Abstract
The measurement of fine (diameter: 100 nanometers–2.5 micrometers) and ultrafine (UF: < 100 nanometers) titanium dioxide (TiO2) particles is instrument dependent. Differences in measurements exist between toxicological and field investigations for the same exposure metric such as mass, number, or surface area because of variations in instruments used, operating parameters, or particle-size measurement ranges. Without appropriate comparison, instrument measurements create a disconnect between toxicological and field investigations for a given exposure metric. Our objective was to compare a variety of instruments including multiple metrics including mass, number, and surface area (SA) concentrations for assessing different concentrations of separately aerosolized fine and UF TiO2 particles. The instruments studied were (1) DustTrak™ DRX, (2) personal DataRAMs™ (PDR), (3) GRIMM™, and (4) diffusion charger (DC). Two devices of each field-study instrument (DRX, PDR, GRIMM, and DC) were used to measure various metrics while adjusting for gravimetric mass concentrations of fine and UF TiO2 particles in controlled chamber tests. An analysis of variance (ANOVA) was used to apportion the variance to interinstrument (between different instrument-types), inter-device (within instrument), and intra-device components. Performance of each instrument-device was calculated using root mean squared error compared to reference methods: close-faced cassette and gravimetric analysis for mass and scanning mobility particle sizer (SMPS) real-time monitoring for number and SA concentrations. Generally, inter-instrument variability accounted for the greatest (62.6% or more) source of variance for mass, and SA-based concentrations of fine and UF TiO2 particles. However, higher intra-device variability (53.7%) was observed for number concentrations measurements with fine particles compared to inter-instrument variability (40.8%). Inter-device variance range(0.5–5.5%) was similar for all exposure metrics. DRX performed better in measuring mass closer to gravimetric than PDRs for fine and UF TiO2. Number concentrations measured by GRIMMs and SA measurements by DCs were considerably (40.8–86.9%) different from the reference (SMPS) method for comparable size ranges of fine and UF TiO2. This information may serve to aid in interpreting assessments in risk models, epidemiologic studies, and development of occupational exposure limits, relating to health effect endpoints identified in toxicological studies considering similar instruments evaluated in this study.
Keywords: Real-time instruments, aerosol mass concentration, aerosol number concentration, aerosol surface area concentration, particle morphology
Graphical Abstract
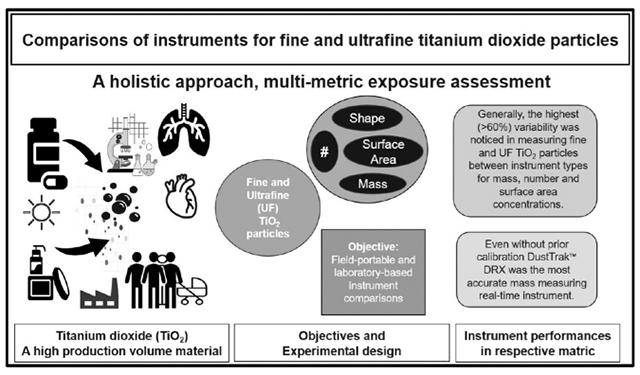
Introduction
Exposure to titanium dioxide (TiO2) particles has been studied extensively (Nicole, Berndnowack, and Nowack 2008) because of its applications in numerous production materials such as photocatalysts, antibacterial agents, and personal care products (Aitken et al. 2006; Nohynek et al. 2010; Vaquero et al. 2016). TiO2 is often categorized by its particle size as ultrafine (UF: diameter < 100 nanometer [nm]) or fine (diameter 100 nm to 2.5 micrometer [μm]), the latter is sometimes referred to as pigment grade (NIOSH 2011). Based upon animal inhalation studies, UF TiO2 particles are classified as “possibly carcinogenic to humans” (IARC 2006). UF and fine TiO2 particles are widely used in paints (60%), with annual US production estimated at 1,000,000 tons (USGS 2022). Widespread usages of these materials raise considerable exposure risk concerns (Robichaud et al., 2009). Variability in UF and fine TiO2 particle properties (i.e., size, refractive index) and exposure potential among workers in multiple industries requires a standardized approach for sampling TiO2 particles (Chen and Selloni 2014; Hamilton et al. 2009; Shi et al. 2013).
A multi-metric approach to assess TiO2 particle exposure
Harmful effects induced by UF TiO2 particles have been well-reported in the literature for their correlation with unique physicochemical characteristics such as size, shape, mass, surface area, number, form, and chemical composition (Liu et al. 2021; Hou et al. 2019; Lovén et al. 2021; Higashikubo et al. 2021; Hoshino, Fujioka, and Oku 2004; Silva et al. 2013; NIOSH/CDC, 2009; Hamilton et al. 2009; Demir 2020; Oberdörster et al. 2005a). Toxicological studies reported that UF particles may initiate a greater toxicological response than fine particles at the site of deposition for the same mass loading (Oberdörster 1996; Oberdörster et al. 2000, 2005b; Stoeger et al. 2006). UF TiO2 particles reportedly initiate inflammatory and immunological responses in the lungs (Grassian et al. 2007; Haar et al. 2006) and translocate to systemic sites within 24 hr of alveolar deposition (Nemmar et al. 2001; Oberdörster et al. 2004, 2002). The elevated inflammatory potential of UF TiO2 particles may be attributed to elevated surface area compared with fine particles for the same mass (NIOSH 2011; Nurkiewicz et al. 2008; Stoeger et al. 2006). Sager and Castranova (2009) reported that surface area, not mass, of UF TiO2 particles may be a more appropriate metric for dose estimation of pulmonary toxicity. Given the importance of physical properties of UF TiO2 such as size, shape or surface area, the traditional mass-based mono-metric approach to exposure assessment may not be the most protective of the respiratory health of workers. Further, the number concentration alone may underestimate exposure risk due to particle interaction (e.g., agglomeration) of UF TiO2 (Leskinen et al. 2012). Exposure assessors should consider using a size-oriented, multi-metric approach.
Global agencies such as World Health Organization (WHO), Organization for Economic Co-operation and Development (OECD), European Committee for Standardization (CEN) have provided guidance to measure and assess occupational exposure of UF particles for workers health (WHO, OECD, CEN EN: 17058 and 16966). Although the National Institute for Occupational Safety and Health (NIOSH) has recommended exposure limits based on mass (fine: 2.4 milligrams per cubic meter (mg/m3) and UF: 0.3 mg/m3) of TiO2 particles, this agency also suggested a tiered-approach strategy as “nanoparticle emission assessment technique (NEAT 1.0; Methner et al. 2010a) and nanomaterial emission assessment technique (NEAT 2.0; Methner et al. 2010b).” These NEAT approaches involve using nonspecific real-time direct reading instruments such as number, mass, or size along with filter-based time-integrated sampling instruments (i.e., gravimetric mass-measurement), coupled with off-line analyses of particle morphology by electron microscopy (Eastlake et al. 2016; Methner et al. 2010a). Using a multi-metric approach for environmental monitoring, Spinazzè et al. (2016) assessed occupational exposures of nanometer-sized TiO2 particles at a specific worksite setting involving photocatalytic concrete production. Spinazzè et al. (2016) determined slightly different magnitudes of low-level exposure using different monitoring methods, but these observations were not generalizable because data were limited to a single facility. Previously, multi-metric approach was considered for measurement of TiO2 at work places (Lee et al. 2011; Kaminski et al. 2015; Xu et al., 2016; Fonseca et al. 2021) and other material including nanometer sized particles (Bekker et al. 2014; Gomez et al. 2015; McGarry et al. 2013; Dylla and Hassan, 2012; Golanski et al. 2011; Koponen, Jensen, and Schneider 2009). However, instrument comparisons to exploring underlying variabilities between those assessments and measurements have been a knowledge gap to considering fine and UF TiO2 particles, especially at reportedly harmful concentration levels in toxicological studies.
Misalignment between laboratory and field investigations assessing TiO2 particles
Toxicological studies demonstrated adverse health outcomes from exposure to fine and UF TiO2 particles in well-controlled lab experiments (Bermudez et al. 2004; Higashikubo et al. 2021; Hou et al. 2019; Knuckles et al. 2012; Kumar, Yano, and Fedulov 2022; Li et al. 2010; Liu et al. 2021; Lovén et al. 2021; Nurkiewicz et al. 2008; R et al. 1995; Warheit et al. 2007; Yu et al. 2019). In these lab-based toxicological evaluations, aerosolized TiO2 particles were measured using sensitive, and often expensive, instruments with limited portability. In addition to a cascade impactor (MOUDI, MSP Co., Shoreview, MN), Nurkiewicz et al. (2008) used a less portable electrical mobility classifier (scanning mobility particle sizer [SMPS], TSI Inc., Shoreview, MN), and an aerodynamic particle sizer (APS, TSI Inc.) to measure TiO2-particle size distributions. Using the MOUDI and gravimetric mass measurements, Nurkiewicz et al. (2008) observed greater microvascular dysfunction after lung deposition with UF (1.5–12 mg/m3) than with fine (3–12 mg/m3) TiO2 particles in a rat model. Occupational exposure data from a packaging workshop also suggested that out of total mass (3.17 mg/m3) of TiO2 particles, UF (1.22 mg/m3) might contribute (39%) to the cardiopulmonary effects among workers (Zhao et al. 2018). Without comparing measurement methods, Knuckles et al. (2012) used a SMPS to monitor particle number concentration and an electrical low pressure impactor (ELPI, Dekati, Tampere, Finland) to monitor the real-time size distribution and relative mass concentration of fine and UF TiO2 particles in a chamber. These instruments are more suited for lab measurements, which limits the utility of their results to extrapolate with measurements conducted at workplaces. Unlike lab-based animal toxicological studies, field assessments have not demonstrated significant positive associations between occupational exposure to TiO2 particles and adverse health-risk outcomes among workers (Boffetta et al. 2001; Fryzek et al. 2003). Out of many possible reasons for misalignment between health effects observed in animal models and observed in epidemiological studies, our focus was on the use of different types of instruments to assess TiO2 particle characteristics.
To assess occupational exposures to TiO2 particles, measurements using advanced lab-based instruments may not be pragmatic because of factors such as cost, availability, and portability. Industrial hygienists might use real-time direct reading instruments for occupational exposure assessments that measure the same metric of TiO2 particles but operate on different principles than lab-based instruments. Numerous real-time instruments are available for exposure monitoring, but comparisons of relative performance of these instruments are generally limited to single exposure metrics (LeBouf et al. 2011a, 2011b; Jørgensen 2019; Stabile et al. 2014; Watson et al. 2011). Investigators documented marked differences among number concentration measurements while comparing instruments of the same brand, such as TSI Inc. (Asbach et al. 2009; Jeong and Evans 2009) or different brands, such as TSI Inc., ELPI, and Dekati. (Maricq et al. 2000). Unlike for fine particle exposure, accounting for surface area of UF particles may be more biologically relevant than accounting for mass (Sager and Castranova 2009). Researchers have considered intra- and inter-instrument comparisons for airborne particles (Hanlon, Galea, and Verpaele 2021; Pelliccioni and Gherardi 2021; Todea et al. 2015, 2017; Viana et al. 2015) including real-time instruments (Bellagamba et al. 2020; Boccuni et al. 2020; Tombolini et al. 2021). However, well-needed instrument comparisons considering multi-metric harmonized approach for different concentration levels of fine and UF TiO2 particles have not been sufficiently addressed yet.
Workplace area exposures are likely a heterogeneous mixture as well as agglomeration of fine and UF TiO2 particles (Brouwer et al. 2013; Kaminski et al. 2015). Instrument measurements are affected by particle properties such as size distribution, material type, or time-dependent evolution at various concentration levels, the representativeness of the calibration and challenge aerosol under investigation; hence, assessment approaches need to be comparable across these measurement techniques. These differences in instruments may affect interpretation during risk assessments and epidemiological investigations or even affect the development of occupational exposure limits, thereby leading to potential bias when establishing limits for TiO2 particle exposures. To remedy these pitfalls, a clear understanding of simultaneous dose-metrics and instruments involved in exposure assessment of TiO2 particles is paramount.
