Abstract
Background and aims
We analyzed the effects of age and sex on the relationship between muscle mass and serum creatinine levels in an apparently healthy population, including older adults.
Materials and methods
We retrospectively evaluated 1,502 individuals from the Korea National Health and Nutrition Examination Survey (KNHANES) and 4,586 individuals from the Health Check (HC) groups. We utilized data from the KNHANES and HC groups on serum creatinine levels and skeletal muscle mass index (SMI), determined using dual X-ray absorptiometry or bioelectric impedance analysis.
Results
A significant negative correlation between SMI and age was observed in both the KNHANES and HC groups in males but not in females. In males, serum creatinine levels showed a significant negative correlation with age in both the KNHANES (r = −0.522, P < 0.0001) and HC groups (r = −0.451, P < 0.0001). In females, there was no significant correlation between serum creatinine levels and age in the KNHANES (r = −0.016, P = 0.5985) and HC group (r = −0.011, P = 0.5618).
Conclusions
Serum creatinine levels decrease more significantly in older males than in older females due to sex-specific muscle mass decline.
Keywords: Creatinine, eGFR, Muscle mass, Older adults, Kidney function evaluation, Sex-specific
Highlights
-
•
Muscle mass declines with age, influenced by both age and sex.
-
•
Serum creatinine levels decrease more in elderly male due to sex-specific muscle decline.
-
•
eGFR assessment needs to consider sex disparities of muscle decline in older adults.
1. Introduction
Glomerular filtration rate (GFR) is a widely used modality for assessing kidney function. However, the direct measurement of GFR using exogenous markers is difficult. Consequently, clinical practice predominantly relies on the estimated GFR (eGFR), employing serum creatinine levels as the primary parameter. Approximately 98 % of total body creatinine is pooled within the muscles [1], and a proportion of free creatine in muscle spontaneously and fairly constantly converts into its anhydride waste product, creatinine [2]. It is a classic unresolved issue that the dependence of creatinine on muscle mass often makes kidney function evaluation difficult in individuals with below-average muscle mass, such as children, older adults, or those with muscle wasting diseases [3,4].
Sarcopenia refers to the involuntary degeneration of skeletal muscle mass and strength attributed to the aging process [5]. Muscle mass reportedly declines by approximately 3–8% every 10 years after the age of 30 years, with an acceleration of 2–3% per year, showing a higher rate of decline after the age of 60 years [[6], [7], [8]]. Evidence suggests that skeletal muscle mass and strength decline linearly from around the age of 40 years and can result in a maximum mass loss of 50 % by the age of 80 years [8]. Consequently, sarcopenia often results in serious health complications in older adults.
As serum levels of creatinine relies on muscle mass, sarcopenia may affect kidney function measurements. In other words, creatinine is a byproduct of muscle metabolism, and eGFR is derived from creatinine levels based mostly on the non-aged populations [9,10]. Therefore, if an individual's muscle mass deviates from the standard range, eGFR may not accurately reflect the actual kidney function. Given the variability in muscle mass based on factors such as age, sex, protein intake, physical fitness level, and muscle wasting diseases, serum creatinine concentrations can exhibit disparities among individuals with the same level of kidney function [4,11]. Sex, age, and race have been proxy parameters of muscle mass in estimating GFR. However, recent studies have suggested that GFR could be accurately estimated by new race-free equations based on creatinine and cystatin C. This shift is supported by the understanding that race is a social construct rather than biological one, and its incorporation overlooks the diversity within and among racial groups [9,10]. Nevertheless, these parameters aim to compensate for most of the interindividual variability related to muscle mass. However, eGFR calculations based on creatinine levels are prone to bias, as they have the propensity to overestimate GFR in accordance with the decline in muscle mass that accompanies the aging process [4,12]. This bias is particularly accentuated in older adults with progressive muscle wasting, which is characteristic of chronic disorders. This inherent limitation could lead to erroneous classification of kidney function, consequently impeding the optimal management of individuals.
In this study, the relationships among muscle mass, serum creatinine, age, and sex were analyzed using data from the Korea National Health and Nutrition Examination Survey (KNHANES) as well as Health Check (HC) records obtained from a university hospital. Consequently, we aimed to establish a comprehensive understanding of the impact of age and sex on the relationship between muscle mass and serum creatinine levels in an apparently healthy population.
