Abstract
Background
To investigate the efficacy of an alternative negative pressure treatment for the treatment of enteroatmospheric fistula transformed from small intestinal leakage due to incision dehiscence after abdominal surgery.
Methods
Patients with an enteroatmospheric fistula from small intestinal leakage owing to incision dehiscence following abdominal surgery between January 2010 and December 2019 were retrospectively reviewed.
Results
A total of 83 patients (mean age: 38.3 ± 11.6 years; Body mass index: 19.9 ± 2.2 kg/m2) were enrolled. Of the 83 patients, 59 (71.1 %) achieved fistula closure. High-output fistula (Hazard ratio = 0.48; 95 % Confidence interval: 0.29–0.81; P = 0.006) and abdominal wall thickness >2 cm (Hazard ratio = 2.76; 95 % Confidence interval: 1.35–5.67; P = 0.006) were identified as factors affecting fistula closure. Lastly, 11/83 (13.3 %) patients exhibited re-dehiscence.
Conclusion
Appropriately applying the alternative negative pressure treatment may enable fistula closure in patients with enteroatmospheric fistula resulting from small intestinal leakage caused by incision dehiscence.
Keywords: Enteroatmospheric fistula, Outcomes, Treatment, Surgery, Infections
1. Introduction
Postoperative intestinal leakage can lead to organ/space surgical site infection (SSI) [1,2]. This type of SSI is characterized by high morbidity and mortality [3,4], possibly requiring additional surgery [5] to control the infection and even prevent death [6]. Moreover, intestinal leakage below the incision site may become exposed following incision dehiscence, creating a direct connection to the external environment and facilitating easier fluid drainage from the incision site. In such cases, the initial intestinal leakage develops into an enteroatmospheric fistula (EAF) [7]. EAFs are associated with risks such as septic abdomen, shock, malnutrition, and electrolyte disorder, leading to a mortality rate of 20%–44 % [8].
The management of EAF is a highly challenging situation for surgeons. In previous literature, EAF isolation from the exposed open abdomen wound followed by the creation of a stoma analog has been indicated as an optimal approach to reduce the associated mortality [9,10]. However, EAF cannot be closed spontaneously and must be resected with definitive surgery [11].
A double-lumen irrigation-suction tube (DLIST) is a negative pressure treatment device previously described in our studies [1,12,13]. DLIST has been proven effective in treating SSIs [1], controlling intestinal fistula infection [12], and promoting intestinal fistula closure [13]. Furthermore, the excellent irrigation ability of DLIST in controlling sources can be used to achieve closure of the separate fascia at the early stage of incision dehiscence. This feature of DLIST can potentially re-transform the EAF into an intestinal fistula that connects with the outside environment via the sinus, thereby increasing closure possibility. In this study, an alternative negative pressure treatment for EAF intervention is described, and its effectiveness is evaluated.
2. Methods
2.1. Study design
This retrospective study was performed at two tertiary hospitals managed by the same chief surgeon, with the same EAF treatment employed in the two hospitals. From January 2010 to December 2019, patients who underwent the alternative negative pressure treatment for EAF resulting from small intestinal leakage caused by incision dehiscence after abdominal surgery were included. Patients fulfilling any of the following exclusion criteria were excluded: (1) more than one fistula, (2) inflammatory bowel disease (IBD), and (3) incomplete data.
2.2. Outcomes
The primary outcome was fistula closure. Secondary outcomes included (1) incision healing, (2) changes in fistula output, and (3) abdominal hernia incidence within 1 year post-discharge.
2.3. Procedure of the alternative negative pressure treatment
Each patient underwent a computed tomography (CT) scan on admission, and bedside exploration of the incision was performed. Further, the purulent cavity under the split incision was evaluated. Next, gastroenterography was conducted, and the fistula location was determined. The surgeon then advised the placement of a DLIST beneath the incision. However, the patient was allowed to decide on receiving the treatment, and the intervention was administered after the patient provided signed informed consent. The patient lay supine during the procedure. After local anesthesia, iodophor disinfection and paving sterile sheet, the location of the fistula was explored and DLIST was placed near the fistula (distance <0.5 cm to fistula). Lastly, the incision fascia and skin was closed using continuous stitching.
A DLIST consists of irrigation and suction catheters and an end-closed supporting pipe riddled with 3-mm diameter holes about 1 cm apart (Fig. 1A). In this procedure, the closed end of the supporting pipe is placed near the fistula (distance <0.5 cm) in abdomen, while the suction catheter is inserted into the supporting pipe from the other opening end to supply the negative pressure. The diameter of the suction catheter is half that of the end-closed supporting pipe.
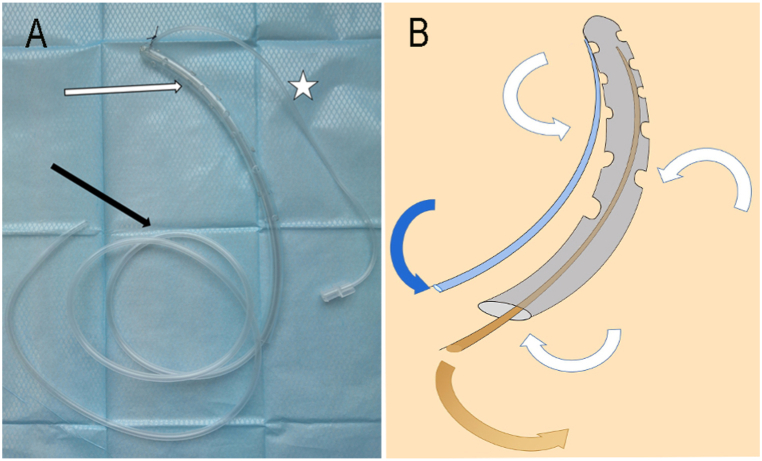
Fig. 1.
A. Double-lumen irrigation–suction tube consist of irrigation catheter (.), suction catheter (marked by black arrow), and end-closed supporting pipe which was riddled with holes with diameter of about 3 mm (the distance between each hole is about 1 cm, marked by white arrow).
