Highlights
-
•
Heat stress is a major environmental problem affecting poultry production around the world.
-
•
Heat stress triggers a series of negative physiological responses, such as depressed immune response, endocrine disorders, and reproductive problems.
-
•
Methionine amino acid has an important role in initiating the translation of proteins associated with body functions.
-
•
Several studies have shown that the dietary supplementation of certain essential amino acids can mitigate the problems resulting from Heat Stress in birds.
-
•
To date, no reports about using nanomethionine in mitigating the effect of heat stress in poultry are present.
Key words: broiler, nanomethionine, growth performance, gene expression, histopathology
Abstract
This study investigated the effects of nanomethionine (nano-meth) on performance, antioxidants, and gene expression of HSP70, HSP90 and Heat Shock factor-1 (HSF-1) from the liver, and TLR4 from the jejunum, of broiler chickens reared under normal temperatures or under heat stress. Three hundred 1-day-old chicks were randomly assigned to 5 treatment groups. Group 1 served as control. Under normal temperature, birds in group 2 received nano-meth (10 mL/L of drinking water) from d1 until the experiment ended. Group 3 birds were heat-stressed (HS) and did not receive any supplementation. Group 4 received nano-meth in the same dose from d1 old until experiment ended, and the birds were exposed to HS. Group 5 birds were HS and received supplementation of nano-meth during the HS period only. Nano-meth improved (P < 0.0001) final body weight, weight gain, feed conversion ratio, and also decreased (P < 0.0001) the effect of HS on growth performance. Reduction (P < 0.0001) in malondialdehyde and changes in antioxidant enzymes GPX and CAT activity indicated the antioxidant effect of nano-meth. Nano-meth supplementation caused an increase in the expression of HSP70 , HSP90 and HSF1, and a downregulation of TLR4 gene expression. Additionally, nano-meth-supplemented groups showed marked improvement in the histological liver structure, intestinal morphology and villus height compared to control or HS groups.
INTRODUCTION
As climate conditions change, high ambient temperatures during summers increasingly impact all living organisms worldwide. High ambient temperatures stress poultry and impair their ability to maintain a homoeothermic body temperature since they lack sweat glands and instead rely on evaporative cooling (panting) to keep themselves cool (Brugaletta et al., 2022). Hyperthermia can develop in chickens when they are exposed to high ambient temperatures (Chowdhury et al., 2012; Lan et al., 2016) which results in heat stress (HS) when heat production exceeds the animal's heat dissipation capacity (Sejian et al., 2018).
HS is a major problem affecting worldwide poultry production and reducing producers' profits (Liu et al., 2020). HS triggers a series of negative physiological responses, such as depressed immune response, endocrine disorders, and reproductive problems (Mirzaei et al., 2022, Waheed et al., 2020). HS also negatively impacts poultry productivity (Abioja and Abiona, 2021; Uyanga et al., 2022). It decreases food intake and body weight (BW) gain, ultimately hindering production and increasing mortality (Liu et al., 2020). Other processes affected by HS include antioxidant activity and free radical generation; these free radicals, such as reactive oxygen and nitrogen species, are highly toxic substances capable of reacting with important macromolecules, such as proteins and lipids (Hu et al., 2019). Even a transient state of oxidative stress is believed to affect animal performance negatively (Surai et al., 2019). Previous studies have investigated the use of antioxidant compounds, such as vitamins A and E (Souza et al., 2011), and amino acids (Del Vesco et al., 2015), in broiler diets to mitigate the adverse effects of HS. Several reports have shown that the dietary supplementation of certain essential amino acids can mitigate the problems resulting from HS in birds (Willemsen et al., 2011; Dai et al., 2012).
Methionine (Meth) is a proteinogenic amino acid that is important in initiating the translation of proteins associated with body functions (e.g., muscle deposition). Its direct and indirect antioxidant properties help minimize the harmful effects of free radicals (Adedokun et al., 2014; Aledo, 2019; Rehman et al., 2019). Meth is involved in glutathione synthesis (Tamanna et al., 2019), and is one of the most readily oxidized residues in proteins (Kim et al., 2014). The beneficial effects of methionine supplementation on antioxidant activity in heat-stressed birds have been demonstrated in previous studies (Del Vesco and Gasparino, 2013; Gasparino et al., 2018). Meth is considered the first limiting amino acid in poultry due to its high protein synthesis and feather development demand. Meth requirements, on an as-fed basis during the production stage of broiler chickens, have been shown to vary 0.50 to 0.52% during the starter phase, 0.38 to 0.45% during the grower phase, and 0.32 to 0.44% during the finisher phase (NRC, 1994; Cobb-700, 2012; KFSP, 2012; Ross-308, 2014). DL-methionine is the most commonly used synthetic Meth product (Alagawany et al., 2014, 2016; Soomro et al., 2017, 2018; Abbasi et al., 2018; Kim et al., 2019). There have been no reports about the effects of nanomethionine (nano-meth) in chicken. Therefore, this study attempts to determine the effects of nano-meth supplementation during normal temperatures and HS on growth performance and gene expression in broiler chickens.
