by intraperitoneal injection of diethylnitrosamine in C57BL/6 mice fed on choline deficient, l-amino acid- defined, high-fat diet. Giri SR, Bhoi B, Trivedi C, et al. BMC Cancer. 2023;23:59. doi: 10.1186/s12885-023-10530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saroglitazar for nonalcoholic fatty liver disease: a single centre experience in 91 patients. Padole P, Arora A, Sharma P, Chand P, Verma N, Kumar A. J Clin Exp Hepatol. 2022;12:435–439. doi: 10.1016/j.jceh.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Role of Saroglitazar in non diabetic non alcoholic fatty liver disease patients: a retrospective observational study. Jaiswal A, Jain K, Singh AK. https://www.jcdr.net/articles/PDF/15738/52065_CE[Ra1]_F(SHU)_PF1(SC_KM)_PFA(SC_KM)_PN(KM).pdf J Clin Diagnosis Res. 2021;15:0–23. [Google Scholar]
- 22.Clinical case series of decrease in shear wave elastography values in ten diabetic dyslipidemia patients having NAFLD with Saroglitazar 4 mg: an Indian experience. Roy S. Case Rep Med. 2020;2020:4287075. doi: 10.1155/2020/4287075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Safety and efficacy of Saroglitazar in nonalcoholic fatty liver patients with diabetic dyslipidemia-a prospective, interventional, pilot study. Rajesh NA, Drishya L, Ambati MM, et al. J Clin Exp Hepatol. 2022;12:61–67. doi: 10.1016/j.jceh.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saroglitazar in patients with non-alcoholic fatty liver disease and diabetic dyslipidemia: a prospective, observational, real world study. Goyal O, Nohria S, Goyal P, Kaur J, Sharma S, Sood A, Chhina RS. Sci Rep. 2020;10:21117. doi: 10.1038/s41598-020-78342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An observational study of reduction in glycemic parameters and liver stiffness by Saroglitazar 4 mg in patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Mitra A. Cureus. 2020;12:0. doi: 10.7759/cureus.9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Efficacy and safety of saroglitazar in real-world patients of non-alcoholic fatty liver disease with or without diabetes including compensated cirrhosis: a tertiary care center experience. Chaudhuri S, Dutta A, Chakraborty SB. JGH Open. 2023;7:215–220. doi: 10.1002/jgh3.12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saroglitazar, a dual PPAR α/γ agonist, improves atherogenic dyslipidemia in patients with non-cirrhotic nonalcoholic fatty liver disease: a pooled analysis. Siddiqui MS, Parmar D, Sheikh F, et al. Clin Gastroenterol Hepatol. 2023;21:2597–2605. doi: 10.1016/j.cgh.2023.01.018. [DOI] [PubMed] [Google Scholar]
- 28.Saroglitazar improves nonalcoholic fatty liver disease and metabolic health in liver transplant recipients. Siddiqui MS, Parmar D, Shaikh F, et al. Liver Transpl. 2023;29:979–986. doi: 10.1097/LVT.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 29.An investigator initiated prospective, single arm observational study to evaluate the safety and efficacy of Saroglitazar 4 mg in patients with non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH) Hajare SD, Gokak VP, Ghorpade S, Patil A, Jadhav A. https://www.ijcmr.com/uploads/7/7/4/6/77464738/ijcmr_2873.pdf Int J Contemp Med Res. 2019;6 [Google Scholar]
- 30.Safety and efficacy of dual PPAR-alpha agonist, saroglitazar, in management of NAFLD with diabetes and/or dyslipidemia in liver transplant recipients. Dhampalwar S, Choudhary NS, Saraf N, Bhangui P, Rastogi A, Soin A. https://www.jcehepatology.com/article/S0973-6883(23)00326-2/fulltext J Clin Exp Hepatol. 2023;13:0–3. [Google Scholar]
- 31.Non-invasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Caussy C, Reeder SB, Sirlin CB, Loomba R. Hepatology. 2018;68:763–772. doi: 10.1002/hep.29797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Effects of saroglitazar in the treatment of non-alcoholic fatty liver disease or non-alcoholic steatohepatitis: a systematic review and meta-analysis. Bandyopadhyay S, Samajdar SS, Das S. Clin Res Hepatol Gastroenterol. 2023;47:102174. doi: 10.1016/j.clinre.2023.102174. [DOI] [PubMed] [Google Scholar]