B. The supporting pipe (Grey pipe), which is perforated with holes, is internally connected to the outside atmosphere. Consequently, when the continuous saline was irrigated through the irrigation catheter at 100–150 mL/h, along with drainage from the suction catheter (Brown tube) under continuous negative pressure of 110–150 mmHg, intestinal fluid (Show with brown arrows) is drained and the inside of the supporting pipe quickly fills with air (Show with white arrows) and saline (Show with blue arrows).
Fig. 1B shows the working diagram of the DLIST. The supporting pipe, which is perforated with holes, is internally connected to the outside atmosphere. Consequently, when the continuous saline was irrigated through the irrigation catheter at 100–150 mL/h, along with drainage from the suction catheter under continuous negative pressure of 110–150 mmHg, intestinal fluid is drained and the inside of the supporting pipe quickly fills with air and saline.
The alternative negative pressure treatment procedure in our study involved fascia closure and DLIST placement between the fascia and the intestine for continuous negative pressure drainage. As illustrated in Fig. 2 (A - D), the technique is performed before the development of a frozen abdomen. During this approach, the incision is explored, and the fistula is located. The fascia and skin are then stitched, and the DLIST is placed between the viscera and fascia (Fig. 2 [B - D]), while the DLIST end is placed near the fistula (distance <0.5 cm). Finally, the suture is removed at least 14 days later.
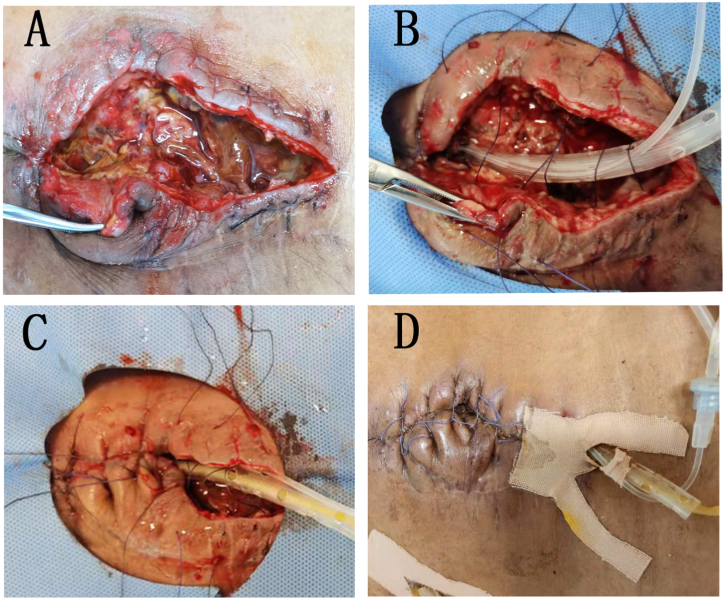
Fig. 2.
A. EAF resulting from small intestinal leakage (incision dehiscence area was about 10*4 cm), the fistula was marked with black arrow.
B.The DLIST was placed under the fascia layer, and the end of DLIST was placed near the fistula. C. The fascia and skin were closed. D. Three days after intervention, the acute inflammatory reaction around the incision subsided.
2.4. Nutrition strategy
Total parenteral nutrition (PN) was administered after DLIST placement. The basic energy supply was calculated according to formula (30 kcal/kg/day), along with adjustments based on fistula output and nutritional indicators (e.g., albumin and prealbumin levels, weight loss, and body mass index [BMI]). Additionally, somatostatin was used to reduce the output in patients with high-output fistula [13] after DLIST placement. In principle, after incision suturing, DLIST placement, and the contraction of sinus granulation, the fistula output gradually decreased and somatostatin was weaned off in most patients. Correspondingly, total EN was gradually resumed for patients who could be tapered off somatostatin within 4 weeks of PN.
However, patients with continued high-output fistula who could not be withdrawn from somatostatin 4 weeks after DLIST placement were administered chyme reinfusion (CR) using a Foley tube, which was inserted into the distal small intestine of the EAF under X-ray guidance [14]. Next, total EN was resumed with CR, and somatostatin was gradually ceased. In such cases, fistula closure was not expected, and a definitive surgery was planned.
2.5. Fistula monitoring
Before DLIST placement, gastroenterography was performed. The length from the ligament of Treitz to the fistula location was then estimated. After DLIST placement, careful monitoring was crucial, including daily examination of the incision and the properties of the flushing solution, along with biweekly CT and routine gastroenterography. Additionally, after 2–3 days with no detection of intestinal fluid in the DLIST, CT and gastroenterography were conducted to determine the diagnosis of fistula closure.
During treatment, gastroenterography was performed to confirm closure. Subsequently, the DLIST was removed, and the patients were discharged upon achieving closure. However, definitive surgery was planned for patients without closure even after at least 3 months of treatment. The criteria for this surgery included normal findings for at least 1 month, encompassing laboratory tests, BMI, and physical strength.
2.6. Definition
The following definitions were used in our study. First, SIRS (systemic inflammatory response syndrome) was established when two of the following criteria were met: 1. Body temperature >38 °C or <36 °C; 2. Heart rate >90 beats/min; 3. Breathing rate >20 times/min or pCO2 <32 mmHg; and 4. White blood cell count >12 × 109/L or <4 × 109/L. Second, a high-output fistula was defined according to the American Association for Parenteral and Enteral Nutrition guidelines [15]. Accordingly, high-output fistula was diagnosed when the output reached >500 mL/day, which can in turn increase the difficulty of infection source control, nutritional support, fistula skin care, and organ function maintenance [15]. Third, hernia was easily diagnosed in our study based on its clinical manifestations. Fourth, SSIs are infections directly resulting from surgical interventions and can be categorized as superficial incisional SSI, deep incisional SSI, and organ/space SSI [4]. Organ/space SSI was defined as clinical symptoms of intraperitoneal infection without evidence of intestinal leakage [4]. Lastly, sepsis was defined as a life-threatening organ dysfunction attributed to a dysregulated host response to infection. The clinical criteria for sepsis included suspected or documented infection and an acute increase of two or more Sequential Organ Failure Assessment points as a proxy for organ dysfunction [16].