Objectives of the study
The aim of this study was to provide comparisons of instruments used in lab and field investigations in measuring mass, number, or surface area of fine and UF TiO2 particles. The comparisons were also performed at concentrations relevant to previous toxicological studies. Compared with previous studies, a strength of the current study was inclusion of a multi-metric (mass, number, and surface area) approach using multiple instruments: real-time direct reading, filter-based time-integrated sampling, and off-line microscopic analysis of particle morphology for fine and UF TiO2 particles. Our objectives were to (1) assess relative instrument performance in terms of root mean squared error (RMSE) and (2) determine the apportionment of measurement variance: (1) between two sets of instruments for the same exposure metric (inter-instrument comparison), (2) between two devices of a particular instrument (inter-device within instrument comparison), and (3) within a device of a particular instrument (intra-device comparison). Herein, our approach was to compare total concentrations by instruments using multimetric measurements that are documented in previous literature including toxicological and field investigations for TiO2 aerosols. Therefore, considerations were not to include the best sampling instruments for measuring fine and/or UF particles in any application. In throughput, the investigator focuses on variability between measurements and interprets the results appropriately while evaluating sampling strategy for given application(s) using any subset of aerosol sampling instruments (studied in this presentation or elsewhere).
Materials and methods
Field portable and laboratory grade instruments
The instrument types were selected based upon representative of multiple metrices for assessing exposure scenarios associated with toxicological effects (Figure 1). For the purposes of this study, subsets of herein studied instruments are categorized as field portable or lab grade to explore underlying variabilities between their measurements. Although it is recognized that some traditional lab instruments such as SMPS now have field portable versions available. The metrology of the field and lab instruments employed in this study are described in Table 1. Two identical devices of the same type of all the field portable instruments were used to enable analyses of inter-device within instrument variability. All instruments were calibrated prior to conducting the tests according to default settings recommended by respective manufacturer of the instrument. Instruments that required external certification were presented in supplemental material. However, TiO2 particle measurements might vary based upon particle characteristics such as size, shape, absorption and scattering of light, and refractive index (Hinds 1999) compared to that of calibration test particles mentioned in Table 1. To avoid influence of external manipulations, instruments were not pre-set for TiO2 particles and measurements were not affected by adjustments such as calibration factor. Portable aerosol photometers (DRX: DustTrak™ DRX, Model 8533, TSI Inc., Shoreview, MN; PDR: personal DataRAM™, Model PDR-1200, Thermo Electron Corporation, Franklin, MA) estimate mass concentration based upon the calculation of material’s refractive index, its density and size distribution of the TiO2 aerosol. Four liters per minute (LPM) sampling flow rate was utilized with cyclone to obtain a particle size cut off of 2.5 μm (i.e., fine particles < 2.5 μm) at which the cyclone collection efficiency or transmission is 50% that allow only particles smaller than ~ 2.5 μm to pass into the PDR-1200 sensing stage for measurement. Although a photometer, DRX, unlike PDR, operates differently to provide size segregated mass measurement regardless of cyclone. Real-time aerosol spectrometers (GRIMM: Aerosol spectrometer and dust monitor, Model 1.108, GRIMM Technologies Inc., Douglasville, GA) estimate particle number concentrations based upon laser light scattering. Diffusion chargers (DC: Model 2000CE, EcoChem, League City, TX) measure active surface area that is defined as the area of the particle that interacts with the surrounding gas or ions and is accessible only from the outside.
Figure 1.
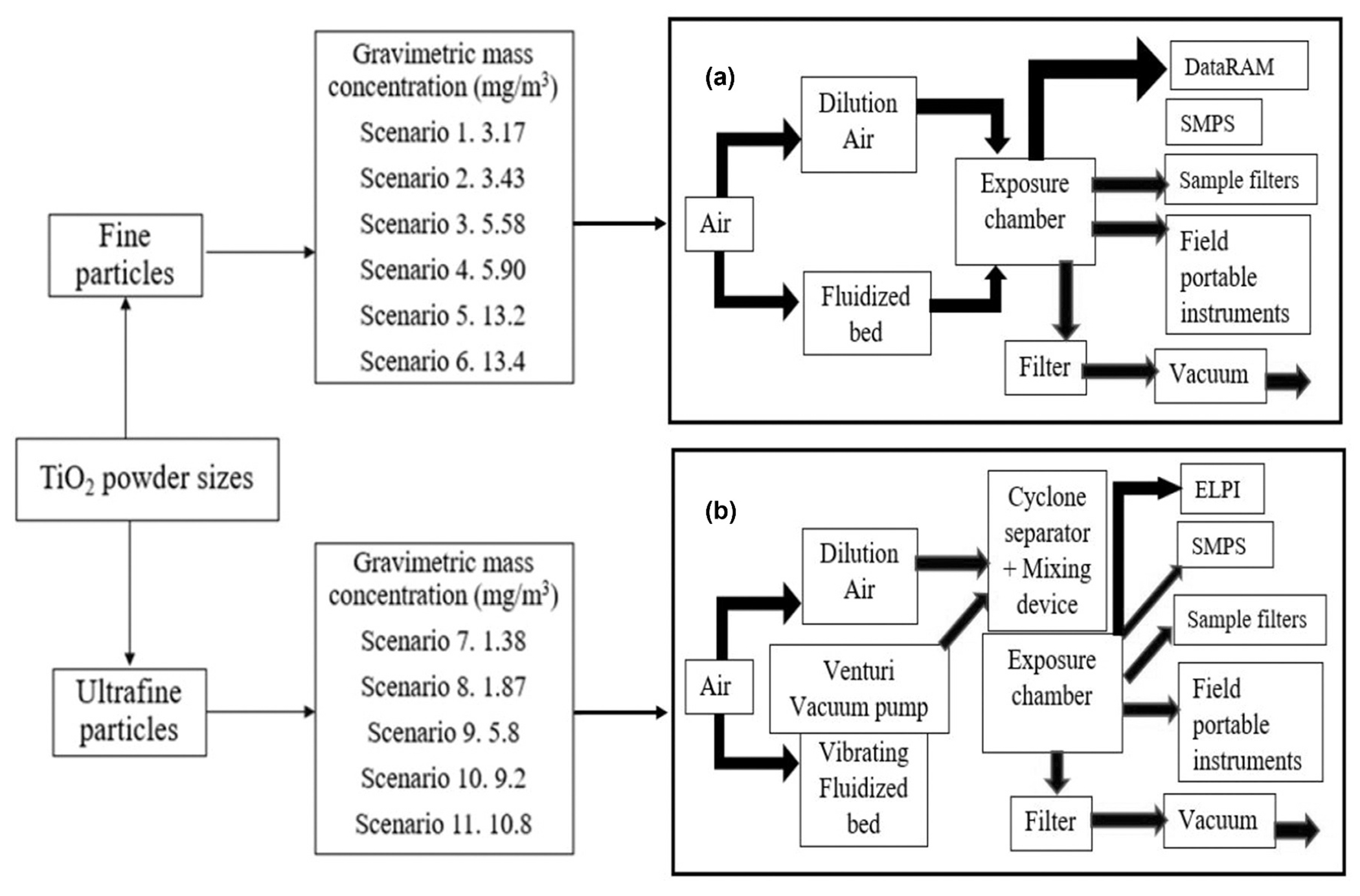
Exposure scenarios and experimental setups: Panel (a) generation of fine TiO2 aerosols. Panel (b) generation of UF TiO2 aerosols. DataRAM (Panel A) and ELPI (Panel B) were used to determine the spatial variability of exposure chamber concentrations.
Table 1.
Characteristics of field portable and reference/laboratory grade instruments.
| Type of instrument | Instruments (# of devices) | Metric | Sampling flow rate (LPM) | Particle size range (nm) | Measurement range | Data log period | Calibrated using reference particle type | Operating principle |
|---|---|---|---|---|---|---|---|---|
| Field portable | DRX (2) | Mass | 3.0 | 100–15,000 | 0.001–150 mg/m3 | 10 sec | Arizona road dust*** | Light-scattering (90°) laser photometer |
| PDR (2) | Mass | 4 | < 2,500 | 0.001–400 mg/m3 | 10 sec | ISO fine test dust** | Active flow nephelometer at wavelength of 880 nm | |
| *GRIMM (2) | Number | 1.2 | 300–20,000 | 1–2,000,000 counts/liter | 1 min | Polystyrene latex | Laser diode spectrometer (90°) at wavelength of 780 nm | |
| DC (2) | Active surface area | 1 | A few – > 10,000 | 1–2,000 mm2/m3 | 10 sec | Not available | Corona mediated unipolar ion discharges | |
| Reference/ Laboratory grade | Gravimetric collection | Mass | 1 | All | 1–20 mg/m3 | 60 min | Not applicable | Offline filter-based mass collection in accordance with NMAM 0500 |
| *SMPS (1) | Number and surface area | 0.3 | 15–660 | 107counts per cm3 | 3 min | Spherical salt particles with smooth surface | Electrical mobility spectrometer |
LPM = liters per minute; nm = nanometer;
= Instruments that provide particle size distribution;
ISO fine test dust (mass median diameter 2,000–3,000 nm; geometric standard deviation 2.5; specific gravity 2.5–2.6, as aerosolized, refractive index 1.54);
Arizona Road Dust (or ISO 12103–1, A1 test Dust ranged from 0–10,000 nm size)
The filter-based sampling technique is the most versatile of the particle collection methods (Cohen, Charles, and McCammon 2001). Gravimetric filter weight is the most commonly used method to determine TiO2 particles in air (NIOSH. 1980, 1994, 1998). Therefore, gravimetric mass concentration was considered the reference method for mass concentration comparisons and employed to estimate the instrument bias among mass-concentration measuring field study instruments (DRX and PDR). SMPS measures the electrical mobility diameter of charged particles in an air stream. SMPS number concentrations can be converted to mobility-based surface area concentrations using an internal algorithm that is based on the following assumptions (TSI 2009a, 2009b): particles are spherical and have a unit positive charge from a bipolar equilibrium charging mechanism. Previous investigators determined SMPS as a reliable instrument to characterize particle concentration with superior size resolution for wide-scale lab-based scientific applications (Jørgensen 2019; Leskinen et al. 2012). Therefore, SMPS measurements were used as a reference method in our study for number (GRIMM) and surface area (DC) concentration comparisons.
As presented in Table 1, field study and lab instruments used to measure particles have different operating parameters and scan ranges that need to be considered when comparing data between instruments. SMPS assembly included classifier (Model # 3080), differential mobility analyzer (Model # 3081), and particle counter (Model # 3775) with 3 min of data logging period for measurement of particle size from 15 nm to 661 nm. Use SMPS rather SMPS assembly was considered or simplicity throughout in the document. Because data logging periods differed, an average of the measurements of the instrument with a shorter data logging period was used for comparisons to the measurement of the instrument with a longer data logging period. For example, GRIMM has a shorter data logging period (1 min) than SMPS (3 min) to measure number concentration of particles. An average of three measurements of GRIMM was compared with every measurement of SMPS for the trial. Secondly, both GRIMM and SMPS provide particle size distribution based upon number concentration. The size range for GRIMM (300–20,000 nm) is different than SMPS (0.015–0.661 μm or 15–661 nm). Therefore, data for both GRIMM and SMPS were adjusted to make the size range compatible. For GRIMM, only data from 300–650 nm were included for analysis of exposure scenarios with both TiO2 powder sizes. For SMPS, only data from 300–637 nm were included for analysis of exposure scenarios with both powder sizes. For inter-instrument comparisons within the same exposure metric, total concentrations measured by different types of instruments were converted to uniform units.
Experimental setup
The experimental design consisted of collecting concurrent, side-by-side measurements of aerosolized TiO2 for all the instruments for 11 exposure scenarios (6 gravimetric mass concentration levels for fine TiO2 particles and 5 gravimetric mass concentration levels for UF TiO2 particles). These 11 exposure scenarios were reconstructed from a toxicological study by Nurkiewicz et al. (2008) who observed adverse health effects attributed to exposure fine and UF TiO2 at different levels of gravimetric mass concentrations among rats in a controlled lab experiment. To match with NIOSH established exposure limit format for fine and UF TiO2 particles, different concentration levels of fine and UF TiO2 particles we also examined separately. As presented in Figure 1, reconstructed exposure scenarios in our study included different levels of mass concentrations of fine (3.17–13.39 mg/m3) and UF (1.38–10.8 mg/m3) TiO2 particles. After achieving a steady state concentration inside the chamber, gravimetric mass was collected using a closed-face cassette with 0.2 μm Teflon® filters (47 millimeter [mm] diameter for fine and 25 mm diameter for UF TiO2) for 60 min at a flow rate of one liter per minute (LPM). Particle size distribution for both sizes of aerosolized fine (Supplemental Figure S1) and UF (Supplemental Figure S2) TiO2 were evaluated and all particles for both UF and fine TiO2 were less than 2.5 μm (i.e., respirable particles). As particle size distribution for all the exposure scenarios were detected no more than 2.5 μm in the chamber, closed-face cassette was consider as a sampler compared to other samplers to evaluate “total” amount of respirable particles (IARC monographs Volume 93).