MATERIALS AND METHODS
Experimental Design and Feeding Program
The Institutional Animal Care and Use Committee of University of Alexandria approved the experimental protocol used in this study (Permit #2022/013/125).
A total of 300 Cobb500 broiler chicks (1-day-old) were used in this study. The chicks were distributed randomly into 5 treatment groups (60 chicks/treatment) with 3 replicates per treatment (20 chicks per replicate). The treatment groups consisted of the following, birds in the first group (G1) received drinking water without any supplementation and were reared under normal temperatures (control). In contrast, birds in group 2 (G2) were supplemented with nano-meth from 1-day old until the end of the experiment and were reared under normal temperatures. In group 3 (G3), birds were exposed to HS at 28 d of age, and did not receive supplementation to their drinking water. Group 4 (G4) was supplemented with nano-meth in their drinking water from 1-day old and experienced HS. Group 5 (G5) was supplemented with nano-meth only during HS periods, with HS induced at 28 d of age and birds exposed to 34°C for 3 d (i.e., 72 h continuously) (Abo-Samaha et al., 2021). Nano-meth was added at a concentration of 5% (10 mL/L of this concentration) to supplement the birds’ drinking water. Nano-meth was prepared according to Hojo et al. (2011).
The chicks were reared on the floor in an environmentally controlled room. The temperature was gradually decreased from 32°C to 24°C in 3.5°C increments weekly. A continuous lighting program was provided on the day of arrival, followed by a 23L:1D lighting schedule thereafter. All groups received the basal diet shown in Table 1 per the recommendations of the National Research Council (NRC, 1994). The formulated diets were analyzed for major nutrients. Broiler chicks were fed on starter, grower, and finisher diets during the first 2 wk, wk 3 to 4, and the last week of the experiment, respectively. The ingredient composition and chemical analyses of the basal diets used for the starter, grower, and finisher periods are presented in Table 1. The experiment used formulated diets that were isoenergetic and isonitrogenous and met the nutrient requirements of the birds.
Table 1.
Ingredient composition and chemical analysis of the used experimental diets.
| Ingredients | Starter | Grower | Finisher |
|---|---|---|---|
| Yellow corn (7.8% CP) | 52.63 | 60.1 | 66.90 |
| Soybean meal (44% CP) | 34.5 | 27.5 | 24 |
| Corn gluten (60% CP) | 6.5 | 7 | 5 |
| Vegetable oil1 | 2.5 | 2 | 1 |
| MCP2 | 1.2 | 0.8 | 0.65 |
| Lime-stone3 | 1.85 | 1.8 | 1.65 |
| Lysine4 | 0.05 | 0.05 | 0.05 |
| DL-Methionine5 | 0.07 | 0.05 | 0.05 |
| Choline6 | 0.05 | 0.05 | 0.05 |
| Threonine7 | 0.05 | 0.05 | 0.05 |
| Mycotoxin adsorpant8 | 0.05 | 0.05 | 0.05 |
| Salt | 0.25 | 0.25 | 0.25 |
| Premix (mineral and vitamin)9 | 0.3 | 0.3 | 0.3 |
| Total | 100 | 100 | 100 |
| Chemical analysis | |||
| Moisture % | 11.7 | 11.9 | 12 |
| Crude protein % | 23.1 | 20.9 | 18.7 |
| Ether extract % | 6.2 | 6 | 5.7 |
| Ash % | 5.9 | 5.7 | 6.1 |
| ME kcal/kg diet | 3036 | 3053 | 3107 |
Mixture of sunflower and soybean oils.
Mono calcium phosphate: contain 16% calcium and 21% phosphorus.
Limestone contains 38% calcium and locally produced.
Lysine 87% produced by Archar Daniels method company De Caur LL. Made in USA.
DL-methionine produced by Evoink Co. Guranted analysis 99.5% DL-methionine.