2.7. Data collection and statistical analysis
Patient characteristics were collected from their clinical records. Additionally, the sex, age, time interval from fistula occurrence to admission, and etiology of the patients were recorded. Moreover, hemoglobin and albumin levels and BMI were measured on admission. Further, fistula output was calculated by weighing daily. Lastly, skin and fascia thickness were calculated using CT scans.
All statistical analyses were performed using Statistical Package for Social Science version 26.0 for Windows (IBM, Analytics, Armonk, NY, USA). The Student's t-test and Mann–Whitney U test were used to compare the continuous variables across groups, while the Fisher's exact test was applied to compare the categorical variables. Finally, multivariate Cox and logistic regressions were used to adjust for the confounding variables. A P value of <0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics
From January 2010 to December 2019, 105 patients underwent negative pressure treatment at one of the two study hospitals. However, 22 patients were excluded (17 had more than one fistula, three had IBD, and two had incomplete data) based on the study exclusion criteria. Eventually, 83 patients were enrolled. The baseline characteristics of the included patients are presented in Table 1. The mean age of the patients was 38.3 ± 11.6 years, and their average BMI was 19.9 ± 2.2 kg/m2. Of the 83 patients, 55 (66.3 %) were males, and 28 were (33.7 %) females. The median interval from the previous abdomen surgery to admission was 21 days (interquartile range [IQR] = 16–28 days), and the median interval from incision dehiscence to admission was 10 days (IQR = 8–12 days). Lastly, 60 (72.2 %) and 36 (43.4 %) patients were diagnosed with SIRS and sepsis, respectively, on admission.
Table 1.
Patients characteristics.
| Clinical variables | Overall patients (n = 83) |
|---|---|
| Demographic data | |
| Female,No. (%) | 28 (33.7) |
| Age, years; (mean ± SD) | 38.3 ± 11.6 |
| BMI, kg/m2, (mean ± SD) | 19.9 ± 2.2 |
| General condition of patients on admission | |
| Peritoneal effusion,No. (%) | 26 (31.3) |
| Pleural effusion,No. (%) | 39 (46.9) |
| SIRS,No. (%) | 60 (72.2) |
| Bilirubin>20 μmol/L,No. (%) | 41 (49.3) |
| Creatinine>106 μmol/L,No. (%) | 8 (9.6) |
| Sepsis,No. (%) | 36 (43.4) |
| Fistula characteristics | |
| Interval from Resection of small intestine to admission, day, (median,IQR) | 21 (16–28) |
| Interval from incision dehiscence to admission, day, (median,IQR) | 10 (8–12) |
| Width of incision dehiscence, No. (%) | |
| <5 cm | 50 (60.2) |
| ≥5 cm | 33 (39.8) |
| Abdominal wall thickness greater than 2 cm,No. (%) | |
| Yes | 58 (69.9) |
| No | 25 (30.1) |
| Length from treitz to fistula,No. (%) | |
| <100 cm | 11 (13.2) |
| ≥100 and < 200 cm | 20 (24.1) |
| ≥200 and < 300 cm | 42 (50.6) |
| >300 cm | 10 (12.1) |
| High output,No. (%) | |
| Yes | 51 (61.5) |
| No | 32 (38.5) |
| Etiology of leakage,No. (%) | |
| Trauma | 47 (56.6) |
| Obstruction | 30 (36.1) |
| Mesenteric thrombosis | 6 (7.2) |
| Laboratory results | |
| Hemoglobin,No. (%) | |
| <90 g/L | 11 (13.3) |
| ≥90 g/L and <120 g/L | 66 (79.5) |
| ≥120 g/L | 6 (7.2) |
| Albumin, No. (%) | |
| <35 g/L | 51 (61.5) |
| ≥35 g/L | 32 (38.5) |
| WBC, No. (%) | |
| <10^9/L | 21 (25.3) |
| ≥10^9/L and <2*10^9/L | 56 (67.5) |
| >2*10^9/L | 6 (7.2) |
| CRP, No. (%) | |
| <50 μg/mL | 38 (45.8) |
| ≥50 μg/mL and <100 μg/mL | 32 (38.5) |
| >100 μg/mL | 13 (15.7) |
| Comorbidity, No. (%) | |
| Hypertensio | 1 (1.2) |
| Diabetes mellitus | 2 (2.4) |
3.2. Closure of fistula
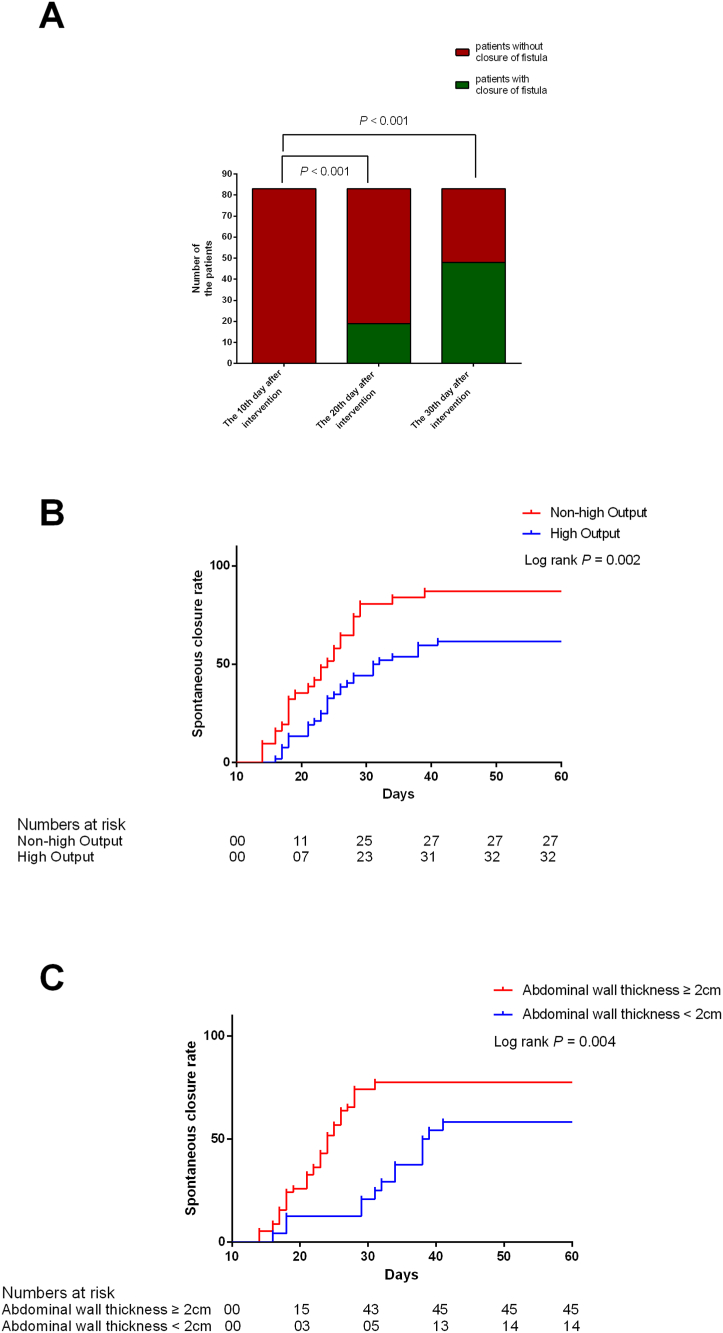
Of the total 83 patients, 59 patients achieved fistula closure, yielding a closure rate of 71.1 %. The median time for closure was 24 days (IQR = 18–28 days). Of the 59 patients with fistula closure, 19 (32.2 %) attained closure within 20 days after DLIST placement. Moreover, 48 of the 59 patients (81.4 %) achieved closure within 1 month of the alternative negative pressure treatment. In the remaining 11 patients who experienced fistula closure after >30 days following DLIST placement, the longest time for closure was 41 days (Fig. 3A). Furthermore, six of the 24 patients without fistula closure received CR due to uncontrollable flow up to 3 weeks after admission.