To occurring TiO2 particle interactions as minimal as possible, temperature (°C) and relative humidity (% RH) were fixed and monitored in the chamber for all exposure scenarios. Temperature and RH for all exposure scenarios of fine particles (mean ± standard deviation) were 22.8 ± 0.5°C and 5.6 ± 0.8%. Temperature and RH for all exposure scenarios of UF particles were 21.7 ± 0.8°C and 5.24 ± 0.6%. Homogeneity of aerosol generation was checked by measuring the number concentration at each port before and after every trial using a condensation particle counter (CPC, Model 3007, TSI Inc., Shoreview, MN). Aerosol concentration stability of the sampling ports was checked to make sure that all the instruments were exposed to a similar aerosol environment through the respective ports of the chamber. The instrument measurements were acquired before and after the sampling period to develop a baseline (i.e., no aerosol) condition prior to the aerosol generation. The length of conductive tubing between the sampling port and instrument was kept the same (1 m) for all devices to minimize diffusion losses to the tube surfaces (Kumar et al. 2008). As fine and UF TiO2 particles were aerosolized in two different chambers due to generation system constraints, it is noteworthy that analysis of the instrument measurements was conducted separately as described previously.
Fine TiO2 particles
Fine TiO2 (< 5 μm, 99.9%+, Sigma-Aldrich, St. Louis, MO) was aerosolized using fluidized bed in a well-characterized 19 L chamber made of mainly plastic and plexiglass at the NIOSH inhalation exposure facility (Chen et al. 2006, 2016). The Fluidized bed aerosol generator (Model 3400A, TSI Inc.) was used to disperse powders over a size range from 0.5 to 40 μm. As HEPA-filtered air passed around the bronze beads and dust particles, it created a boiling action that de-agglomerated the powder for stable aerosol dispersion at given concentrations. For all the exposure scenarios with fine particles, number concentration declined markedly after 650 nm and thus it is conceivable that overall particle size was no more than 1.5 μm (Supplemental Figure S1). The powder was fed into the fluidized bed by a variable speed bead-chain. Filtered air entering from beneath the fluidized bed carried the particles at 12 LPM to the outlet. On the fluidized bed, the continuous motion of the large bronze beads reduced agglomeration of the fine particles (Figure 1A). During sampling, mass concentrations in the exposure chamber were continuously monitored with a DataRAM (DR-4000, Thermo Electron Co., Franklin, MA). Aerosol mass concentration was stable among sampling ports (DataRAM coefficient of variation [CV] 5%).
UF TiO2 particles
The UF TiO2 bulk powder was obtained from Degussa (Aeroxide TiO2, P25, Evonik, Parsippany, NJ) and received with a primary particle size of 21 nm. To reduce the potential formation of agglomerates because of weaker Van Der Waals forces, UF TiO2 powder was carefully prepared for aerosol generation by drying (to avoid agglomeration because of high humidity), sieving (to remove the large agglomerates), and storing (to prevent agglomerate attraction through contact charges) (Nurkiewicz et al. 2008; Yi et al. 2013). The exposure scenarios encompassed a range of UF TiO2 concentrations that were generated in a well-characterized 19 L exposure chamber at West Virginia University (WVU) using a vibrating fluidized bed, a Venturi vacuum pump, and a cyclone separator. The dry powder rested on a filter supported by a metal air distributor in the vibrating fluidized bed. The Venturi vacuum pump, connected to the exit port of the fluidized bed, blew a high velocity air jet across a constriction in a pipe, thus drawing in air and particles into the Venturi vacuum pump. The large agglomerates were broken up by impaction and by the high-speed shear flow in the Venturi vacuum pump. The particles were then ejected into a cyclone separator (Figure 1B).
Stability of the aerosol concentration in the chamber was measured in real time using an electrical low pressure impactor (ELPI™, Dekati Ltd., Kangasala, Finland). Particle size distribution for all the exposure scenarios with UF particles using number concentration drastically declined beyond 650 nm so it is reasonably believed that overall particle size was no more than 1.5 μm size (Supplemental Figure S2). Based upon gravimetric concentrations, mean UF TiO2 particle concentration was calculated within the same day as 5.3–6.6 mg/m3 for a target concentration of 6.0 mg/m3. CV was calculated at 0.02% and 0.17%. UF particle concentration was measured at 4 different locations in the zones just above the cages in the exposure chamber to maintain appropriate environmental parameters inside the chamber (spatial variation). The maximum CV of the aerosol concentrations at different measurement locations from the mean concentration was less than 6% (Yi et al. 2013).
Morphological analysis of fine and UF TiO2 particles
Scanning electron microscopy (SEM, Hitachi S-4800, Tokyo, Japan) was used to characterize the morphology of fine and UF TiO2 particles at 5.0 kilovolts (kV) accelerating voltage and varying magnifications. Morphology of particles such as shape and diameters of agglomerates at two concentrations of TiO2 (fine: 13.2 mg/m3 and UF: 2.4 mg/m3) particles were measured and presented using SEM images.
Data analysis
As illustrated in Figure 2, three metrics (mass, number, and surface area concentrations) were analyzed for each comparison, i.e., inter-instrument, inter-device within same instrument-type, and intra-device comparison, for 11 exposure scenarios, using a mixed model analysis of variance (ANOVA) and RMSEs. Measurements were collected with each instrument to observe background aerosol concentrations before beginning the test scenarios. Data was truncated to align with sample gravimetric mass collection, processed and analyzed using SAS and JMP® 10 (SAS Institute Inc., Cary, NC).
Figure 2.
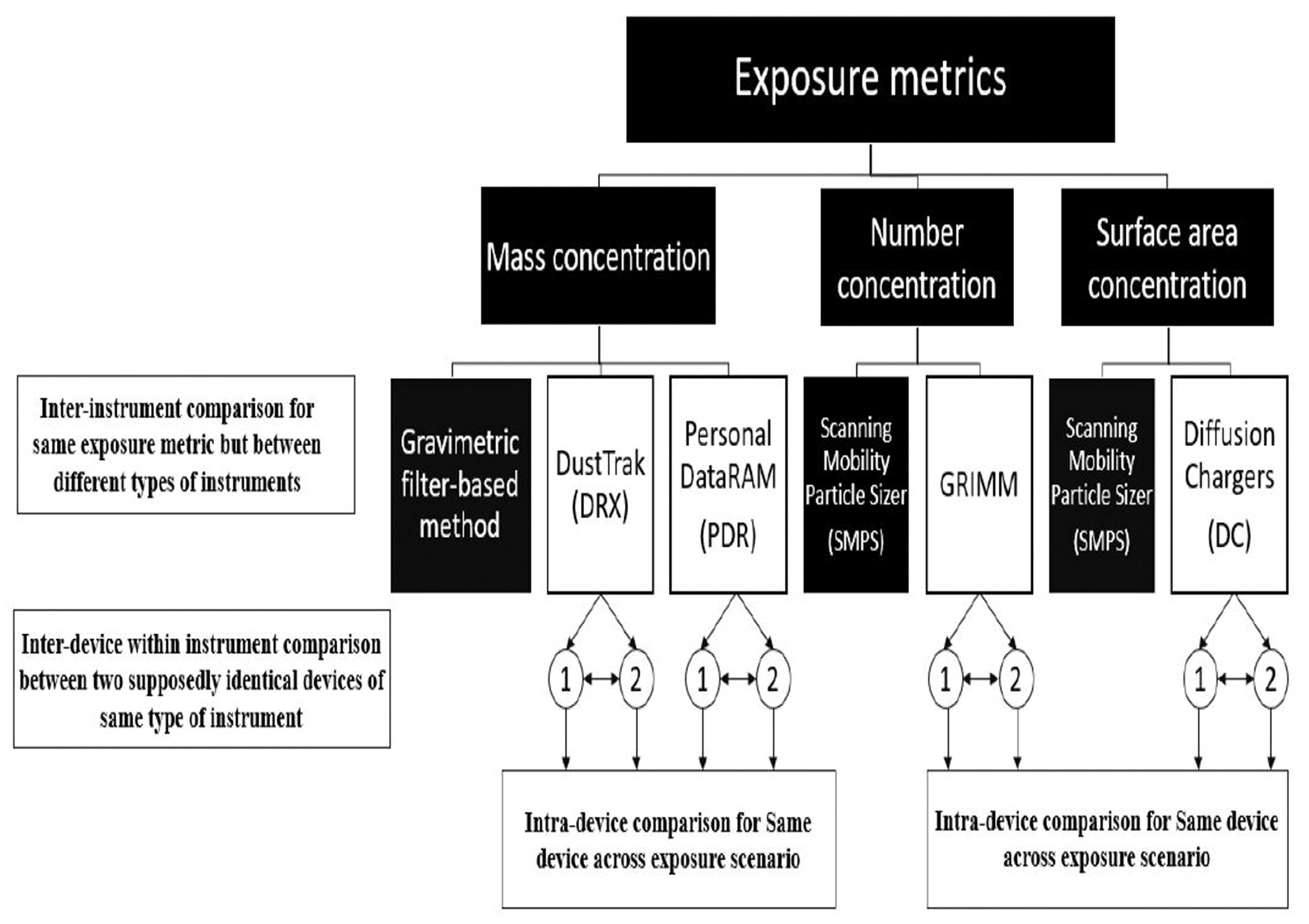
Instrument comparisons to apportion variance sources for each test scenario.
Measurement variability was confounded by aerosol generation variability from the system for both fine and UF TiO2 powder sizes. To reliably estimate the variability between instrument-device measurements, contribution of the aerosol generation system needs to be minimized. Measurement data for each device were plotted versus time to identify a shorter time period where the aerosol concentration was relatively stable. Only measurements during this reduced period were considered in the calculation of variance components to minimize system variability.
An ANOVA model was used to calculate variance components by powder size using restricted maximum likelihood with unbounded variance components. Variance components were estimated from a model with a random effect of instrument and device nested within instrument. Gravimetric mass concentration was used as a fixed effect. The total variance was fractioned into components of interinstrument, inter-device within same instrument-type, and intra-device. The majority of the residual effect of the ANOVA model was contributed by variability of the instrument-device itself and thereby it was considered as intra-device variance. The sum of these three components equals 100%, which enables a rapid interpretation and identification of the major contributors to the variance. The performance of each device was assessed using RMSE, an indicator of accuracy that combines bias and precision of the measurement (Eq. 1):
| (1) |
where RMSE is expressed in percent (%), and the first squared term in the RMSE equation is the bias or % difference from the reference method. Smaller values of RMSE approaching zero can be interpreted as better performance compared to larger values indicating poor performance. The precision of the instrument measurement was assessed using the coefficient of variation (CV) from a period of relatively stable (i.e., less than 6%) aerosol concentration for all the exposure scenarios. Therefore, CV for the instrument measurement was calculated as the standard deviation divided by the mean of the instrument measurements times 100% without considerable influence by experimental set up. Gravimetric mass concentrations were employed as the reference method to calculate bias for the DRX and PDR. However, for number and surface area concentrations, no reference method exists. Therefore, SMPS measurements (measured number and calculated surface area concentrations) were used as the reference method for the number concentrations measured by GRIMM and active surface area measured by DC.
Results
Results for three variance components for instrument comparisons by exposure metric (mass, number, and surface area) by powder size, fine and UF TiO2 particles (Figure 2) are described using mixed model ANOVA. Three variance components (inter-instrument, inter-device within same instrument-type, and intra-device variance components) are displayed in Table 2. To further addressing intra-device variance component, RMSEs are presented as an indicator of each device performance by powder size and gravimetric mass concentration levels.
Table 2.
Apportionment of variance components between instruments, between devices, and within devices using a mixed model ANOVA.
| TiO2 Powder size | Exposure metrics | Variance components (%) |
||
|---|---|---|---|---|
| Inter-instrument | Inter-device | Intra-device | ||
| Fine | Mass | 83.9 | 0.0 | 16.1 |
| Number | 40.8 | 5.5 | 53.7 | |
| Surface Area | 84.8 | 0.0 | 15.2 | |
| UF | Mass | 62.6 | 0.5 | 36.9 |
| Number | 86.9 | – | 13.1 | |
| Surface Area | 73.4 | 0.0 | 26.6 | |
TiO2 = Titanium dioxide; UF = Ultrafine; – = not evaluated due to measurements with only one GRIMM
Instrument comparisons using mixed model ANOVA
As expected, generally, the greatest variability was attributed to inter-instrument comparisons with the exception of number concentrations of fine TiO2 particles. Among all instrument comparisons, the smallest variability was attributed to interdevice variability (0.0%–5.5%), which indicated consistent results between devices of the same instrument type.