2. Materials and methods
2.1. Study design and participants
We utilized data (total 1,956 participants; 809 males and 1,147 females) from the 2011 KNHANES data, in which a standardized Jaffe kinetic serum creatinine assay traceable to isotope dilution mass spectrometry (IDMS) with two-digit decimal reporting and skeletal muscle mass index (SMI) using dual-energy X-ray absorptiometry (DXA) was used. KNHANES is an annual nationwide cross-sectional survey conducted by the Korea Disease Control and Prevention Agency (KDCA) [13]. The survey employs a rolling sampling design involving a complex, stratified, multistage, probability cluster survey of a representative sample of non-institutionalized Korean citizens residing in South Korea. The study protocol (2011-02CON-06-C) was approved by the KDCA Ethics Committee, and written informed consent was obtained from all participants or their parents.
We also employed a standardized enzymatic serum creatinine assay with Cobas c702 (Roche Diagnostics, Mannheim, Germany) traceable to IDMS and SMI using BC 720 (Accuniq, SELVAS, Daejeon, South Korea) and multi-frequency bioelectrical impedance analysis (BIA) to evaluate individuals who visited the health promotion center of Yongin Severance Hospital from March 2020 to November 2021 (n = 6,572; 3459 males and 3,113 females), referred to as the HC group. SMI was calculated as the sum of the appendicular muscle mass of the four limbs divided by the height squared in either DXA or BIA. Individuals <30 years of age, high body mass index (≥30 kg/m2) or decreased kidney function whose eGFR was <90 mL/min/1.73 m2 based on serum creatinine levels according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [2,9] were excluded from the study. We employed the CKD-EPI (2009) equation, which had previously been validated as suitable for Korean population as demonstrated by two studies comparing it with measured GFR using 51Cr-EDTA [14,15]. Specifically, we utilized the CKD-EPI (2009) Non-Black equation, as all participants in our study were of Korean ethnicity. After exclusion, we finally evaluated 1,502 participants (456 males and 1,046 females) in the KNHANES group and 4,586 participants (1,967 males and 2,619 females) in the HC group. The requirement for informed consent was waived due to the retrospective nature of the study. This study was approved by the Institutional Review Board of the Yongin Severance Hospital, South Korea (IRB No. 9-2021-0095).
2.2. Statistical analysis