Choline chloride 60% with vegetable carrier (corn powder) produced by Shandyuong Pharmaceutical Co. China.
Crystalline L-Thr (98.5% Thr, PT. Cheil Jedang, Indonesia). 6Choline: choline chloride 60% with vegetable carrier (corn powder) produced by Shandyuong Pharmaceutical Co. China.
Beta-2-x.
Each 3 kg contains: Vit A (12,000,000 IU), Vit D (2,000,000 IU), Vit E(10 g), Vit K3 (2 g), Vit B1 (1 g), Vit B2 (5 g), VitB6 (1.5 g), Vit B12 (10 g), nicotinic acid (30 g), pantothinic acid (10 g), folic acid (1 g), biotin (50 mg), choline chloride 50% (250 mg), iron (30 g), copper (10 g), zinc (50 g), manganese (60 g), iodine (1 g), selenium (0.1 g), cobalt (0.1 g), and carrier lime stone up to 3 kg.
Dry Matter and Crude Nutrients Analysis
The dry matter contents of feed samples were determined by oven-drying at 105°C for 8 h (AOAC, 1990). Ash contents were determined by incineration at 550°C overnight. Crude protein was determined using the Kjeldahl method according to Randhir and Pradhan (1981), and ether extract was determined according to Hanson and Olly (1963).
Growth Performance, Relative Organ Weight, and Rectal Temperature
Chicks were weighed individually for initial and final BW. The chicks' total weight gain (WG) (expressed in grams) was calculated as the difference between initial and final weights on a replicate basis. Feed intake was calculated as the difference between the weight of the offered feed and the remainder and then divided by the number of birds in each group per day and totaled per week. The feed conversion ratio was calculated by dividing the feed consumed (g) by the BW gain (g) during the week. Broilers (9 samples/treatment) were weighed individually before slaughter (live weight). Immediately after slaughter, the liver, gizzard, proventriculus, heart, and abdominal fat were removed from the carcass and weighed to determine the relative weight as relative organ weight (%) = organ weight/live BW × 100. The rectal temperature was measured in both control and HS birds.
Lipid Peroxidation and Antioxidant Profile
Lipid peroxide was measured after the reaction with thiobarbituric acid and expressed as nmol malondialdehyde (MDA) per tissue weight (Ohkawa et al., 1979). Glutathione peroxidase (GPX) activity was analyzed according to Paglia and Valentine (1967), which is based on the reaction of hydrogen peroxide (H2O2) in the presence of NADPH, GSH, and glutathione reductase. The absorbance was measured at 340 nm, and the result was expressed as IU/tissue weight. Catalase (CAT) activity was measured based on the rate of oxidation according to Chiang et al. (2006), and recorded as IU/tissue weight.
qRT-PCR Analysis of Stress-Related Genes
Analyses were conducted for Heat Shock protein 70 (HSP70), Heat Shock protein 90 (HSP90), and Heat Shock factor-1 (HSF-1) from the liver, and Toll-like receptor 4 (TLR4) from the jejunum. Fifty-milligram samples were collected in clean 2.0 mL microfuge tubes, snap-frozen, and stored at −80°C until analyzed. Total RNA from liver samples was extracted using easy-RED total RNA extraction kits (iNtRON Biotechnology, Inc., Seongnam-Si, South Korea) according to the manufacturer's instructions. RNA integrity was verified by agarose gel electrophoresis. A fixed concentration of RNA (2 μg) was used for cDNA synthesis using the Intron-Power cDNA synthesis kit (Cat. no. 25011). For qRT-PCR assay, specific HSP70, HSP90, HSF1, and TLR4 primers were used to amplify these genes from Cobb chicken, with 18S rRNA used as an internal standard housekeeping gene. The primer sequences are displayed in Table 2. The reaction mix for each gene contained 10 μL of SensiFast SYBR Low-Rox master mix (Bioline, London, UK), 0.5 μM of each primer, and 2 μL of cDNA. Tests were conducted in duplicate, using the Stratagene MX300 P real-time PCR system (Agilent Technologies, Santa Clara, CA), with the following thermal cycling conditions: initial denaturation at 95°C for 15 min, followed by 40 cycles at 95°C for 15 s, and annealing for 1 min at gene-specific annealing temperatures. The dissociation curves were analyzed by showing only 1 peak at a specific melting temperature for all tested genes, indicating specifically amplified PCR products. The relative gene expression levels were evaluated using the 2−ΔΔct method as described by Rao et al. (2013). In this context, the fold change for each gene was normalized against the housekeeping gene (18S) and the corresponding values of the control group.