Fig. 3.
A. The closure rate of the overall patients at different time nodes.
B.The closure rate in patients with and without high output fistula. C. The closure rate in patients with and without abdominal wall thickness more than 2 cm.
In terms of the factors affecting fistula closure, an adjusted Cox regression analysis showed that high-output fistula had a negative effect on closure (hazard ratio [HR] = 0.48; 95 % confidence interval [CI]: 0.29–0.81; P = 0.006; Table 2 and Fig. 3B). In contrast, abdominal wall thickness >2 cm exhibited a positive effect on closure (HR = 2.76; 95 % CI: 1.35–5.67; P = 0.006; Table 2 and Fig. 3C). Moreover, in the 60 patients diagnosed with SIRS on admission, the median time required for becoming free of SIRS was 6 days (IQR = 5–9 days). In the case of the 36 patients detected with sepsis on admission, the median time to become sepsis-free was 11 days (IQR = 8–15 days).
Table 2.
Cox-regression analysis of factors associated with closure of fistula.
| Clinical variables | Univariate regression |
p | Multivariate regression |
p | |||
|---|---|---|---|---|---|---|---|
| HR | 95%CI | HR | 95%CI | ||||
| Female | 1.091 | 0.637–1.872 | 0.750 | ||||
| Age | 0.998 | 0.997–0.019 | 0.819 | ||||
| BMI | 1.086 | 1.002–1.213 | 0.014 | 0.969 | 0.839–1.118 | 0.666 | |
| Peritoneal effusion | 0.875 | 0.556–1.365 | 0.854 | ||||
| Pleural effusion | 0.941 | 0.658–1.297 | 0.899 | ||||
| SIRS | 0.708 | 0.929–1.069 | 0.604 | ||||
| Bilirubin>20 μmol/L | 0.942 | 0.687–1.489 | 0.801 | ||||
| Creatinine>100 μmol/L | 0.959 | 0.905–1.069 | 0.419 | ||||
| Interval from abdominal surgery to admission | 0.988 | 0.946–1.032 | 0.592 | ||||
| Interval from incision dehiscence to admission | 0.962 | 0.871–1.063 | 0.448 | ||||
| Width of incision dehiscence | |||||||
| <5 cm | Ref | ||||||
| ≥5 cm | 1.486 | 0.865–2.551 | 0.151 | ||||
| Abdominal wall thickness | |||||||
| <2 cm | Ref | ||||||
| ≥2 cm | 2.426 | 1.319–4.462 | 0.004 | 2.761 | 1.347–5.663 | 0.006 | |
| Length from treitz to fistula | |||||||
| <100 cm | Ref | ||||||
| ≥100 and < 200 cm | 2.152 | 0.787–5.884 | .0.135 | ||||
| ≥200 and < 300 cm | 2.023 | 0.784–5.219 | 0.145 | ||||
| >300 cm | 2.286 | 0.745–7.013 | 0.148 | ||||
| High output | |||||||
| No | Ref | ||||||
| Yes | 0.512 | 0.305–0.858 | 0.011 | 0.482 | 0.286–0.812 | 0.006 | |
| Etiology of leakage | |||||||
| Trauma | Ref | ||||||
| Obstruction | 0.999 | 0.581–1.717 | 0.996 | ||||
| Mesenteric thrombosis | 0.604 | 0.186–1.968 | 0.403 | ||||
| Hemoglobin | |||||||
| <90 g/L | Ref | ||||||
| ≥90 g/L and <120 g/L | 1.271 | 0.599–2.695 | 0.532 | ||||
| ≥120 g/L | 1.355 | 0.407–4.505 | 0.620 | ||||
| Albumin | |||||||
| <35 g/L | Ref | ||||||
| ≥35 g/L | 1.111 | 0.651–1.894 | 0.700 | ||||
| WBC | |||||||
| <10^9/L | Ref | ||||||
| ≥10^9/L and <2*10^9/L | 1.090 | 0.603–1.970 | 0.777 | ||||
| >2*10^9/L | 0.662 | 0.191–2.287 | 0.514 | ||||
| CRP | |||||||
| <50 μg/mL | Ref | ||||||
| ≥50 μg/mL and <100 μg/mL | 0.659 | 0.378–1.149 | 0.141 | ||||
| >100 μg/mL | 0.558 | 0.245–1.269 | 0.164 | ||||
| Comorbidity | |||||||
| Hypertensio | 0.864 | 0.119–6.249 | 0.884 | ||||
| Diabetes mellitus | 0.268 | 0.037–1.941 | 0.192 | ||||
3.3. Incision healing
In this study population, 11 of the 83 patients experienced re-dehiscence. In these patients, the incomplete healing was due to noninfectious incision dehiscence induced by excessive tension. After the edema was relieved, the fascia and skin on both sides of the incision could not completely close, resulting in final re-dehiscence. Furthermore, the suture-out interval in these 11 patients was 24 days (IQR = 21–28 days). An adjusted logistics regression analysis suggested that abdominal wall thickness <2 cm was the only factor for re-dehiscence (OR = 4.83; 95 % CI: 1.09–21.49; p = 0.039). Additionally, in eight of the 11 patients, the EAF persisted after re-dehiscence. Among these eight patients, two had a low-output fistula, and six received CR.