The Pearson correlation coefficient (r) was used to determine the correlation between parameters. Through multiple regression analysis, we formulated a model to predict actual serum creatinine levels using SMI, age, and sex. Statistical analysis was performed using Analyse-it version 6.15.4 for Microsoft Excel (Analyse-it Software Ltd., Leeds, UK), and the level of significance was defined as P < 0.05.
3. Results
3.1. Correlation between serum creatinine and SMI
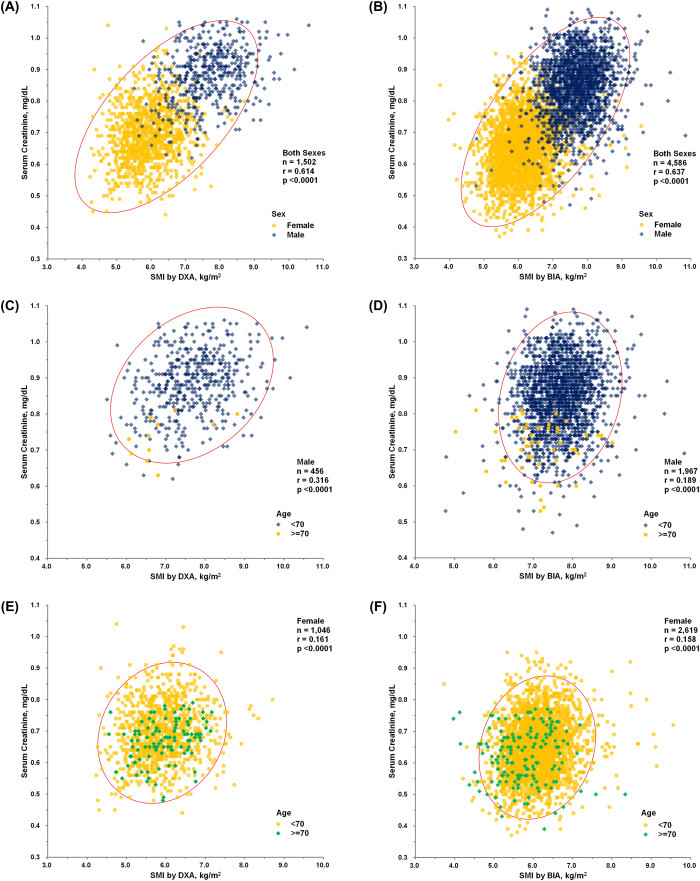
We found a significant correlation between serum creatinine levels and SMI (kg/m2) for both sexes in both KNHANES (n = 1,502, r = 0.614, P < 0.0001) and HC groups (n = 4,586, r = 0.637, P < 0.0001), as determined using Pearson's correlation analysis (Fig. 1A and B). Serum creatinine levels (mg/dL) were also significantly correlated with SMI (kg/m2) in both sexes. In males, a correlation was observed between the KNHANES (n = 456, r = 0.316, P < 0.0001) and HC groups (n = 1,967, r = 0.189, P < 0.0001) (Fig. 1C and D). In females, a correlation was observed between the KNHANES (n = 1,046, r = 0.161, P < 0.0001) and HC groups (n = 2,619, r = 0.158, P < 0.0001) (Fig. 1E and F).
Fig. 1.
(A) Results of Pearson's correlation analysis between serum creatinine level (mg/dL) and SMI using DXA (kg/m2) in participants of both sexes (n = 1,502, r = 0.614, P < 0.0001) of the KNHANES group. (B) Results of Pearson's correlation analysis between serum creatinine level (mg/dL) and SMI using BIA (kg/m2) in participants of both sexes (n = 4,586, r = 0.637, P < 0.0001) of the HC group. (C) Results of Pearson's correlation analysis between serum creatinine level (mg/dL) and SMI using DXA (kg/m2) in KNHANES group males (n = 456, r = 0.316, P < 0.0001). (D) Results of Pearson's correlation analysis between serum creatinine level (mg/dL) and SMI using BIA (kg/m2) in HC group males (n = 1,967, r = 0.189, P < 0.0001). (E) Results of Pearson's correlation analysis between serum creatinine level (mg/dL) and SMI using DXA (kg/m2) in KNHANES group females (n = 1,046, r = 0.161, P < 0.0001). (F) Results of Pearson's correlation analysis between serum creatinine level (mg/dL) and SMI using BIA (kg/m2) in HC group females (n = 2,619, r = 0.158, P < 0.0001).
*Abbreviations: SMI, skeletal muscle mass index as the sum of appendicular muscle mass of the four limbs, adjusted with height squared; DXA, dual-energy X-ray absorptiometry; BIA, bioelectrical impedance analysis; KNHANES, Korea National Health and Nutrition Examination Survey; HC, Health Check.
3.2. Correlation between age and SMI
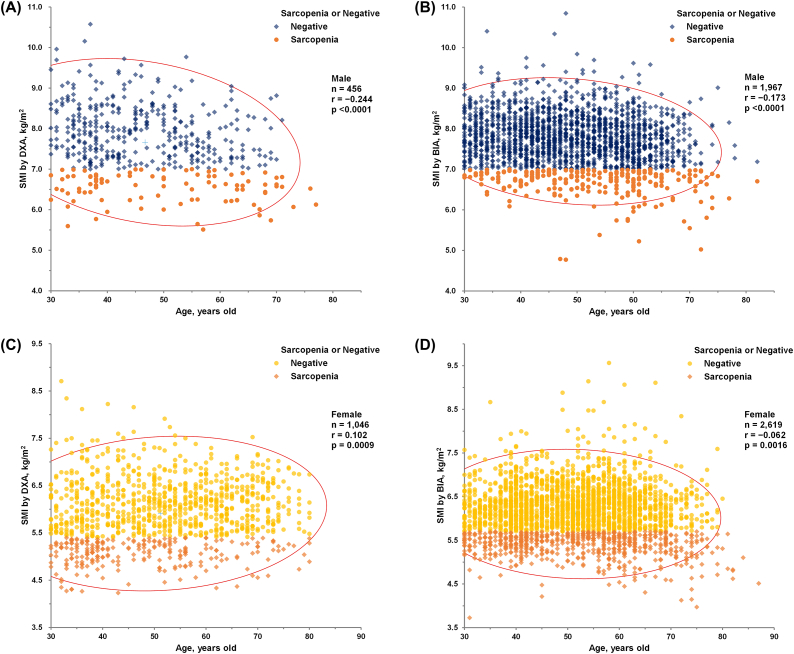
In both the KNHANES (n = 456, r = −0.244, P < 0.0001) and HC groups (n = 1,967, r = −0.173, P < 0.0001), male exhibited a significant negative correlation between SMI (kg/m2) and age, as determined using Pearson's correlation analysis (Fig. 2A and B).