For mass concentration, the mixed model ANOVA resulted in greater inter-instrument variability (83.9%) compared with intra-device variability (16.1%) for all exposure scenarios of fine TiO2 particles. Similar observation was noticed for UF TiO2 particles by resulting in greater interinstrument (62.6%) variability than intra-device variability (36.9%).
For number concentration, the mixed model ANOVA resulted in greater intra-device variability (53.7%) compared to inter-instrument variability (40.8%) for fine TiO2 particles, with inter-device being the smallest variability at 5.5%. Contrastingly, inter-instrument variability (86.9%) was greater than intra-device variability (13.1%) for UF TiO2 particles.
Similar to mass concentration, greater interinstrument variability (84.8%) was observed compared to intra-device variability (15.2%) between active surface area measured by DC and surface area calculated by SMPS for fine TiO2 particles. Similarly, for UF TiO2 particles, greater inter-instrument variability (73.4%) was noted compared to intra-device variability (26.6%). One reason for these discordant measurements might be that DC measures “active” surface area of diffusively charged particles (i.e., “Fuch’s” surface area) and SMPS surface area is calculated from mobility-based particle number concentrations. Previously investigators comparing surface area measurements using SMPS and DC expressed considerable concerns for TiO2 particles under various exposure scenarios such as type of powder, sizes, or shape in lab setups as well as at workplace investigations (Ku 2010; Ku and Kulkarni 2012; Vosburgh, Ku, and Peters 2014). Leskinen et al. (2012) also reported a larger difference (90%–248%) between surface area measurements of TiO2 nanoparticles.
Instrument comparisons using RMSE
As an indicator of device performance, RMSEs for each device by powder size and gravimetric mass concentration levels are presented for mass (Table 3), number (Table 4), and surface area (Table 5) concentrations. Performance “within” a device may be interpreted by comparing RMSEs across experimental conditions (e.g., DRX 1 at 3.43 mg/m3 versus DRX 1 at 3.17 mg/m3). Inter-device performance may be interpreted by comparing RMSEs of two devices for the same type of instrument (e.g., DRX 1 versus DRX 2 at 3.43 mg/m3). Tables 3–5 also include mean concentration values for each device to promote direct comparison to the mean values of the reference measurement. CV (as a part of RMSE, Equation 1) was obtained to provide a measure of the consistency of each device during stable aerosol concentration for every exposure scenario with fine (Supplemental Table 1) and UF TiO2 particles (Supplemental Table 2). Generally, a comparable range of CVs was obtained for all instruments measuring exposure scenarios for mass and number concentration measurements between UF (mass: 1–7%; number: 0.5–7%) and fine (mass: 0.3–2%; number: 0.5–5%). Unlike mass and number measurements, relatively greater CVs were noted for surface area measurements (fine: 1–17%; UF: 0.7–27%). Average and SD of the instrument CV value may be interpreted as instruments ability to consistently measure aerosol exposure metrics of fine and UF TiO2 particles.
Table 3.
Performance (RMSE) of PDR and DRX aerosol mass concentrations.
| TiO2 powder size | Grav. mass conc. (mg/m3) | RMSEs (%) |
Mean mass concentration (mg/m3) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| DRX 1A | DRX 2B | PDR 1C | PDR 2D | DRX 1 | DRX 2 | PDR 1 | PDR 2 | ||
| Fine | 3.17 | 3.8 | 0.8 | 99 | 21 | 3.3 | 3.2 | 6.3 | * |
| 3.43 | 7.2 | 5.9 | 96 | 90 | 3.2 | 3.2 | 6.7 | 6.5 | |
| 5.58 | 2.9 | 3.1 | 109 | 117 | 5.7 | 5.7 | 11.7 | 12.1 | |
| 5.90 | 2.9 | 1.1 | 106 | 111 | 6.1 | 5.9 | 12.1 | 12.5 | |
| 13.2 | 1.4 | 5.3 | 91 | 77 | 13.0 | 12.5 | 25.2 | 23.4 | |
| 13.4 | 6.1 | 3.3 | 93 | 112 | 12.6 | 13.0 | 25.8 | 28.3 | |
| UF | 1.38 | 4.5 | 19 | 91 | 43 | 1.4 | 1.4 | 0.1 | 0.8 |
| 1.87 | 24 | 34 | 51 | 52 | 2.3 | 2.3 | 0.9 | 0.9 | |
| 5.80 | 17 | 35 | 31 | 41 | 6.8 | 7.3 | 4.0 | 3.5 | |
| 9.20 | 10 | 22 | 50 | 43 | 8.3 | 8.3 | 4.6 | 5.3 | |
| 10.8 | 25 | 32 | 27 | 47 | 13.5 | 14.2 | 7.9 | 5.8 | |
RMSEs = Root mean squared errors; UF = Ultrafine; Grav. mass conc. = Gravimetric mass concentration;
= Data not collected due to instrument failure; A = Coefficient of Variation (CV) (Average % ± SD for fine: 0.82% ± 0.29 and UF: 1.80% ± 0.84) for DRX 1; B = CV (Average % ± SD for fine: 1.00% ± 0.0 and UF: 2.20% ± 0.84) for DRX 2; C = CV (Average % ± SD for fine: 0.88% ± 0.29 and UF: 2.80% ± 1.79) for PDR 1; D = CV (Average % ± SD for fine: 0.98% ± 0.57 and UF: 3.60% ± 2.07) for PDR 2
Table 4.
Performance (RMSE) of GRIMM and SMPS aerosol number concentrations.
| TiO2 powder size | Grav. mass conc. (mg/m3) | RMSEs (%) |
Mean number concentration (particles/m3) |
|||
|---|---|---|---|---|---|---|
| GRIMM 1A | GRIMM 2B | GRIMM 1 | GRIMM 2 | SMPSC | ||
| Fine | 3.17 | 51 | 98 | 6.37E+09 | 8.38E+09 | 4.23E+09 |
| 3.43 | 60 | 114 | 6.79E+09 | 9.11E+09 | 4.26E+09 | |
| 5.58 | 74 | 99 | 1.25E+10 | 1.43E+10 | 7.17E+09 | |
| 5.90 | 68 | 75 | 1.36E+10 | 1.42E+10 | 8.11E+09 | |
| 13.2 | 17 | 20 | 1.23E+10 | 1.19E+10 | 1.48E+10 | |
| 13.4 | 25 | 25 | 1.24E+10 | 1.25E+10 | 1.66E+10 | |
| UF | 1.38 | 53 | * | 2.22E+09 | * | 4.75E+09 |
| 5.80 | 47 | * | 9.93E+09 | * | 1.85E+10 | |
| 9.20 | 60 | * | 1.29E+10 | * | 3.24E+10 | |
| 10.8 | 63 | * | 1.31E+10 | * | 3.57E+10 | |
RMSEs = Root mean squared errors; UF = Ultrafine;
= Data not collected; Grav. mass conc. = Gravimetric mass concentration;
= Data not collected due to instrument failure; A = CV (Average % ± SD for fine: 2.33% ± 1.21 and UF: 2.63% ± 1.75) for GRIMM 1; B = CV (Average % ± SD for fine: 1.25% ± 0.61) for GRIMM 2; C = CV (Average % ± SD for fine: 3.00% ± 1.41 and UF: 4.00% ± 3.11) for SMPS
Table 5.
Performance (RMSEs) of DC and SMPS for surface area concentrations.
| TiO2 powder size | Grav. mass conc. (mg/m3) | RMSEs (%) |
Mean surface area concentration (m2/m3) |
|||
|---|---|---|---|---|---|---|
| DC 1A | DC 2B | DC 1 | DC 2 | SMPSC | ||
| Fine | 3.17 | 97 | 98 | 6.29E-05 | 3.32E-05 | 3.57E-03 |
| 3.43 | 96 | 98 | 6.56E-05 | 3.67E-05 | 3.48E-03 | |
| 5.58 | 97 | 98 | 1.03E-04 | 7.51E-05 | 5.91E-03 | |
| 5.90 | 97 | 98 | 9.64E-05 | 6.92E-05 | 6.69E-03 | |
| 13.2 | 98 | 98 | 1.66E-04 | 1.01E-04 | 1.23E-02 | |
| 13.4 | 98 | 99 | 1.82E-04 | 1.01E-04 | 1.39E-02 | |
| UF | 1.38 | 95 | 96 | 1.07E-04 | 9.05E-05 | 4.46E-03 |
| 1.87 | 99 | 99 | 4.38E-05 | 3.29E-05 | 5.80E-03 | |
| 5.80 | 99 | 100 | 7.80E-05 | 5.88E-05 | 1.61E-02 | |
| 9.20 | 98 | 103 | 2.66E-04 | 1.16E-04 | 2.73E-02 | |
| 10.8 | 99 | 99 | 2.08E-04 | 1.49E-04 | 3.01E-02 | |
RMSEs = Root mean squared errors; UF = Ultrafine; Grav. mass conc. = Gravimetric mass concentration; A = CV (Average % ± SD for fine: 5.83% ± 3.13 and UF: 3.54% ± 2.49) for DC 1; B = CV (Average % ± SD for fine: 5.00% ± 3.16 and UF: 9.00% ± 10.98) for DC 2; C = CV (Average % ± SD for fine: 8.00% ± 4.47 and UF: 5.60% ± 3.44) for SMPS
Mass concentration for fine and UF particles
Percentage RMSEs and mean mass concentrations for DRXs and PDRs are presented for each exposure scenario for fine and UF TiO2 particles (Table 3). Mass measuring instruments were precise with average CVs less than 5% but exhibited greater variability for UF (2.60% CV ± 1.54 SD) compared to fine (0.92% CV ± 0.33 SD); although, most of the RMSEs were potentially influenced by mean differences between instruments and gravimetric mass concentration (CVs in supplemental Table S1 (fine) and Table S2 (UF)). Generally, DRXs (fine: 0.8–7.2% and UF: 4.5–35%) displayed lower RMSEs than PDRs (fine: 21–117% and UF: 27–91%) for both powder sizes. Due to discrepancies between size-resolution capabilities (Table 1), DRXs generally might overestimate exposure scenarios with UF (but not with fine), and PDRs overestimate for exposure scenarios with fine particles (but underestimated exposure scenarios with UF particle). Mean differences between PDRs and gravimetric mass concentrations were higher compared with DRXs, which reflected as a wider range of RMSEs for fine (21–117%) than UF (27–91%) TiO2 particles.
Number concentration for fine and UF particles
Performance, measured as RMSE, of GRIMM devices and SMPS for measuring aerosol number concentrations is presented in Table 4. Using SMPS measurements as a reference, GRIMM 2 (20%–114%) performance mostly resulted in greater range for RMSEs compared with GRIMM 1 (17%–;74%) for fine TiO2 particles. Similar to mass measurements, CVs for GRIMM 1 and GRIMM 2 were lower and, therefore, RMSEs were dominated by their mean differences to SMPS measurements. For exposure scenarios with fine TiO2 particles, RMSEs for GRIMM 2 (Average % ± SD: 70% ± 40) were higher than GRIMM 1 (49% ± 23), which was driven by a higher mean difference relative to the SMPS measurements because CVs for GRIMM 2 (1.25% ± 0.61) were less than GRIMM 1 (2.33% ± 1.21). Generally, SMPS (fine: 3.00% ± 1.41 and UF: 4.00% ± 3.11) resulted in higher CV than GRIMM devices (fine: 2.33% ± 1.21 and UF: 2.63% ± 1.75) for all the exposure scenarios of both sizes of TiO2 powder.
Surface area concentration for fine and UF particles
For surface area concentration, RMSEs and mean values for DCs and SMPS are displayed in Table 5. It is noteworthy here that SMPS surface area was calculated (not measured) by SMPS number measurements as a reference value to calculate RMSEs (Eq. 1). Percentage RMSEs are higher (> 97%) for both DCs due to higher mean differences with SMPS for both powder sizes. DC directly measures “active” surface area of the particles, unlike SMPS, which calculates surface area based on an algorithm that depends on mobility-based particle counts. For exposure scenarios with fine TiO2 particles, the DCs (Average % ± SD for DC 1: 5.83% ± 3.13 and DC 2: 5.00% ± 3.16) were more consistent with less CV than SMPS (8.00% ± 4.47). However, for exposure scenarios with UF TiO2 particles, SMPS (5.60% ± 3.44) resulted into comparable CV to DC 1 (3.54% ± 2.49).