Table 2.
Sequences of primers used in real-time PCR.
| Gene | Primer sequence (5′–3′) | Reference | Acc. number | Annealing temp |
|---|---|---|---|---|
| HSP70 | F:CCAAGAACCAAGTGGCAATGAA R: CATACTTGCGGCCGATGAGA |
El-Kassas et al. (2019) | EU747335 | 60 |
| HSP90 | F: GAGTTTGACTGACCCGAGCA R: TCCCTATGCCGGTATCCACA |
El-Kassas et al. (2019) | NM_206959 | 60 |
| HSF1 | F: 5-CAGGGAAGCAGTTGGTTCAC TACACG-3 R: 5-CCTTGGGTTTGGGTTGCTCAGTC-3 |
Abdo et al. (2017) | L06098.1 | 60 |
| TLR4 | F: AGTCTGAAATTGCTGAGCTCAAAT R: GCGACGTTAAGCCATGGAAG |
Iqbal et al. (2005) | AY064697 | 60 |
| 18s | F: 5-CGAAAGCATTTGCCAAGAAT-3 F: 5-GGCATGGTTTATGGTCGG |
Primer 3 | XM_288030.1 | 60 |
Histopathological Study
Representative specimens were collected from the livers and jejunums of control and treated chicks. The collected specimens were washed and soaked in a 10% neutral-buffered formalin solution for 24 h for fixation. Next, fixed specimens were prepared via the paraffin-embedding technique according to Bancroft and Gamble (2013). Briefly, the fixed specimens were dehydrated using ascending concentrations of ethyl alcohol, cleared in 3 changes of xylol, blocked in paraffin wax, and cut into 3 to 5 µm thick sections. The microtomed sections were stained with hematoxylin and eosin stain (H&E stain). Next, blinded examination and imaging were conducted by an experienced pathologist. The representative photomicrographs were taken using a digital camera (Leica EC3; Leica Biosystems, Wetzlar, Germany) connected to a microscope (Leica DM500).
Histomorphometric Analysis of Intestine
After blood was drawn, the chickens were cut open and the entire digestive tracts were separated. Segments of the middle portions of the digestive tracts were collected and images from the intestinal tissues of the chicks were taken using a digital camera connected to a bright field microscope (Nikon E200, Nikon Corp., Tokyo, Japan). The images were then subjected to computerized quantitative histomorphometric analysis. Light microscopic observations were used to assess the effects of nano-meth dietary supplementation on the following parameters: 1) villus height (µm, from the tip to the base of villus); 2) villus width (µm, at the tip; and the crypt/villus junction); 3) crypt depth; 4) Villus height/crypt depth ratio; and 5) tunica muscularis thickness. A computerized image analysis system was used to perform the measurements (Image J software; Bethesda, MD) (Schneider et al., 2012). The villi height, crypt depth, and villi width at the crypt/villus junction and the villi tips were measured (the mean value was determined for 15 villi/sample, × 10).
Statistical Analysis
Statistical analysis was performed using SAS software (SAS, 2014). Data were evaluated for normality before analysis using the Kolmogorov-Smirnov test. Generalized linear models were used to analyze the data with the following statistical model:
where Xij = the observation record; μ = overall mean; Ti = ith level effect of different treatments; eij = random error. Post hoc treatment group comparisons were conducted using Duncan's test. The overall significance level was set as P < 0.05. The gene expression data were analyzed using GraphPad Prism 6 software (GraphPad Software, San Diego, CA). Differences were considered statistically significant at P < 0.05*, P < 0.01**, P < 0.001***, and P < 0.0001****. All values are expressed as the mean ± SEM.
RESULTS
Characterization of Nanomethionine
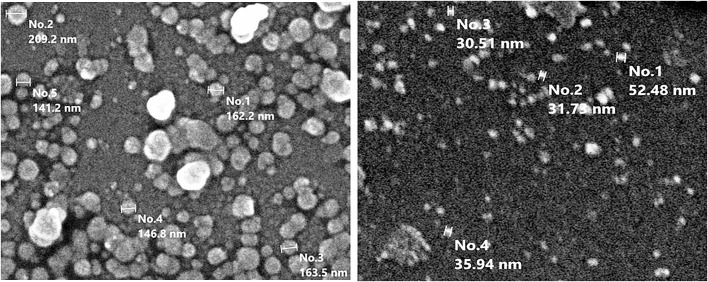
The sizes of the Nano-Meth particles were determined by scanning electron microscopy. The solution was diluted to 50% with deionized water, then 1 drop was spread on a glass slide, and morphology (Figure 1) was observed with a JSM-IT 200 (JOEL) scanning electron microscope operated between 15 and 20 keV.