Fig. 2.
(A) (A) Results of Pearson's correlation analysis between SMI using DXA (kg/m2) and age (years) in KNHANES group males (n = 456, r = -0.244, P < 0.0001). (B) Results of Pearson's correlation analysis between SMI using BIA (kg/m2) and age (years) in HC group males (n = 1,967, r = -0.173, P < 0.0001). (C) Results of Pearson’s correlation analysis between SMI using DXA (kg/m2) and age (years) in KNHANES group females (n = 1,046, r = 0.102, P = 0.0009). (D) Results of Pearson's correlation analysis between SMI using BIA (kg/m2) and age (years) in HC group females (n = 2,619, r = -0.062, P = 0.0016). Participants with sarcopenia are represented by orange symbols from (A) to (D) according to the cutoff values (<7.0 kg/m2 in men and <5.4 kg/m2 in women by DXA, and <7.0 kg/m2 in men and <5.7 kg/m2 in women by BIA) suggested by the AWGS 2019 [5]. Conversely, dark blue or yellow symbols represent negative (non-sarcopenic) subjects by the same cutoff.
In contrast, SMI measured using DXA (kg/m2) showed a weak positive correlation with age in KNHANES group females (n = 1,046, r = 0.102, P = 0.0009, Fig. 2C), which differs from the negative correlation in males. Additionally, SMI measured using BIA (kg/m2) exhibited a weaker negative correlation with age in females from the HC group (n = 2,619, r = −0.062, P = 0.0016, Fig. 2D) compared to males as determined by Pearson's correlation analysis.
3.3. Correlation between age and serum creatinine
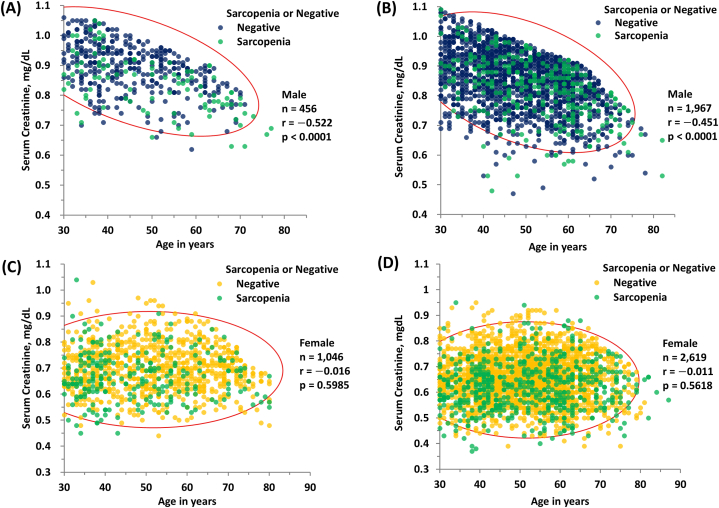
Serum creatinine levels showed a significant negative correlation with age in males, in both KNHANES (n = 456, r = −0.522, P < 0.0001) and HC groups (n = 1,967, r = −0.451, P < 0.0001), as determined using Pearson's correlation analysis (Fig. 3A and B). However, no significant correlation between serum creatinine levels and age was observed in females, in both KNHANES (n = 1,046, r = −0.016, P = 0.5985) and HC groups (n = 2,619, r = −0.011, P = 0.5618), as determined using Pearson's correlation analysis (Fig. 3C, D).