Morphology of fine and UF TiO2 particles
Using SEM images, the morphology of UF (gravimetric mass concentration: 2.4 mg/m3) and fine (gravimetric mass concentration: 13.2 mg/m3) TiO2 particles are displayed in Figures 3A (UF) and 3B (fine). Particle morphology was neither spherical or smooth-surfaced for either powder size because of particle interactions, such as agglomeration, which supports the SMPS observations of a multimodal aerosol number size distribution (Supplemental Figure S1) and UF (Supplemental Figure S2) TiO2 particles. For fine TiO2 particles, we identified a dominant mode at 270 nm for test scenarios with gravimetric mass concentrations greater than 5.6 mg/m3. For UF TiO2 particles, three modes were apparent at 15 nm, 140 nm, and 400 nm, with the latter two modes being the dominant for test scenarios with gravimetric mass concentrations greater than 5.8 mg/m3, indicating a potential increase in agglomeration with increasing mass concentrations.
Figure 3.
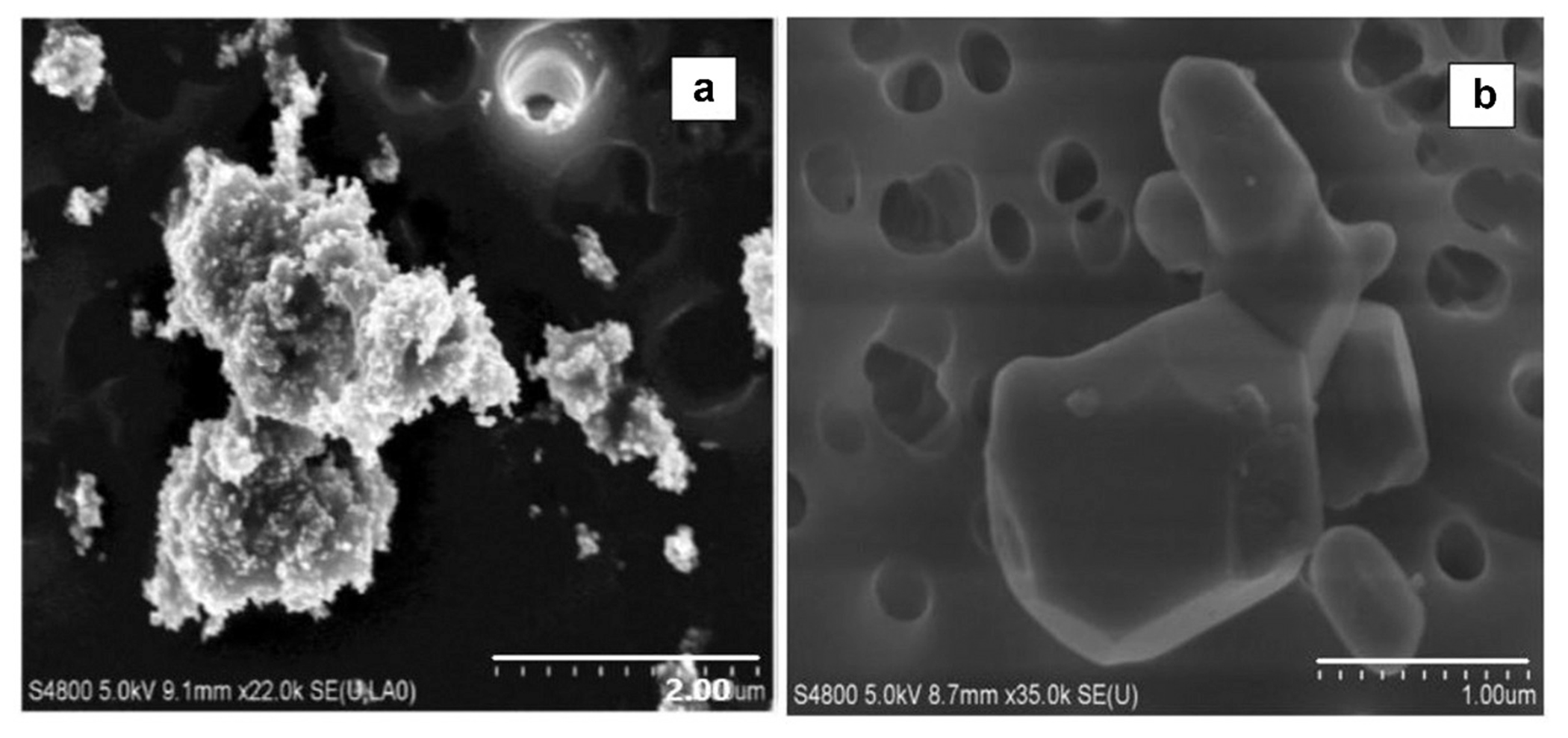
SEM images for (a) UF TiO2 collected in a chamber at a gravimetric mass concentration of 2.4 mg/m3 and (b) fine TiO2 collected in a chamber at a gravimetric mass concentration of 13.2 mg/m3. Note that different scale bars between images are only indicative of the nonspherical nature of UF and fine TiO2 particles.
Discussion
This study considered a holistic approach to sampling TiO2 nanoparticles
The availability of aerosol sampling instruments other than those examined in this review is acknowledged; however, our approach was to explore the measurement variability by different types of instruments documented in previously published papers including representative respective metrics. In this study, one aspect of misalignment between lab and field instruments was addressed by focusing on the performance and comparison of measurements for aerosolized fine and UF TiO2 particles in two different experimental set ups. By considering the measurement of fine and UF TiO2 particles at a wide range of mass concentrations used to assess adverse health outcomes in toxicological studies, the instrument comparisons presented in this study provide realistic and relevant performance data. From an exposure perspective, the range of mass concentrations mimic variability seen in industrial hygiene investigations. By implementing NIOSH’s NEAT approach in assessing the aerosolized TiO2 particles for different metrics, such as mass, number, and surface area, an attempt to bridge the knowledge gaps associated with mono-metric was undertaken based upon previous investigations and the necessity of using a combination of different methods for understanding UF TiO2 aerosols and toxicity was addressed (Lovén et al. 2021). The difference in operating principles of these instruments (Table 1) might have been a potential reason for higher mean differences for both powder sizes. Greatest inter-instrument variability indicated the existence of considerable variabilities in reporting measurements between different types of instruments for mass, number, or surface area concentrations of TiO2 particles consistent with mono-metric instrument comparisons from previous studies (Jørgensen 2019; Ku 2010; LeBouf et al. 2011a; Leskinen et al. 2012; Stabile et al. 2014; Vosburgh, Ku, and Peters 2014). Performance and measurement variability within and between different instruments used to assess UF aerosol characteristics might aid in interpreting occupational exposure data used in risk models, epidemiologic studies, and in development of exposure limits in relation to health effect endpoints identified in toxicological studies that employed dissimilar instruments.
Mass concentration for fine and UF TiO2 particles
Previous investigators documented considerable mass measurement errors in predicting the response of a nephelometer, such as PDRs, because the intensity of scattered light rises with increasing particle size (e.g., from UF to fine particles) (Chakrabarti et al. 2004; Sioutas et al. 2000; Thomas and Gebhart 1994). Because the manufacturer calibrates the PDRs using International Organization for Standardization (ISO) fine aerosolized test dust of a mass median diameter of 2,000–3,000 nm, the instrument underreported the mass of smaller-sized (< 1,000 nm) TiO2 particles in this study. Along with differences in operating principles including how each instrument calibrated, and the potential effect of wall loses might produce deviations in gravimetric analyses. The impact of wall losses on total particle mass for different particle type or composition was recognized by NIOSH’s guidance in 2014 (NMAM Chapter O, 5th Ed) and documented by Puskar et al. (1991) for pharmaceutical dust. However, variability attributed to wall losses in gravimetric sampling were beyond the scope of this study.
The differing apportionment of variability between powder sizes was likely because of better agreement between instruments for UF, which decreased inter-instrument variability and raised intra-device variability. PDRs consistently measured increased mass concentrations for all fine TiO2 test scenarios because of scattering effects of the material. In situ measurements of particle mass by PDRs, rather than gravimetric mass collection on a filter, incorporate additional mass due to condensational growth of the particles associated with water uptake by hygroscopic components (McMurry, Zhang, and Lee 1996; Sloane 1984). Thus, nephelometers tend to overestimate particle mass when condensational particle growth is favored in high RH environments. Particle growth in high humidity environments was studied and modeled by Sloane (1984) and Lowenthal et al. (1995). Relative humidity did not markedly affect our study results as it was low at approximately 5% RH. However, influence of RH in aerosol measurement such as hygroscopic growth in size, concentrations, or agglomeration needs to be considered in field and toxicology lab studies when using nephelometers.
Our analysis with all the exposure scenarios with UF particles presented limited ability of photometers such as DRX and PDR in measuring smaller sized particles likely governed by Rayleigh scattering. The same limitation of realtime photometers (e.g., DRX and PDR) in accurately and consistently measuring UF particles was also reported by Ostraat, Thornburg, and Malloy (2012). Particle measuring instruments are calibrated using various types of reference particles such as Arizona road dust for DRX and ISO test dust for PDR. The test particles used to calibrate these photometers may possess different characteristics than TiO2 particles. Variability in TiO2 particle measurements may be explained by contribution of differences in particle characteristics such as size, shape, absorption and scattering of light, and refractive index (Hinds 1999) compared to that of calibration test particles. In addition, the difference in refractive index between TiO2 (2.61) and ISO test dust (1.54) for PDRs might explain deviations (1.69 = 2.61/1.54) in the mass measurements. GörneGörner, Berner, and Fabries (1995) proposed a calibration index theoretically predicting photometer response for any aerosol based upon aerosol parameters such as particle density, size distribution, and refractive index of the calibration and test aerosol as well as photometer parameters such as the wavelength and angles of light. However, a simpler approach is recommended to correct positive and negative biases by calibrating each photometer measurement using the mass collected on the internal filter of the device. This was not done in this case as this would demonstrate significant bias associated with not calibrating PDR measurements with the test aerosol.
Number concentration for fine and UF TiO2 particles
The greater variability between different types of instruments observed in this study was consistent with other aerosol investigations conducted using number concentration of fine and UF particles. Jørgensen (2019) reported that number concentrations of UF particles (not TiO2) measured by Nanoscan SMPS were considerably overestimated (1.44–2-fold higher) than SMPS for exposure scenarios, which were similar to occupational hygiene studies. Further, number concentration measurements between instruments (e.g., CPC 3007 and SMPS 3938) from the same brand type (i.e., TSI Inc.) were not comparable with each other in Jørgensen (2019) study. Number concentration measuring instruments are calibrated using specific types of reference particles such as polystyrene latex for GRIMM and spherical salt particles of smooth surface for SMPS. Differences between operating principles (Table 1) as well as the fact that the instruments were measuring TiO2 but calibrated with a different material might account for some of the observed difference in response for fine and UF TiO2 particles.
It is noteworthy that to more accurately compare measured number concentrations, only considered particle sizes between GRIMMs (300–650 nm) and SMPS (300–637 nm) were considered. As GRIMMs’ size resolution limits the ability to measure number concentration < 300 nm (unlike SMPS: approximately 15 nm), it is reasonable to expect higher inter-instrument variability (86.9%) for exposure scenarios of UF TiO2 particles. GRIMM measures the size range of fine particles better than UF particles because of its scan range from 300–20,000 nm. SMPS measures smaller-sized UF particles better than fine particles because of its scan range from 15–661 nm (Table 1). Comparing number concentration measuring instruments, Asbach et al. (2009) reported considerable inter-instrument variability (CPC vs SMPS) and a reliable inter-device agreement between CPCs. For UF, inter-instrument variability was 86.9% between GRIMMs and SMPS (Table 2), which was attributed to low intra-device variability and failure of GRIMM 2 for the UF trials. Different operating principles for both these instruments and measurement size ranges (Table 1) might have also contributed to inter-instrument variability between GRIMMs and SMPS. For UF, SMPS electrical mobility-based measurement was greater than the GRIMM optical-based instrument. For fine, GRIMM and SMPS exhibited lower apportioned inter-instrument variance (40.8%) than intradevice variance (53.7%), which was attributed to an increase in %RMSE for GRIMM 2 in the lower three mass concentrations (Table 4). Similar to the approach presented in our study, Leskinen et al. (2012) evaluated number and surface area concentration of two sizes of TiO2 agglomerates and powder types using common aerosol monitoring instruments. Leskinen et al. (2012) concluded that larger differences between outputs of different instruments might be attributed to the nature-type such as chemical property of the test particles, especially biologically active TiO2 particles with complex morphology due to interactions such as particle agglomerations.