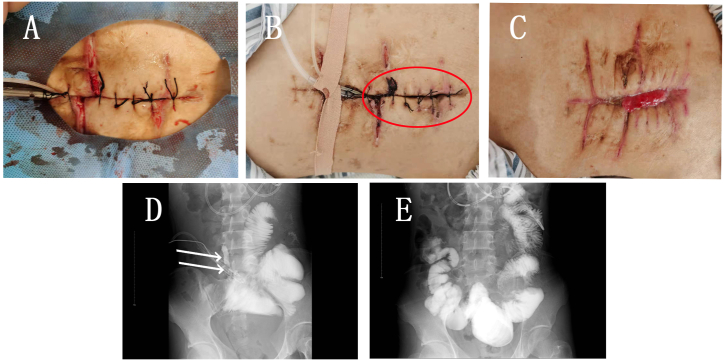
The remaining three of the 11 patients achieved fistula closure before re-dehiscence (Fig. 4 [A - C]). In these three patients, the initial fistula output before DLIST placement was 210, 240, and 220 mL/day, respectively, whereas the output on the 10th day after DLIST placement was 30, 60, and 40 mL/day, respectively. Fistula closure was confirmed under gastrointestinal radiography (Fig. 4 [D, E]) within 20 days in all three patients (17, 18, and 18 days, respectively), and the suture was removed 21, 21, and 22 days after DLIST placement, respectively.
Fig. 4.
A. during the intervention, edema around the incision was still obvious.
B. Three days after intervention, after the edema subsided, the incision could not be completely closed (marked in blue circle).
C. 17 days after intervention, the closure was detected while the incision was split again and the suture was removed on the 21st day after intervention. On the 26th day, the incision granulation grows further.
D. On the second day after the intervention, the contrast medium was injected through naso intestinal tube, and the leakage of contrast medium was observed (marked by white arrow).
E. 17 days after intervention, the closure was detected under X ray.
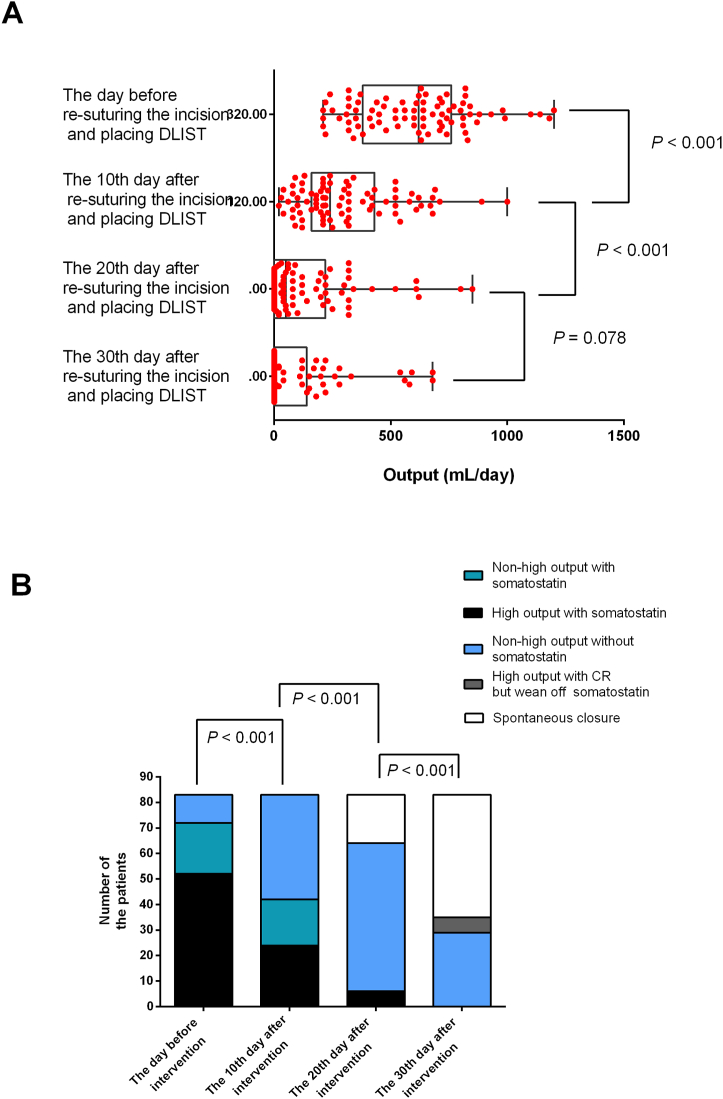
3.4. Output of fistula
The average fistula output was 598.3 ± 242.7 mL/day on the day before DLIST placement, which decreased significantly within 1 month (Fig. 5A). Furthermore, the number of patients requiring somatostatin treatment decreased over time. In particular, somatostatin was administered in 72 (86.8 %) of the total 83 patients before DLIST placement, with 52 (62.7 %) demonstrating high-output fistula after somatostatin treatment. Moreover, on the 10th day after DLIST placement, the number of patients with somatostatin treatment decreased to 42 (50.6 %), while the number of patients with high-output fistula after somatostatin use also decreased to 24 (28.9 %) (Fig. 5B). Somatostatin was stopped within 30 days after DLIST placement in all patients. However, six patients had a high-output fistula and received CR to maintain nutritional status and an internal environment.