Fig. 3.
(A) Results of Pearson's correlation analysis between serum creatinine (mg/dL) and age (years) in KNHANES group males (n = 456, r = 522, P < 0.0001). (B) Results of Pearson's correlation analysis between serum creatinine (mg/dL) vs. age (years) in HC group males (n = 1,967, r = 0.451, P < 0.0001). (C) Results of Pearson's correlation analysis between serum creatinine (mg/dL) and age (years) in KNHANES group females (n = 1,046, r = 0.016, P = 0.5985). (D) Results of Pearson's correlation analysis between serum creatinine (mg/dL) and age (years) in HC group females (n = 2,619, r = 0.011, P = 0.5618). Participants with sarcopenia are represented by orange symbols from (A) to (D) according to the cutoff values (<7.0 kg/m2 in men and <5.4 kg/m2 in women by DXA, and <7.0 kg/m2 in men and <5.7 kg/m2 in women by BIA) suggested by the AWGS 2019 [5]. Conversely, dark blue or yellow symbols represent negative (non-sarcopenic) subjects by the same cutoff.
*Abbreviations: KNHANES, Korea National Health and Nutrition Examination Survey; HC, Health Check; AWGS 2019, Asian Working Group for Sarcopenia consensus update in 2019.
3.4. Prediction model for creatinine
Serum creatinine levels was predicted using the following equation using multiple regression analysis with an adjusted R2 = 0.506, suggesting that 50.6 % of the variation in serum creatinine level could be predicted according to sex, SMI using DXA, and age in the KNHANES group.
Serum creatinine (mg/dL) = 0.5933 + 0.02637*SMI using DXA + 0.1343*Male Sex – 0.001065*Age
Serum creatinine levels was predicted using the following equation using multiple regression analysis with an adjusted R2 = 0.545, suggesting that 54.5 % of the variation in serum creatinine level could be predicted according to sex, SMI using BIA, and age in the HC group.
Serum creatinine (mg/dL) = 0.5855 + 0.02315*SMI using BIA + 0.1568*Male Sex – 0.001521*Age
4. Discussion
It is well known that muscle mass gradually declines with age [8,16,17]. However, the rate of muscle mass loss is influenced not only by age, but also sex. The high muscle mass in young males and the steep decline in estimated muscle mass in older males are attributed to the decrease in testosterone with its anabolic effects and protein synthesis [[18], [19], [20]]. However, these age-related effects are relatively absent in females who are less affected by testosterone, as our study demonstrates that muscle mass in older females either remains relatively stable or even increases (Fig. 3A, B, C, D), regardless of KNHANES or HC groups.
Sex-specific differences in muscle mass decline contribute to a greater reduction in serum creatinine levels among older males. Despite the increasing representation of older adult individuals in the general population, the aspect of sex-related variations in age-related creatinine decline has unfortunately received insufficient attention. Serum creatinine holds a pivotal role as a marker for estimating kidney function and serves as a cornerstone in calculating the estimate eGFR, a parameter inherently tied to muscle mass. Thus, in turn, influences the classification of chronic kidney disease [[21], [22], [23]].
The existing equations for eGFR in relation to serum creatinine level were predominantly developed from younger populations, where most participants were under 65 years of age [21]. Although, these equations have been employed in large-scale studies involving over 1,000 participants aged 65 years and older [9,24], and have included age coefficients, which is uniformly applied across both genders, to account for the lower serum creatinine levels observed in older populations with comparable measured GFR [24], they may not adequately address the sex-specific differences in serum creatinine levels among older individuals. The significant decline in muscle mass in older males, contrasted with the relatively stable (or even increasing) muscle mass in older females, should be taken into account when calculating eGFR. This potential disparity underscores the possibility of incorrect kidney function classification and subsequent misapplication of clinical intervention, especially in elderly women. It might necessitate a distinct application of age-related parameters in elderly women when compared to males.