Surface area concentration for fine and UF TiO2 particles
For surface area concentration, inter-instrument variance between DC and SMPS (> 70%) for both TiO2 particle sizes (Table 2) was likely due to differences in surface area measurement principles. DC measures active (“outer envelope”) surface area concentration of particles, unlike SMPS, which measures particle number concentration and calculates surface area concentration. SMPS number concentration measurements are based upon the assumption that particle shape is spherical, which is not consistent with the compact particle agglomerate morphology for TiO2 in this study. These results were consistent with earlier findings that also suggested that DCs underestimated surface areas for both spherical monomers and agglomerates (Ku 2010; Ku and Kulkarni 2012). Although small (approximately 1%) consistently greater RMSE were detected for DC 2 data compare with DC1; this difference may have been attributed to an elevated flow rate of DC 2, approximately 25% higher (1.25 LPM) than optimal (1 LPM), although the manufacturer’s instruction manual states this would not affect results. Flow rate variation is not incorporated in the internal calculation of surface area concentration. This elevated flow rate might produce a reduction in surface area concentrations reported by the instrument. Surface area concentration measurements of DC and SMPS observed in this study were not as comparable to each other as a cumulative surface area metric for a similar study material (LeBouf et al. 2011a). This may have been due to differences between the two studies in terms of devices used and generation procedures. Vosburgh, Ku, and Peters (2014) found considerable deviations while measuring different concentrations of aerosols of interest when moving or vibrating a DC at a workplace and stated that DC surface areas were significantly different compared to reference surface areas.
Evaluating instruments from the same manufacturer (i.e., TSI Inc., Shoreview, MN), Leskinen et al. (2012) also reported greater differences (90%–248%) between a nanoparticle surface area monitor (NSAM, TSI model: 3550) and SMPS for measuring the surface area of TiO2 nanopowder. Leskinen et al. (2012) argued that NSAM overestimated surface areas of larger, and non-spherical TiO2 agglomerates compared with spherical TiO2 particles measured by SMPS. The charging efficiency unipolar or bipolar of different shapes of particles spherical vs non-spherical is a crucial phase in the measuring process for these instruments. SMPS uses bipolar charging while DC uses corona mediated unipolar ion discharging. The operating principles of these instruments assume a certain charging efficiency such as charge distribution and use this assumption to convert the measured values to the particle distribution. As the larger agglomerates expressed different dimension than spherical smooth-surfaced particles, the charging efficiency of the unipolar charger differs for larger agglomerates and spherical smooth-surfaces particles (Shin et al. 2010). As it is not practically possible to calibrate the instruments using all possible agglomerates, typically, the instruments are calibrated using specific types of reference particles (e.g., PSL, spherical salt particles, oil) and, therefore, the response for real particles (e.g., large agglomerates) might be different.
Limitations
A multi-metric approach including the morphological analysis enabled to complete our approach for characterizing airborne TiO2 particles at specific exposure scenarios. However, it is understood that there have been other sampling instruments available in the market than considered in this study which might produce different measurement variabilities. Some limitations and assumptions in the study affected apportionment of variance components. In modeled ANOVA, the inter-instrument and intra-device variance accounted for nearly all of the apportioned variance (approximately 95%–100%) for both fine and UF TiO2 sizes. Proportion of inter-instrument variance (fine 83.9% and UF 62.6%) contributed by DRX and PDR was not analyzed in ANOVA model, but a separate set of analysis was conducted to evaluating performance of each instrument. Further, only one GRIMM device was used for UF powder size due to device failure and inter-device variability was not assessed due to measurements with only one GRIMM (Table 2). Performance including bias (i.e., mean differences) and precision (i.e., CV) measured by RMSE was complimentary to the apportioned variance and aided in the interpretation of intra-device variability.
The distinction of fine and UF TiO2 from background aerosols was performed by subtracting the background concentrations (mean pre- and postactivity concentrations) and assuming that concentrations of TiO2 particles were relatively stable during the measurements to minimize its influence on instrument variability (Jensen et al. 2015; Koivisto et al. 2012a, 2014, 2012b; Koponen, Koivisto, and Jensen 2015). In real-world scenarios, instrument measurements (considered in this study or not) might face more complex conditions, such as a mixture of fine and UF particles or a variety of relative humidity and morphologies and thus, might exhibit more deviations in variabilities than reported in our investigation.
Further, SMPS assumes a spherical particle shape with a smooth surface when assessing number concentration and electrical mobility diameter. Morphological analysis of aerosols in this study indicated the non-spherical shape of both TiO2 powder sizes, which likely contributed to instrument performance and variability. Chen et al. (2016) documented limitations of SMPS in measuring size distribution and total number concentrations of UF TiO2 particles compared with microscopic analysis. Regarding morphological analysis, temperature and RH effects on TiO2 particle growth or agglomeration were not tested in this study. Research focused on morphological changes due to fundamentals of aerosol agglomeration might better explain misalignments between existing instrument variability and, thereby, deliver more reliable exposure assessment of TiO2 particles. In addition, evaluating correction factors for different types of instruments is required to generalize applications for measuring TiO2 particles across field and lab studies. Recently available portable instruments including TSI APS 3321, TSI portable SMPS and ELPI that were not examined in our study might show comparable measurements for TiO2 particles, but these comparisons are yet to be analyzed in future research.
Conclusions
Industrial hygienists and occupational health professionals might use the information from this study to better understand the variability within and between instruments for measuring nanomaterial aerosol properties using multiple metrics. DRX aerosol mass concentrations were closer to gravimetric mass concentrations than PDRs for both TiO2 powder sizes. Considerable variability in number concentrations (GRIMM) and in surface area concentrations (DC) from the field study instruments was observed when compared with the lab instrument (SMPS). This information may be employed to aid in interpreting occupational exposure data used in risk models, epidemiologic investigations, and development of exposure limits, in relation to health effect endpoints identified in toxicological studies that used dissimilar instruments. Depending on particle type and chemical specificity such as TiO2, future studies need to develop adjustment factors among instruments to convert field instrument measurements to lab-based or traditional reference concentrations that provide a real-time mass measurement for compliance monitoring, and more accurate surface area and number concentration data for exposure assessments. Data suggest a multi-metric, standardized approach to evaluate exposures among workers involved with high production volume material such as TiO2 particles consistent with NIOSH’s NEAT strategy.
Supplementary Material
Acknowledgments
The authors would like to thank Christopher Coffey and Matthew Duling for reviewing this manuscript.
Disclosure statement
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention (CDC). Mention of any company or product does not constitute endorsement by NIOSH, CDC. In addition, citations to websites external to NIOSH do not constitute NIOSH endorsement of the sponsoring organizations or their programs or products. Furthermore, NIOSH is not responsible for the content of these websites. All web addresses referenced in this document were accessible as of the publication date.
Funding
This work was supported by internal funding from the National Institute for Occupational Safety and Health (NIOSH) and external funding from the National Institutes for Health (NIH).
Footnotes
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15287394.2022.2150730
Data availability statement
Data inquiries can be directed to Anand Ranpara, aranpara@hsc.wvu.edu.
References
- Aitken RJ, Chaudhry MQ, Boxall ABA, and Hull M. 2006. Manufacture and use of nanomaterials: Current status in the UK and global trends. Occup Med 56:300–06. doi: 10.1093/occmed/kql051. [DOI] [PubMed] [Google Scholar]
- Asbach C, Kaminski H, Fissan H, Monz C, Dahmann D, Mühlhopt S, Paur H, Kiesling H, Herrmann F, Voetz M, et al. 2009. Comparison of four mobility particle sizers with different time resolution for stationary exposure measurements. J Nanopart Res 11:1593–609. doi: 10.1007/s11051-009-9679-x. [DOI] [Google Scholar]
- Bekker C, Brouwer DH, van Duuren-Stuurman B, Tuinman IL, Tromp P, and Fransman W. 2014. Airborne manufactured nano-objects released from commercially available spray products: Temporal and spatial influences. J Expo Sci Environ Epidemiol 24:74–81. doi: 10.1038/jes.2013.36. [DOI] [PubMed] [Google Scholar]
- Bellagamba I, Boccuni F, Ferrante R, Tombolini F, Marra F, Sarto MS, and Iavicoli S. 2020. Workers’ exposure assessment during the production of graphene nanoplatelets in R&D laboratory. Nanomaterials 10:1520. doi: 10.3390/nano10081520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez E, Mangum JB, Wong BA, Asgharian B, Hext PM, Warheit DB, and Everitt JI. 2004. Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol Sci 77:347–57. doi: 10.1093/toxsci/kfh019. [DOI] [PubMed] [Google Scholar]
- Boccuni F, Ferrante R, Tombolini F, Natale C, Gordiani A, Sabella S, and Lavicoli S. 2020. Occupational exposure to graphene and silica nanoparticles. Part I: Workplace measurements and samplings. Nanotoxicology 14:1280–300. doi: 10.1080/17435390.2020.1834634. [DOI] [PubMed] [Google Scholar]
- Boffetta P, Gaborieau V, Nadon L, Parent M-E, Weiderpass E, and Siemiatycki J. 2001. Exposure to titanium dioxide and risk of lung cancer in a population-based study from Montreal. Scand J Work Environ Health 27:227–32. doi: 10.5271/sjweh.609. [DOI] [PubMed] [Google Scholar]
- Brouwer DH, van Duuren-Stuurman B, Berges M, Bard D, Jankowska E, Moehlmann C, Pelzer J, and Mark D. 2013. Workplace air measurements and likelihood of exposure to manufactured nano-objects, agglomerates, and aggregates. J Nanoparticle Res 15:2090. doi: 10.1007/s11051-013-2090-7. [DOI] [Google Scholar]
- Chakrabarti B, Fine PM, Delfino R, and Siouta C. 2004. Performance evaluation of the active-flow personal DataRAM PM2.5 mass monitor (Thermo Anderson pDR-1200) designed for continuous personal exposure measurements. Atmos Environ 38:3329–40. doi: 10.1016/j.atmosenv.2004.03.007. [DOI] [Google Scholar]
- Chen B, Frazer D, Stone S, Schwegler-Berry D, Cumpston J, McKinney W, Lindsley B, Frazer A, Donlin M, Vandestouwe K, et al. 2006. Development of a small inhalation system for rodent exposure to fine and ultrafine titanium dioxide aerosols. Proc 7th in Aerosol Conf 858–59. [Google Scholar]