Fig. 5.
A. The change of amount of output at different time nodes.
B. Number of patients with non-high output fistula, high output fistula, and closure at different time nodes.
3.5. Abdominal hernia after fistula closure
Patients were followed up for 1 year after discharge to investigate abdominal hernia incidence. No patients died during the follow-up period. Among the 59 patients with closure, only four (6.8 %; all four were male) had abdominal hernia within 1 year post-discharge. Additionally, these four patients exhibited a weight gain of 6, 8, 8, and 9 kg, respectively. In the case of the remaining 55 patients, a mean weight gain of 4.6 ± 2.1 kg was observed. The characteristics of the four patients with abdominal hernia are shown in Table 3.
Table 3.
Characteristics of patients who achieved the closure but had incisional hernia within one year after discharged.
| Patients number | Age | Initial BMI (kg/m2) | Abdominal wall thickness ≥ 2 cm | width of the incision dehiscence ≥ 5 cm | gained weight one year after discharge | BMI (kg/m2) one year after discharge | Incisional hernia size (cm*cm) |
|---|---|---|---|---|---|---|---|
| 1 | 34 | 17.71 | No | Yes | 8 | 21.01 | 3*8 |
| 2 | 41 | 19.28 | Yes | Yes | 8 | 21.39 | 3*10 |
| 3 | 28 | 17.48 | No | Yes | 9 | 20.72 | 3*10 |
| 4 | 35 | 18.87 | Yes | No | 6 | 21.95 | 3*6 |
4. Discussion
EAF is a unique type of enterocutaneous fistula (ECF) that connects to the outside environment without a sinus. In negative pressure wound therapy (NPWT), the EAF is isolated from the exposed open abdomen wound to form a stoma analog.17 Although this strategy can effectively reduce abdominal cavity pollution caused by the EAF, the treatment procedure is lengthy. Additionally, most studies [9,10,[17], [18], [19], [20], [21]] have indicated an extremely slim possibility of fistula closure, with a reported global ECF closure rate of 19%–92 % [[22], [23], [24], [25], [26], [27], [28]]. The main reason for the difficulty in attaining EAF closure may be the ineffectiveness of granulation accumulation in blocking the fistula, thereby allowing the mucosa to grow out from the fistula eventually. Therefore, a definitive surgery with a challenging process is ultimately required.
Our study is the first to focus on the early stages of EAF formation, proposing an alternative method to improve the prognosis by transforming EAF into an ordinary ECF (with a sinus tract). The core component of this treatment method was the draining and suturing of the fascia before the dehisced fascia atrophied and adhered to the granulation tissue. This strategy promoted sinus tract formation and consequently fistula closure. After treatment, EAF closure could be detected, demonstrating a substantial overall rate of fistula closure of 71.1 %. This approach was noteworthy because it shortened the entire treatment process and decreased procedure difficulty in patients with early incision dehiscence and EAF. Compared with NPWT with equal-diameter micro-pores,17,30 DLIST has lateral holes with a larger diameter. Due to the difference in structures, the effect of NPWT on the micro-deformation of granulation tissue and angiogenesis is relatively stronger, while DLIST is more helpful in removing large amounts of viscous and thick fluid. Moreover, adequately draining viscous fluid contributes to the following effects: (1) direct reduction of peripheral inflammation and tension at the re-sutured incision, facilitating incision healing and sinus formation and (2) indirect provision of conditions for the growth of granulation tissue and sinus formation. Furthermore, the development of granulation tissue around the fistula leads to sinus narrowing, resulting in reduced output and fistula closure. In one of our earlier studies [13], drainage of >2000 mL of colonic fluid and pus was performed within 48 h using DLIST. Additionally, another of our previous studies [12] obtained an closure rate as high as 86 % under good nutritional conditions by leveraging the continuous flush and drain capability of DLIST. Moreover, the hazards linked to intra-abdominal catheters with negative pressure and new fistula formation should be considered during this treatment. However, no new fistulas were observed after DLIST placement in our study. This outcome could be because the suction catheter supplying the negative pressure was not in direct tissue contact. Further, the supporting pipe, which was covered with holes, was internally connected to the ambient atmosphere. Thus, when the suction catheter provided a negative pressure of 110–150 mmHg, intestinal fluid was drained out, quickly filling the inside of the supporting pipe with air and saline. Thus, this working mechanism of DLIST does not lead to progressive negative pressure on the tissue and avoids sustained tissue damage and the formation of new intestinal fistulas.
In this study, our negative pressure strategy was associated with an abdominal hernia incidence rate of 6.8 % in patients with fistula closure. Many risk factors are linked to abdominal incision hernia, particularly relating to abdominal pressure [29], the strength of abdominal wall incision [30], and healing ability [31]. Based on the above risk factors, we believe that the leading cause of abdominal hernia may be a rapid body weight increase over a short duration. Although the incision and fascia were re-sutured in our study, severe incision infection and weaker than normal integrity and tensile capacity of the fascia were observed. Consequently, incision healing was mainly due to the formation of granulation tissue.
Our study has several limitations that should be considered. First, this study was a single-arm observation with no controls. However, previous research suggests that the possibility of closure without intestinal fistula excision is highly slim in EAF. Therefore, the high closure rate of over 70 % in our study illustrates the advantages of the proposed treatment method. Second, our sample size was relatively small, potentially leading to bias. However, EAF from small intestinal leakage due to incision dehiscence following abdominal surgery has a low incidence rate. Moreover, the present study is the first to explore methods to achieve fistula closure in this condition. Third, our study findings may not be generalizable to other populations because specific characteristics in our patients differ from those of international populations. For example, the relatively young population, lack of patients with obesity, and low rate of diabetes and IBD in our study represent a distinct subset. Finally, the application of DLIST is not relatively broad. Our study patients were in the early stage of EAF formation. Thus, frozen abdomen formation had not yet occurred, enabling a split incision that was not excessively wide to allow the fascia to be stitched and pulled together. Nonetheless, the present study aimed to shed light on our experience, presenting an alternative method for treating EAF before the stage requiring additional staff and material resources. Therefore, the current study results suggest that our alternative negative pressure approach may serve as a treatment modality for EAF management, warranting future research on this aspect.