Currently, it is recommended to use both creatinine and cystatin C as markers to estimate GFR rather than creatinine alone [[25], [26], [27]]. However, creatinine remains the most commonly used indicator, whereas cystatin C has limitations in individuals with diabetes, obesity, inflammation, thyroid dysfunction, and smoking history [28]. There remain many factors that must be assessed for accurate eGFR calculation, such as the inclusion of larger numbers of older adults. In addition, the age-related decline of kidney function with age is well-known, although it remains unclear whether it is an age-associated disease or a part of normal aging process. Age-related muscle mass decline could potentially mask the age-related kidney function decline [29,30]. In a previous report, it was noted that age-related muscle mass decline might obscure the age-related kidney function decline. This was demonstrated by a steeper decline of eGFR using cystatin C in comparison to eGFR using creatinine by either MDRD, or CKD-EPI equation, in the elderly population aged over 80 years [31]. Furthermore, sex-related differences in muscle mass decline in older adults would be another important consideration for precise eGFR calculation.
This study had several limitations. Firstly, there could be inherent variability when attempting to establish a relationship among muscle mass, serum creatinine levels, and age, using eGFR calculated by the CKD-EPI formula based on serum creatinine levels, as opposed to using directly measured GFR to exclude cases of kidney dysfunction. Secondly, it's worth noting that this study is based on a limited population of Korean ethnicity, and as such, its finding may not be definitely extrapolated to other populations. Thirdly, due to the cross-sectional and retrospective nature of this study, we could not track prospective decline of muscle mass and serum creatinine levels in each individual as they aged.
In conclusion, sex differences in muscle mass decline in older adult populations are important considerations for the accurate calculation of eGFR. The significant decline in muscle mass in older males and the relatively stable (or even increasing) muscle mass in older females highlight the necessity of reevaluating distinct age coefficients in eGFR calculations for different sexes, including those aged >65 years. Considering the current trend of increasingly aging populations and the distribution of age groups utilizing healthcare facilities, it is necessary to dedicate further attention and research to ensure the accurate calculation of eGFR in older populations. Sex disparities in muscle mass decline among older populations should be recognized as another crucial factor in achieving an accurate eGFR assessment.
Funding sources
This study was supported by the Sejin Joint Research Grant from the Department of Laboratory Medicine, Yonsei University College of Medicine, Republic of Korea.
Data availability
The authors do not have permission to share data.
CRediT authorship contribution statement
Jisook Yim: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. Nak-Hoon Son: Conceptualization, Investigation. Taeyoung Kyong: Conceptualization, Investigation. Yongjung Park: Investigation, Visualization, Writing – original draft, Writing – review & editing. Jeong-Ho Kim: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful for generous help by Ms. Young-Kyoung Woo in Health-Check Center in Yongin Severance Hospital.
References
- 1.Heymsfield S.B., Arteaga C., McManus C., Smith J., Moffitt S. Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am. J. Clin. Nutr. 1983;37(3):478–494. doi: 10.1093/ajcn/37.3.478. [DOI] [PubMed] [Google Scholar]
- 2.Lamb E.J., Jones G.R.D. In: Tietz Textbook of Laboratory Medicine. seventh ed. Rifai N., Chiu R.W.K., Young I., Burnham C.-A., Wittwer C.T., editors. Elsevier; 2023. Kidney function test; p. 352.e360. [Google Scholar]