- Chen B, Schwegler-Berry D, Cumpston A, Cumpston J, Friend S, Stone S, and Keane M. 2016. Performance of a scanning mobility particle sizer in measuring diverse types of airborne nanoparticles: Multi-walled carbon nanotubes, welding fumes, and titanium dioxide spray. J Occup Environ Hyg 13:501–18. doi: 10.1080/15459624.1148267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, and Selloni A. 2014. Introduction: Titanium dioxide (TiO2) nanomaterials. Chem Rev 114:9281–82. doi: 10.1021/cr500422r. [DOI] [PubMed] [Google Scholar]
- Cohen B, Charles S Jr, and McCammon S 2001. Air sampling instruments for evaluation of atmospheric contaminants (9 ed.). [Google Scholar]
- Demir E 2020. An in vivo study of nanorod, nanosphere and nanowire forms of titanium dioxide using Drosophila melanogaster: Toxicity, cellular uptake, oxidative stress and DNA damage. J Toxicol Environ Health A 83:456–69. doi: 10.1080/15287394.2020.1777236. [DOI] [PubMed] [Google Scholar]
- Dylla H, and Hassan MM. 2012. Characterization of nanoparticles released during construction of photocatalytic pavements using engineered nanoparticles. J. Nanopart. Res 14:825. doi: 10.1007/s11051-012-0825-5. [DOI] [Google Scholar]
- Eastlake A, Beaucham C, Martinez K, Dahm M, Sparks C, Hodson L, and Geraci C. 2016. Refinement of the nanoparticle emission assessment technique into the nanomaterial exposure assessment technique (NEAT 2.0. J Occup Environ Hyg 13:708–17. doi: 10.1080/15459624.2016.1167278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca AS, Viitanen A-K, Kanerva T, Säämänen A, Aguerre-Chariol O, Fable S, Dermigny A, Karoski N, Fraboulet I, Koponen IK, et al. 2021. Occupational exposure and environmental release: The case study of pouring TiO2 and filler materials for paint production. Int. J. Environ. Res. Public Health 18:418. doi: 10.3390/ijerph18020418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryzek JP, Chadda B, Marano D, White K, Schweitzer S, McLaughlin JK, and Blot WJ. 2003. A cohort mortality study among titanium dioxide manufacturing workers in the United States. J Occup Environ Med 45:400–09. doi: 10.1097/01.jom.0000058338.05741.45. [DOI] [PubMed] [Google Scholar]
- Golanski L, Gaborieau A, Guiot A, Uzu G, Chatenet J, and Tardif F. 2011. Characterization of abrasion-induced nanoparticle release from paints into liquids and air. J. Phys. Conf. Ser 304:012062. [Google Scholar]
- Gomez V, Levin M, Saber AT, Irusta S, Dal Maso M, Hanoi R, Santamaria J, Jensen KA, Wallin H, and Koponen IK. 2015. Comparison of dust release from epoxy and paint nanocomposites and conventional products during sanding and sawing. Ann. Occup. Hyg 58:983–94. doi: 10.1093/annhyg/meu046. [DOI] [PubMed] [Google Scholar]
- Görner P, Berner D, and Fabries JF. 1995. Photometer measurement of polydisperse aerosols. J Aerosol Sci 26:1282–302. doi: 10.1016/0021-8502(95)00049-6. [DOI] [Google Scholar]
- Grassian VH, O’Shaughnessy P, Adamcakova-Dodd T, Pettibone A, M J, and Thorne PS. 2007. Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm. Environ Health Perspect 115:397–402. doi: 10.1289/ehp.9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haar C, Hassing I, Bol M, Bleumink R, and Pieters R. 2006. Ultrafine but not fine particulate matter causes airway inflammation and allergic airway sensitization to co-administered antigen in mice. Clin Exp Allergy 36:1469–79. doi: 10.1111/j.1365-2222.2006.02586.x. [DOI] [PubMed] [Google Scholar]
- Hamilton RF, Wu N, Porter D, Buford M, Wolfarth M, and Holian A. 2009. Particle length-dependent titanium dioxide nanomaterials toxicity and bioactivity. Part Fibre Toxicol 6:35. doi: 10.1186/1743-8977-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon J, Galea KS, and Verpaele S. 2021. Review of workplace based aerosol sampler comparison studies, 2004–2020. Int. J. Environ. Res. Public Health 18:6819. doi: 10.3390/ijerph18136819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashikubo I, Handika RA, Kawamoto T, Shimizu H, Thongyen T, Piriyakarnsakul S, Muhammad A, Hata M, and Furuuchi M. 2021. Worker’s personal exposure to PM0.1 and PM4 titanium dioxide nanomaterials during packaging. Aerosol Air Qual. Res 21:200606. doi: 10.4209/aaqr.2020.10.0606. [DOI] [Google Scholar]
- Hinds WC 1999. Aerosol Technology: Properties, Behavior and Measurement of Airborne Particles. New York: John Wiley & Sons. [Google Scholar]
- Hoshino A, Fujioka K, and Oku T. 2004. Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modification. Nano Lett 4:2163–69. doi: 10.1021/nl048715d. [DOI] [Google Scholar]
- Hou J, Wang L, Wang C, Zhang S, Liu H, Li S, and Wang X. 2019. Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J Environ Sci 75:40–53. doi: 10.1016/j.jes.2018.06.010. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC). 2006. Titanium dioxide (IARC Group 2B), Summary of data reported, Feb. [Google Scholar]
- International Agency for Research on Cancer (IARC monograph volume 93). Agents Classified by the IARC Monographs, 1–129. http://monographs.iarc.fr/ENG/Classification/index.php. [Google Scholar]
- International Agency for Research on Cancer (IARC): Titanium dioxide (IARC Group 2B), Summary of data reported. 2006. Feb. [Google Scholar]
- Jensen ACØ, Levin M, AJ K, KI K, AT S, and Koponen IK. 2015. Exposure assessment of particulate matter from abrasive treatment of carbon and glass fiber-reinforced epoxy-composites—two case studies. Aerosol Air Qual Res 15:1906–16. doi: 10.4209/aaqr.2015.02.0086. [DOI] [Google Scholar]
- Jeong CH, and Evans GJ. 2009. Inter-comparison of a fast mobility particle Sizer and a scanning mobility particle Sizer incorporating an ultrafine water-based condensation particle counter. Aerosol Sci Technol 43:364–73. doi: 10.1080/02786820802662939. [DOI] [Google Scholar]
- Jørgensen RB 2019. Comparison of four nanoparticle monitoring instruments relevant for occupational hygiene applications. J Occup Med Toxicol 14:28. doi: 10.1186/s12995-019-0247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski H, Beyer M, Fissan H, Asbach C, and Kuhlbusch TAJ. 2015. Measurements of nanoscale TiO2 and Al2O3 in industrial workplace environments – Methodology and results. Aerosol Air Qual. Res 15:129–41. doi: 10.4209/aaqr.2014.03.0065;. [DOI] [Google Scholar]
- Knuckles TL, Yi J, Frazer DG, Leonard HD, Chen BT, Castranova V, and Nurkiewicz TR. 2012. Nanoparticle inhalation alters systemic arteriolar vasoreactivity through sympathetic and cyclooxygenase-mediated pathways. Nanotoxicology 6:724–35. doi: 10.3109/17435390.2011.606926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivisto AJ, Lyyränen J, Auvinen A, Vanhala E, Hämeri K, Tuom T, and Jokiniemi J. 2012a. Industrial worker exposure to airborne particles during the packing of pigment and nanoscale titanium dioxide. Inhal Toxicol 24:839–49. doi: 10.3109/08958378.2012.724474. [DOI] [PubMed] [Google Scholar]
- Koivisto AJ, Palomäki JE, Viitanen A-K, Siivola KM, Koponen IK, Yu M, Kanerva AHT, Hussein T, Savolainen KM, and Hameri KL. 2014. Range finding risk assessment of inhalation exposure to nano diamonds in a laboratory environment. Int J Environ Res Public Health 11:5382–402. doi: 10.3390/ijerph110505382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivisto AJ, Yu M, Hämeri K, and Seipenbusch M. 2012b. Size resolved particle emission rates from an evolving indoor aerosol system. J Aerosol Sci 47:58–69. doi: 10.1016/j.jaerosci.2011.12.007. [DOI] [Google Scholar]
- Koponen IK, Jensen KA, and Schneider T. 2009. Sanding dust from nanoparticle-containing paints: Physical characterization. J. Phys. Conf. Ser 151:012048. doi: 10.1088/1742-6596/151/1/012048. [DOI] [Google Scholar]
- Koponen IK, Koivisto AJ, and Jensen KA. 2015. Worker exposure and high time-resolution analyses of process-related dust concentrations at mixing stations in two paint factories. Ann Occup Hyg 59:749–63. doi: 10.1093/annhyg/mev014. [DOI] [PubMed] [Google Scholar]
- Ku BK 2010. Determination of the ratio of diffusion charging-based surface area to geometric surface area for spherical particles in the size range of 100–900nm. J Aerosol Sci 41:835–47. doi: 10.1016/j.jaerosci.2010.05.008. [DOI] [Google Scholar]
- Ku BK, and Kulkarni P. 2012. Comparison of diffusion charging and mobility-based methods for measurement of aerosol agglomerate surface area. J Aerosol Sci 47:100–10. doi: 10.1016/j.jaerosci.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Paul F, Jonathan S, and Rex B. 2008. Treatment of losses of ultrafine aerosol particles in long sampling tubes during ambient measurements. Atmos Environ 42:8819–26. doi: 10.1016/j.atmosenv.2008.09.003. [DOI] [Google Scholar]
- Kumar M, Yano N, and Fedulov AV. 2022. Gestational exposure to titanium dioxide, diesel exhaust and concentrated urban air particles affects levels of specialized pro-resolving mediators in response to allergen in asthma-susceptible neonatal lungs. J Toxicol Environ Health A 85:243–61. doi: 10.1080/15287394.2021.2000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBouf RF, Ku BK, Chen BT, Cumpston JL, Frazer DG, and Stefaniak AB. 2011a. Measuring surface area of airborne titanium dioxide powder agglomerates: Relationships between gas adsorption, diffusion and mobility-based methods. J Nanopart Res 13:7029–39. doi: 10.1007/s11051-011-0616-4. [DOI] [Google Scholar]
- LeBouf RF, Stefaniak AB, Chen BT, Frazer DG, and Virji MA. 2011b. Measurement of airborne nanoparticle surface area using a filter-based gas adsorption method for inhalation toxicology experiments. Nanotoxicology 5687–99. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kwon M, Ji JH, Kang CS, Ahn KH, Han JH, and Yu IJ. 2011. Exposure assessment of workplaces manufacturing nanosized TiO2 and silver. Inhal. Toxicol 23:226–36. doi: 10.3109/08958378.2011.562567. [DOI] [PubMed] [Google Scholar]
- Leskinen J, Joutsensaari J, Lyyränen J, Koivisto J, Ruusunen J, Järvelä M, Tuomi T, Hämeri K, Auvinen A, and Jokiniemi J. 2012. Comparison of nanoparticle measurement instruments for occupational health applications. J Nanopart Res 14:718. doi: 10.1007/s11051-012-0718-7. [DOI] [Google Scholar]
- Li N, Duan Y, Hong M, Zheng L, Fei M, Zhao X, Wang J, Cui Y, Liu H, Cai J, et al. 2010. Spleen injury and apoptotic pathway in mice caused by titanium dioxide nanoparticles. Toxicol Lett 195:161–68. doi: 10.1016/j.toxlet.2010.03.1116. [DOI] [PubMed] [Google Scholar]
- Liu XD, Wang C, Meng XJ, Pan XF, J LI, Niu DS, and Chen ZJ. 2021. A non-targeted metabolomics study on urine of occupational exposure people with titanium dioxide nanoparticles. Chinese J Indust Hyg Occup Dis 9:328–32. doi: 10.3760/cma.j.cn121094-20200323-00149. [DOI] [PubMed] [Google Scholar]
- Lovén K, Franzén SM, Isaxon C, Messing ME, Martinsson J, Gudmundsson A, Pagels J, Hedmer M, Nano L, and Franzén SM. 2021. Emissions and exposures of graphene nanomaterials, titanium dioxide nanofibers, and nanoparticles during down-stream industrial handling. J Expo Sci Environ Epidemiol 31:736–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenthal HD, Rogers FC, Saxena P, Watson JG, and Chow JC. 1995. Sensitivity of estimated light extinction coefficients to model assumptions and measurement errors. Atmos Environ 29:751–66. doi: 10.1016/1352-2310(94)00340-Q. [DOI] [Google Scholar]
- Maricq MM, Podsiadlik DH, and Chase RE. 2000. Chase 2000 size distributions of motor vehicle exhaust PM: A comparison between ELPI and SMPS measurements. Aerosol Sci Technol 33:239–60. doi: 10.1080/027868200416231. [DOI] [Google Scholar]
- McGarry P, Morawska L, Knibbs LD, and Morris H. 2013. Excursion guidance criteria to guide control of peak emission and exposure to airborne engineered particles. J. Occup. Environ. Hyg 10:640–51. doi: 10.1080/15459624.2013.831987. [DOI] [PubMed] [Google Scholar]
- McMurry PH, Zhang X, and Lee QT. 1996. Issues in aerosol measurement for optical assessments. J Geophys Res 101:19188–97. [Google Scholar]
- Methner M, Hodson L, Dames A, and Geraci C. 2010a. Nanoparticle emission assessment technique (NEAT) for the identification and measurement of potential inhalation exposure to engineered nanomaterials-Part B: Results from 12 field studies. J Occup Environ Hyg 7:163–76. doi: 10.1080/15459620903508066. [DOI] [PubMed] [Google Scholar]