5. Conclusion
The appropriate application of our alternative negative pressure treatment may help achieve fistula closure in patients with EAF resulting from small intestinal leakage caused by incision dehiscence.
Institutional review board statement
This study was reviewed and approved by the Ethics Committee of the Jinling Hospital (2021DZKY03-0167).
Informed consent statement
Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Data sharing statement
Data will be made available on request.
CRediT authorship contribution statement
Ran Sun: Writing – review & editing, Writing – original draft. Xin Xu: Writing – original draft, Software, Methodology, Investigation. Shikun Luo: Software, Methodology, Investigation, Formal analysis, Data curation. Risheng Zhao: Validation, Supervision, Software, Formal analysis, Data curation. Weiliang Tian: Software, Resources, Methodology, Investigation. Ming Huang: Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation. Zheng Yao: Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e22045.
Contributor Information
Risheng Zhao, Email: dr_zhaorisheng@163.com.
Ming Huang, Email: huangmingdoc@163.com.
Zheng Yao, Email: yaozheng774@outlook.com.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Suragul W., Rungsakulkij N., Vassanasiri W., Tangtawee P., Muangkaew P., Mingphruedhi S., Aeesoa S. Predictors of surgical site infection after pancreaticoduodenectomy. BMC Gastroenterol. 2020;20(1):201. doi: 10.1186/s12876-020-01350-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao Z., Tian W., Xu X., Zhao R., Huang M., Zhao Y., Chen X. The double-lumen irrigation-suction tube in the management of incisional surgical site infection after enterocutaneous fistula excisions: an observational study. J. Invest. Surg. : the official journal of the Academy of Surgical Research. 2021;34(7):791–797. doi: 10.1080/08941939.2019.1693667. [DOI] [PubMed] [Google Scholar]
- 3.Vahedian J., Jahanian S., Banivaheb B., Hemmati N., Ghavamipour M., Chegini M., Alemrajabi M. A new method for surgical abdominal mass closure after abdominal fascial dehiscence using Nasogastric tube and Hemovac perforator: a case-Series study. World J. Surg. 2018;42(10):3106–3111. doi: 10.1007/s00268-018-4607-9. [DOI] [PubMed] [Google Scholar]
- 4.Berríos-Torres S.I., Umscheid C.A., Bratzler D.W., Leas B., Stone E.C., Kelz R.R., Reinke C.E., Morgan S., Solomkin J.S., Mazuski J.E., Dellinger E.P., Itani K., Berbari E.F., Segreti J., Parvizi J., Blanchard J., Allen G., Kluytmans J., Donlan R., Schecter W.P. Healthcare infection control Practices advisory committee. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA surgery. 2017;152(8):784–791. doi: 10.1001/jamasurg.2017.0904. [DOI] [PubMed] [Google Scholar]
- 5.Girard E., Abba J., Boussat B., Trilling B., Mancini A., Bouzat P., Létoublon C., Chirica M., Arvieux C. Damage control surgery for non-traumatic abdominal emergencies. World J. Surg. 2018;42(4):965–973. doi: 10.1007/s00268-017-4262-6. [DOI] [PubMed] [Google Scholar]
- 6.Tartaglia D., Costa G., Camillò A., Castriconi M., Andreano M., Lanza M., Fransvea P., Ruscelli P., Rimini M., Galatioto C., Chiarugi M. Damage control surgery for perforated diverticulitis with diffuse peritonitis: saves lives and reduces ostomy. World J. Emerg. Surg. : WJES. 2019;14:19. doi: 10.1186/s13017-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majercik S., Kinikini M., White T. Enteroatmospheric fistula: from soup to nuts. Nutr. Clin. Pract. : official publication of the American Society for Parenteral and Enteral Nutrition. 2012;27(4):507–512. doi: 10.1177/0884533612444541. [DOI] [PubMed] [Google Scholar]
- 8.Wainstein D.E., Calvi R.J., Rezzonico F., Deforel M.L., Perrone N., Sisco P. Management of enteroatmospheric fistula: a ten-year experience following fifteen years of learning. Surgery. 2023;173(4):1079–1085. doi: 10.1016/j.surg.2022.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Terzi C., Egeli T., Canda A.E., Arslan N.C. Management of enteroatmospheric fistulae. Int. Wound J. 2014;11(Suppl 1):17–21. doi: 10.1111/iwj.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Saverio S., Tarasconi A., Inaba K., Navsaria P., Coccolini F., Costa Navarro D., Mandrioli M., Vassiliu P., Jovine E., Catena F., Tugnoli G. Open abdomen with concomitant enteroatmospheric fistula: attempt to rationalize the approach to a surgical nightmare and proposal of a clinical algorithm. J. Am. Coll. Surg. 2015;220(3):e23–e33. doi: 10.1016/j.jamcollsurg.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 11.Coccolini F., Roberts D., Ansaloni L., Ivatury R., Gamberini E., Kluger Y., Moore E.E., Coimbra R., Kirkpatrick A.W., Pereira B.M., Montori G., Ceresoli M., Abu-Zidan F.M., Sartelli M., Velmahos G., Fraga G.P., Leppaniemi A., Tolonen M., Galante J., Razek T.…Catena F. The open abdomen in trauma and non-trauma patients: WSES guidelines. World J. Emerg. Surg. : WJES. 2018;13:7. doi: 10.1186/s13017-018-0167-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang F., Liu D., Xu X., Tian W., Yao Z., Wang C., Zhao R. A double-lumen irrigation-suction tube placed during operation could reduce the risk of grade C anastomotic leakage resulting from selective sigmoid colon cancer radical resection. Langenbeck's Arch. Surg. 2020;405(7):1007–1016. doi: 10.1007/s00423-020-01959-z. [DOI] [PubMed] [Google Scholar]