- 3.Groothof D., Post A., Polinder-Bos H.A., Erler N.S., Flores-Guerrero J.L., Kootstra-Ros J.E., Pol R.A., de Borst M.H., Gansevoort R.T., Gans R.O.B., Kremer D., Kieneker L.M., Bano A., Muka T., Franco O.H., Bakker S.J.L. Muscle mass and estimates of renal function: a longitudinal cohort study. J Cachexia Sarcopenia Muscle. 2022;13(4):2031–2043. doi: 10.1002/jcsm.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nankivell B.J., Nankivell L.F.J., Elder G.J., Gruenewald S.M. How unmeasured muscle mass affects estimated GFR and diagnostic inaccuracy. EClinicalMedicine. 2020;29–30 doi: 10.1016/j.eclinm.2020.100662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L.K., Woo J., Assantachai P., Auyeung T.W., Chou M.Y., Iijima K., Jang H.C., Kang L., Kim M., Kim S., Kojima T., Kuzuya M., Lee J.S.W., Lee S.Y., Lee W.J., Lee Y., Liang C.K., Lim J.Y., Lim W.S., Peng L.N., Sugimoto K., Tanaka T., Won C.W., Yamada M., Zhang T., Akishita M., Arai H. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020;21(3):300–307 e302. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Volpi E., Nazemi R., Fujita S. Muscle tissue changes with aging. Curr. Opin. Clin. Nutr. Metab. Care. 2004;7(4):405–410. doi: 10.1097/01.mco.0000134362.76653.b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jang H.C. How to diagnose sarcopenia in Korean older adults? Ann Geriatr Med Res. 2018;22(2):73–79. doi: 10.4235/agmr.2018.22.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkinson D.J., Piasecki M., Atherton P.J. The age-related loss of skeletal muscle mass and function: measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 2018;47:123–132. doi: 10.1016/j.arr.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., 3rd, Feldman H.I., Kusek J.W., Eggers P., Van Lente F., Greene T., Coresh J., Ckd E.P.I. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inker L.A., Eneanya N.D., Coresh J., Tighiouart H., Wang D., Sang Y., Crews D.C., Doria A., Estrella M.M., Froissart M., Grams M.E., Greene T., Grubb A., Gudnason V., Gutierrez O.M., Kalil R., Karger A.B., Mauer M., Navis G., Nelson R.G., Poggio E.D., Rodby R., Rossing P., Rule A.D., Selvin E., Seegmiller J.C., Shlipak M.G., Torres V.E., Yang W., Ballew S.H., Couture S.J., Powe N.R., Levey A.S. C. Chronic kidney disease Epidemiology, new creatinine- and cystatin C-based equations to estimate GFR without race. N. Engl. J. Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaman T., Filipowicz R., Beddhu S. Implications and importance of skeletal muscle mass in estimating glomerular filtration rate at dialysis initiation. J. Ren. Nutr. 2013;23(3):233–236. doi: 10.1053/j.jrn.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iacomelli I., Giordano A., Rivasi G., Rafanelli M., Tortu V., Cartei A., Rostagno C., Di Bari M., Marchionni N., Mossello E., Ungar A. Low creatinine potentially overestimates glomerular filtration rate in older fracture patients: a plea for an extensive use of cystatin C? Eur. J. Intern. Med. 2021;84:74–79. doi: 10.1016/j.ejim.2020.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Korea Disease Control and Prevention Agency, Korea National Health and Nutrition Examination Survey (KNHANES). https://knhanes.kdca.go.kr/knhanes/eng/index.do (accessed 13 July 2023).
- 14.Jeong T.D., Lee W., Yun Y.M., Chun S., Song J., Min W.K. Development and validation of the Korean version of CKD-EPI equation to estimate glomerular filtration rate. Clin. Biochem. 2016;49(9):713–719. doi: 10.1016/j.clinbiochem.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Jeong T.D., Cho E.J., Lee W., Chun S., Hong K.S., Min W.K. Accuracy assessment of five equations used for estimating the glomerular filtration rate in Korean adults. Ann Lab Med. 2017;37(5):371–380. doi: 10.3343/alm.2017.37.5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson L.V. Age-related muscle dysfunction. Exp. Gerontol. 2009;44(1–2):106–111. doi: 10.1016/j.exger.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson A.S., Janssen I., Sui X., Church T.S., Blair S.N. Longitudinal changes in body composition associated with healthy ageing: men, aged 20-96 years. Br. J. Nutr. 2012;107(7):1085–1091. doi: 10.1017/S0007114511003886. [DOI] [PubMed] [Google Scholar]