- Methner M, Hodson L, and Geraci C. 2010b. Nanoparticle emission assessment technique (NEAT) for the identification and measurement of potential inhalation exposure to engineered nanomaterials -Part A. J Occup Environ Hyg 7:127–32. doi: 10.1080/15459620903476355. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Vanbilloen H, Hoylaerts M, Hoet P, Verbruggen A, and Nemery B. 2001. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am J Resp Crit Care Med 164:16665–11668. doi: 10.1164/rccm2101036. [DOI] [PubMed] [Google Scholar]
- Nicole M, Berndnowack, and Nowack B. 2008. Exposure modeling of engineered nanoparticles in the environment. Environ Sci Technol 42:4447–53. doi: 10.1021/es7029637. [DOI] [PubMed] [Google Scholar]
- NIOSH. 1980. Metals reduction plant. Ashtabula, Cincinnati, OH: NIOSH/Hazard Evaluations and Technical Assistance Branch/Division of Surveillance/Hazard Evaluations and Field Studies. [Google Scholar]
- NIOSH. 1994. Particulates not otherwise regulated, total: method 0500, Issue 2, dated 15 August 1994, 1–3. Centers for disease Control and Prevention.U. S. Department of Health and Human Services. https://www.cdc.gov/niosh/docs/2003-154/pdfs/0500.pdf [Google Scholar]
- NIOSH. 1998. Method 0600. Particulates not otherwise regulated, respirable, 1–6. National Institute for Occupational Safety and Health. Centers for disease Control and Prevention. Public Health Service. U. S. Department of Health and Human Services. [DOI] [PubMed] [Google Scholar]
- NIOSH. 2011. CURRENT INTELLIGENCE BULLETIN 63. Occupational Exposure to Titanium Dioxide. Centers for Disease control and prevention. U.S. Department of Human Health and Services (NIOSH), Cincinnati, Ohio, USA. Publication No. 2011–160. 10.26616/NIOSHPUB2011160 [DOI] [Google Scholar]
- NIOSH/CDC. 2009. Approaches to Safe Nanotechnology Managing the Health and Safety Concerns Associated with NMAM guidance. National Institute for Occupational Safety and Health. Centers for disease Control and Prevention. Public Health Service. U. S. Department of Health and Human Services. https://www.cdc.gov/niosh/docs/2003-154/cassetteguidance.html (Chapter O, 5th Ed). [Google Scholar]
- Nohynek GJ, Antignac E, Thomas R, and Toutain H. 2010. Safety assessment of personal care products/cosmetics and their ingredients. Toxicol Appl Pharmacol 243:239–59. doi: 10.1016/j.taap.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Nurkiewicz TR, Porter DW, Hubbs AF, Cumpston JL, Chen BT, Frazer DG, and Castranova V. 2008. Nanoparticle inhalation augments particle-dependent systemic microvascular dysfunction. Part Fibre Toxicol 5:1. doi: 10.1186/1743-8977-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G 1996. Significance of particle parameters in the evaluation of exposure-dose-response relationships of inhaled particles. Inhal Toxicol 8:73–89. [PubMed] [Google Scholar]
- Oberdörster G, Finkelstein N, Johnston C, Gelein R, Cox C, Baggs R, and Elder A. 2000. Acute pulmonary effects of ultrafine particles in rats and mice. disc. 75-7486. Issue number 96. Res Rep Health Effect Inst 5–74. [PubMed] [Google Scholar]
- Oberdörster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, Carter J, Karn B, Kreyling W, Lai D, et al. 2005a. Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Part Fibre Toxicol 2:8. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Oberdörster E, and Oberdörster J. 2005b. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113:823–39. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, and Cox C. 2004. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol 16:437–45. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, Kreyling W, and Cox C. 2002. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A 65:1531–43. doi: 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- Organisation For Economic Co-operation And Development (OECD), Paris. 2015. Harmonized Tiered Approach to Measure and Assess the Potential Exposure to Airborne Emissions of Engineered Nano-Objects and Their Agglomerates and Aggregates at Workplaces; Series on the Safety of Manufactured Nanomaterials; ENV/JM/MONO No. 55; [Google Scholar]
- Ostraat ML, Thornburg JW, and Malloy GJQ. 2012. Measurement strategies of airborne nanomaterials. Environ Eng Sci 30:126–32. doi: 10.1089/ees.2012.0331. [DOI] [Google Scholar]
- Pelliccioni A, and Gherardi M. 2021. Development and validation of an intra-calibration procedure for MiniDISCs measuring ultrafine particles in multi-spatial indoor environments. Atmos Environ 246. doi: 10.1016/j.atmosenv.2020.118154. [DOI] [Google Scholar]
- Puskar MA, Harkins JM, Moomey JD, and Hecker LH. 1991. Internal wall losses of pharmaceutical dusts during closed-face, 37-mm polystyrene cassette sampling. Am Ind Hyg Assoc J 52:280–86. doi: 10.1080/15298669191364730. [DOI] [PubMed] [Google Scholar]
- Robichaud CO, Uyar AE, Darby MR, Zucker LG, and Mark R. 2009. Estimates of Upper Bounds and Trends in Nano-TiO2 Production as a Basis for Exposure Assessment. Wiesner Environmental Science & Technology 43 (12):4227–4233. doi: 10.1021/es8032549. [DOI] [PubMed] [Google Scholar]
- H. R, U. F, Rittinghausen S, Creutzenberg O, Bellmann B, Koch W, and Levsen K. 1995. Chronic inhalation exposure of Wistar rats and two different strains of mice to diesel engine exhaust, carbon black and titanium dioxide. Inhal Toxicol 7:533–56. doi: 10.3109/08958379509015211. [DOI] [Google Scholar]
- Sager TM, and Castranova V. 2009. Surface area of particle administered versus mass in determining the pulmonary toxicity of ultrafine and fine carbon black: Comparison to ultrafine titanium dioxide. Part Fibre Toxicol 6:15 . doi: 10.1186/1743-8977-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Magaye R, Castranova V, and Zhao J. 2013. Titanium dioxide nanoparticles: A review of current toxicological data. Part Fibre Toxicol 10:15. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin WG, Wang W, Mertler M, Sachweh B, Fissan H, and Pui DYH. 2010. The effect of particle morphology on unipolar diffusion charging of nanoparticle agglomerates in the transition regime. J Aerosol Sci 41:975–86. doi: 10.1016/j.jaerosci.2010.07.004. [DOI] [Google Scholar]
- Silva RM, TeeSy C, Franzi L, Weir A, Westerhoff P, Evans JE, and Pinkerton KE. 2013. Biological response to nano-scale titanium dioxide (TiO2): Role of particle dose, shape and retention. J Toxicol Environ Health A 76:953–72. doi: 10.1080/15287394.2013.826567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioutas C, Kim S, Chang MC, Terrell LL, and Gong H. 2000. Field evaluation of a modified data ram mie scattering monitor for real-time PM2.5 mass concentration measurements. Atmos Environ 34:4829–38. doi: 10.1016/S1352-2310(00)00244-2. [DOI] [Google Scholar]
- Sloane CS 1984. Optical properties of aerosols of mixed composition. Atmos Environ 18:871–78. doi: 10.1016/0004-6981(84)90273-7. [DOI] [Google Scholar]
- Spinazzè A, Cattaneo A, Limonta M, Bollati V, Bertazzi PA, and Cavallo DM. 2016. Titanium dioxide nanoparticles: Occupational exposure assessment in the photocatalytic paving production. J Nanopart Res 18:15. doi: 10.1007/s11051-016-3462-6. [DOI] [Google Scholar]
- Stabile L, Cauda E, Marini S, and Buonanno G. 2014. Metrological assessment of a portable analyzer for monitoring the particle size distribution of ultrafine particles. Ann Occup Hyg 58:860–76. doi: 10.1093/annhyg/meu025. [DOI] [PubMed] [Google Scholar]
- Stoeger T, Reinhard C, Schroeppel STA, Schulz H, Schulz H, Schulz H, Schulz H, and Schulz H. 2006. Instillation of six different ultrafine carbon particles indicates a surface area threshold dose for acute lung inflammation in mice. Environ Health Perspect 114:328–33. doi: 10.1289/ehp.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, and Gebhart J. 1994. Correlations between gravimetry and light-scattering photometry for atmospheric aerosols. Atmos Environ 28:935–38. doi: 10.1016/1352-2310(94)90251-8. [DOI] [Google Scholar]
- Todea AM, Beckmann S, Kaminski H, and Asbach C. 2015. Accuracy of electrical aerosol sensors measuring lung deposited surface area concentrations. J Aerosol Sci 89:96–109. doi: 10.1016/j.jaerosci.2015.07.003. [DOI] [Google Scholar]
- Todea AM, Beckmann S, Kaminski H, Bard D, Bau S, Clavaguera S, Dahmann D, Dozol H, Dziurowitz N, Elihn K, et al. 2017. Inter-comparison of personal monitors for nanoparticles exposure at workplaces and in the environment. Sci Total Environ 605–606:929–45. doi: 10.1016/j.scitotenv.2017.06.041. [DOI] [PubMed] [Google Scholar]
- Tombolini F, Boccuni F, Riccardo F, Natale C, Luigi M, Mantero E, Aedr A, Leoncini L, Pellegrini V, Sabella S, et al. 2021. Integrated and multi-technique approach to characterize airborne graphene flakes in the workplace during the production phases. Nanoscale 13. doi: 10.1039/D0NR07114E. [DOI] [PubMed] [Google Scholar]
- TSI. 2009a. Scanning Mobility Particle Sizer Spectrometer Nanoparticle Aggregate Mobility Analysis Software Module, Ed. A. N. Smps-002. TSI Incorporated, Shoreview, MN. [Google Scholar]
- TSI. 2009b. Series 3080 Electrostatic Classifiers: Operation and Service Manual. TSI Incorporated, Shoreview, MN. https://tsi.com/getmedia/b15c636e-69ce-4434-9322-31d056a10f02/SMPS-002appnote?ext=.pdf [Google Scholar]
- U.S. Geological Survey. Mineral Commodity Summaries, January2022. https://pubs.usgs.gov/periodicals/mcs2022/mcs2022-titanium.pdf
- Vaquero -CG-C, Galarza N, López de Ipiña JL, and López de Ipiña JL. 2016. Exposure assessment to engineered nanoparticles handled in industrial workplaces: The case of alloying nano-TiO2 in new steel formulations. J Aerosol Sci 102:1–15. doi: 10.1016/j.jaerosci.2016.08.011. [DOI] [Google Scholar]
- Viana M, Rivas I, Reche C, Fonseca AS, Pérez N, Querol X, Alastuey A, Álvarez-Pedrerol M, and Sunyer J. 2015. Field comparison of portable and stationary instruments for outdoor urban air exposure assessments. Atmospheric Environment 123:347–57. doi: 10.1093/toxsci/kfh019. [DOI] [Google Scholar]
- Vosburgh DJH, Ku BK, and Peters TM. 2014. Evaluation of a diffusion charger for measuring aerosols in a workplace. Ann Occup Hyg 58:424–436 436. doi: 10.1093/annhyg/met082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warheit DB, Webb TR, Reed KL, Frerichs S, and Sayes CM. 2007. Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: Differential responses related to surface properties. Toxicology 230:90–104. doi: 10.1016/j.tox.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Watson JG, Chow JC, Sodeman DA, Lowenthal DH, Chang M-CO, Park K, and Wang X. 2011. Comparison of four scanning mobility particle sizers at the Fresno Supersite. Particuology 9:204–09. doi: 10.1016/j.partic.2011.03.002. [DOI] [Google Scholar]
- WHO guidelines on nanomaterials workers health. https://apps.who.int/iris/bitstream/handle/10665/259671/9789241550048-eng.pdf
- Xu H, Zhao L, Chen Z, Zhou J, Tang S, Kong F, Xinwei L, Yan L, Zhang J, and Jia G. 2016. Exposure assessment of workplace manufacturing titanium dioxide particles. Journal of Nanoparticle Research 18:288. doi: 10.1007/s11051-016-3508-9. [DOI] [Google Scholar]
- Yi J, Chen B, Schwegler-Berry D, Frazer D, Castranova V, McBride C, Knuckles TL, Stapleton PA, Minarchick VC, and Nurkiewicz TR. 2013. Wholebody nanoparticle aerosol inhalation exposures. J Visual Exp 75:e50263. doi: 10.3791/50263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Mu Y, Zhang X, Li J, Lee C, and Wang H. 2019. Molecular mechanisms underlying titanium dioxide nanoparticles (TiO2 NP) induced autophagy in mesenchymal stem cells ((MSC). J Toxicol Environ Health A 82:997–1008. doi: 10.1080/15287394.2019.1688482. [DOI] [PubMed] [Google Scholar]
- Zhao L, Zhu Y, Chen Z, Xu H, Zhou J, Tang S, Xu Z, Kong F, Li X, Zhang Y, et al. 2018. Cardiopulmonary effects induced by occupational exposure to titanium dioxide nanoparticles. Nanotoxicology 12:169–84. doi: 10.1080/17435390.2018.1425502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data inquiries can be directed to Anand Ranpara, aranpara@hsc.wvu.edu.