- 13.Yao Z., Tian W., Xu X., Huang Q., Zhao Y. An innovative method for placing a double-lumen irrigation-suction tube in the management of abdominal infection: a case report. Medicine. 2018;97(9) doi: 10.1097/MD.0000000000010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Layec S., Seynhaeve E., Trivin F., Carsin-Mahé M., Dussaulx L., Picot D. Management of entero-atmospheric fistulas by chyme reinfusion: a retrospective study. Clinical nutrition (Edinburgh, Scotland) 2020;39(12):3695–3702. doi: 10.1016/j.clnu.2020.03.030. [DOI] [PubMed] [Google Scholar]
- 15.Kumpf V.J., de Aguilar-Nascimento J.E., Diaz-Pizarro Graf J.I., Hall A.M., McKeever L., Steiger E., Winkler M.F., Compher C.W., FELANPE. American Society for Parenteral and Enteral Nutrition ASPEN-FELANPE Clinical Guidelines. JPEN. J. Parenteral and Enteral Nutrition. 2017;41(1):104–112. doi: 10.1177/0148607116680792. [DOI] [PubMed] [Google Scholar]
- 16.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., Hotchkiss R.S., Levy M.M., Marshall J.C., Martin G.S., Opal S.M., Rubenfeld G.D., van der Poll T., Vincent J.L., Angus D.C. The Third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Saverio S., Tarasconi A., Inaba K., Navsaria P., Coccolini F., Costa Navarro D., Mandrioli M., Vassiliu P., Jovine E., Catena F., Tugnoli G. Open abdomen with concomitant enteroatmospheric fistula: attempt to rationalize the approach to a surgical nightmare and proposal of a clinical algorithm. J. Am. Coll. Surg. 2015;220(3):e23–e33. doi: 10.1016/j.jamcollsurg.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 18.Marinis A., Gkiokas G., Anastasopoulos G., Fragulidis G., Theodosopoulos T., Kotsis T., Mastorakos D., Polymeneas G., Voros D. Surgical techniques for the management of enteroatmospheric fistulae. Surg. Infect. 2009;10(1):47–52. doi: 10.1089/sur.2008.044. [DOI] [PubMed] [Google Scholar]
- 19.Marinis A., Gkiokas G., Argyra E., Fragulidis G., Polymeneas G., Voros D. Enteroatmospheric fistulae"--gastrointestinal openings in the open abdomen: a review and recent proposal of a surgical technique. Scandinavian journal of surgery : SJS : official organ for the Finnish Surgical Society and the Scandinavian Surgical Society. 2013;102(2):61–68. doi: 10.1177/1457496913482252. [DOI] [PubMed] [Google Scholar]
- 20.Huang J., Ren H., Jiang Y., Wu X., Ren J. Technique advances in enteroatmospheric fistula isolation after open abdomen: a review and outlook. Frontiers in surgery. 2021;7 doi: 10.3389/fsurg.2020.559443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giudicelli G., Rossetti A., Scarpa C., Buchs N.C., Hompes R., Guy R.J., Ukegjini K., Morel P., Ris F., Adamina M. Prognostic factors for enteroatmospheric fistula in open abdomen treated with negative pressure wound therapy: a multicentre experience. J. Gastrointest. Surg. : official journal of the Society for Surgery of the Alimentary Tract. 2017;21(8):1328–1334. doi: 10.1007/s11605-017-3453-7. [DOI] [PubMed] [Google Scholar]
- 22.Quinn M., Falconer S., McKee R.F. Management of enterocutaneous fistula: outcomes in 276 patients. World J. Surg. 2017;41(10):2502–2511. doi: 10.1007/s00268-017-4063-y. [DOI] [PubMed] [Google Scholar]
- 23.Garden O.J., Dykes E.H., Carter D.C. Surgical and nutritional management of postoperative duodenal fistulas. Dig. Dis. Sci. 1988;33(1):30–35. doi: 10.1007/BF01536627. [DOI] [PubMed] [Google Scholar]
- 24.Haffejee A.A. Surgical management of high output enterocutaneous fistulae: a 24-year experience. Curr. Opin. Clin. Nutr. Metab. Care. 2004;7(3):309–316. doi: 10.1097/00075197-200405000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Hollington P., Mawdsley J., Lim W., Gabe S.M., Forbes A., Windsor A.J. An 11-year experience of enterocutaneous fistula. Br. J. Surg. 2004;91(12):1646–1651. doi: 10.1002/bjs.4788. [DOI] [PubMed] [Google Scholar]
- 26.Leang Y.J., Bell S.W., Carne P., Chin M., Farmer C., Skinner S., Wale R., Warrier S.K. Enterocutaneous fistula: analysis of clinical outcomes from a single Victorian tertiary referral centre. ANZ J. Surg. 2018;88(1–2) doi: 10.1111/ans.13686. E30–E33. [DOI] [PubMed] [Google Scholar]
- 27.Li J., Ren J., Zhu W., Yin L., Han J. Management of enterocutaneous fistulas: 30-year clinical experience. Chinese medical journal. 2003;116(2):171–175. [PubMed] [Google Scholar]
- 28.Martinez D., Zibari G., Aultman D., McMillan R., Mancini M.C., Rush B.M., McDonald J.C. The outcome of intestinal fistulae: the Louisiana State University Medical Center--Shreveport experience. Am. Surg. 1998;64(3):252–254. [PubMed] [Google Scholar]
- 29.Itatsu K., Yokoyama Y., Sugawara G., Kubota H., Tojima Y., Kurumiya Y., Kono H., Yamamoto H., Ando M., Nagino M. Incidence of and risk factors for incisional hernia after abdominal surgery. Br. J. Surg. 2014;101(11):1439–1447. doi: 10.1002/bjs.9600. [DOI] [PubMed] [Google Scholar]
- 30.Fujii T., Tsutsumi S., Matsumoto A., Fukasawa T., Tabe Y., Yajima R., Asao T., Kuwano H. Thickness of subcutaneous fat as a strong risk factor for wound infections in elective colorectal surgery: impact of prediction using preoperative CT. Dig. Surg. 2010;27(4):331–335. doi: 10.1159/000297521. [DOI] [PubMed] [Google Scholar]
- 31.Cata J.P., Wang H., Gottumukkala V., Reuben J., Sessler D.I. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. British journal of anaesthesia. 2013;110(5):690–701. doi: 10.1093/bja/aet068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.