- 18.Zirkin B.R., Tenover J.L. Aging and declining testosterone: past, present, and hopes for the future. J. Androl. 2012;33(6):1111–1118. doi: 10.2164/jandrol.112.017160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barone B., Napolitano L., Abate M., Cirillo L., Reccia P., Passaro F., Turco C., Morra S., Mastrangelo F., Scarpato A., Amicuzi U., Morgera V., Romano L., Calace F.P., Pandolfo S.D., De Luca L., Aveta A., Sicignano E., Trivellato M., Spena G., D'Alterio C., Fusco G.M., Vitale R., Arcaniolo D., Crocetto F. The role of testosterone in the elderly: what do we know? Int. J. Mol. Sci. 2022;23(7) doi: 10.3390/ijms23073535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snyder P.J., Peachey H., Berlin J.A., Hannoush P., Haddad G., Dlewati A., Santanna J., Loh L., Lenrow D.A., Holmes J.H., Kapoor S.C., Atkinson L.E., Strom B.L. Effects of testosterone replacement in hypogonadal men. J. Clin. Endocrinol. Metab. 2000;85(8):2670–2677. doi: 10.1210/jcem.85.8.6731. [DOI] [PubMed] [Google Scholar]
- 21.Carnevale V., Tinti M.G. Use of eGFR in older adults with kidney disease. N. Engl. J. Med. 2022;387(6):574–575. doi: 10.1056/NEJMc2208615. [DOI] [PubMed] [Google Scholar]
- 22.Carnevale V., Tinti M.G., Scillitani A., Nieddu L. Estimated glomerular filtration rate and muscle mass: their relationship in older inpatients. J. Am. Med. Dir. Assoc. 2019;20(11):1469–1471. doi: 10.1016/j.jamda.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Lee R.C., Wang Z., Heo M., Ross R., Janssen I., Heymsfield S.B. Total-body skeletal muscle mass: development and cross-validation of anthropometric prediction models. Am. J. Clin. Nutr. 2000;72(3):796–803. doi: 10.1093/ajcn/72.3.796. [DOI] [PubMed] [Google Scholar]
- 24.Levey A.S., Grams M.E., Inker L.A. Use of eGFR in older adults with kidney disease. Reply, N Engl J Med. 2022;387(6):575. doi: 10.1056/NEJMc2208615. [DOI] [PubMed] [Google Scholar]
- 25.Inker L.A., Schmid C.H., Tighiouart H., Eckfeldt J.H., Feldman H.I., Greene T., Kusek J.W., Manzi J., Van Lente F., Zhang Y.L., Coresh J., Levey A.S., Investigators C.-E. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shlipak M.G., Matsushita K., Arnlov J., Inker L.A., Katz R., Polkinghorne K.R., Rothenbacher D., Sarnak M.J., Astor B.C., Coresh J., Levey A.S., Gansevoort R.T., Consortium C.K.D.P. Cystatin C versus creatinine in determining risk based on kidney function. N. Engl. J. Med. 2013;369(10):932–943. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yim J., Son N.H., Kim K.M., Yoon D., Cho Y., Kyong T., Moon J.Y., Yi T.I., Lee S.G., Park Y., Lee J.J., Kim K.A., Lee J.E., Kim J.H. Establishment of muscle mass-based indications for the cystatin C test in renal function evaluation. Front. Med. 2022;9 doi: 10.3389/fmed.2022.1021936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shlipak M.G., Mattes M.D., Peralta C.A. Update on cystatin C: incorporation into clinical practice. Am. J. Kidney Dis. 2013;62(3):595–603. doi: 10.1093/ajcn/37.3.478. 10.1053/j.ajkd.2013.03.027. [1] Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S. Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am J Clin Nutr 1983;37:478-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindeman R.D., Tobin J.D., Shock N.W. Association between blood pressure and the rate of decline in renal function with age. Kidney Int. 1984;26(6):861–868. doi: 10.1038/ki.1984.229. [DOI] [PubMed] [Google Scholar]
- 30.Braun F., Brinkkoetter P.T. Is the age-related decline in renal function an age-associated disease or part of a normal aging process? Gerontology. 2017;63(4):325–326. doi: 10.1159/000456616. [DOI] [PubMed] [Google Scholar]
- 31.Christensson A., Elmstahl S. Estimation of the age-dependent decline of glomerular filtration rate from formulas based on creatinine and cystatin C in the general elderly population. Nephron Clin. Pract. 2011;117(1):c40–c50. doi: 10.1159/000319646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.