Figure 1.
Characterization of nanomethionine using scanning electron microscopy, before (left) and after (right).
Growth Performance
Data for BW, WG, total feed intake (TFI), and feed conversion ratio (FCR) are presented in Table 3. Final BW was significantly different among groups. Specifically, the heaviest weight was recorded for G2 birds supplemented with nano-meth without exposure to HS (1,936 g). This was heavier than the control (G1) by 174 g; while the lightest weight was recorded for G3 birds (1,536 g), which had been exposed to HS without nano-meth. Supplementation of nano-meth in G4 decreased the effect of HS, where average BW was the same as the control (G1). Moreover, the supplementation of nano-meth only when HS began, as in G5, was also effective in improving the average BW when compared to those exposed to HS without nano-meth supplementation (G3). The WG had the same trend as BW, where the highest gain was observed in G2 and the least gain was observed in G3. TFI was significantly varied among groups, with the highest value seen in G2 (1,775 g), while the lowest value was in G5 (1,465 g). FCR also varied significantly among groups, with the best conversion ratio seen in G2 (1.49), and the worst conversion ratio observed in G3 (1.70). G4, which was exposed to HS with supplementation of nano-meth, performed much better than G3, in terms of final BW, WG, and FCR.
Table 3.
Body weight, total feed intake, total weight gain, and average feed conversion ratio.
| Variable | Group |
P value | ||||
|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | ||
| Initial weight (day old) | 49.75 ± 0.54 | 49.09 ± 0.29 | 49.73 ± 0.38 | 50.25 ± 0.35 | 49.25 ± 0.50 | NS |
| Final weight (32 d) | 1762.65 ± 31.08b | 1936.47 ± 27.82a | 1536.37 ± 25.36d | 1767.68 ± 20.82b | 1688.60 ± 26.51c | <0.0001 |
| Total WG | 1712.90 ± 30.56b | 1887.37 ± 27.54a | 1486.63 ± 25.01d | 1717.42 ± 20.50b | 1639.35 ± 26.06c | <0.0001 |
| TFI | 2631.70 ± 0.00b | 2775.28 ± 0.00a | 2482.05 ± 0.00d | 2574.95 ± 0.00c | 2465.35 ± 0.00e | <0.0001 |
| FCR | 1.54 ± 0.03b | 1.47 ± 0.02b | 1.67 ± 0.03a | 1.49 ± 0.02b | 1.50 ± 0.03b | <0.0001 |
The data presented as mean ± standard error. Means bearing different superscript letters within the same row are significantly different (P < 0.05). NS: nonsignificant. WG: weight gain, TFI: total feed intake, FCR: average feed conversion ratio. G1: control, G2: Nanomethionine in drinking water, G3: heat stress (HS), G4: nanomethionine+HS, G5: nanomethionine during HS.
Rectal Temperature and Carcass Quality
Results for average rectal temperature of broilers are shown in Table 4. Body temperature was increased in G3 until reaching 41.87°C, followed by G4 (41.47°C), and then G5, G2, and G1 (40.90°C, 40.70°C, and 40.50°C, respectively). Dressing percentage (Table 4) varied significantly among groups, where the percentage was improved in G2, G1, and G4, while dressing percentage was decreased in G5 and G3. The organs relative weight (liver, gizzard, proventriculus, and heart) were also significantly different among the groups. Additionally, abdominal fat was significantly varied among groups (P < 0.0001), with the highest value recorded in G1 (control), and lowest value in G3.
Table 4.
Rectal temperature, dressing percentage, and some organs relative weight.
| Variables | Groups |
P value | ||||
|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | ||
| Rectal temp | 40.50 ± 0.06c | 40.70 ± 0.08c | 41.87 ± 0.07a | 41.47 ± 0.15b | 40.90 ± 0.25c | <0.0001 |
| Dressing % | 73.99 ± 0.40a | 74.45 ± 0.29a | 69.22 ± 1.52b | 72.58 ± 0.81a | 69.95 ± 0.18b | <0.0001 |
| Liver | 2.33 ± 0.04a | 2.33 ± 0.09a | 1.74 ± 0.08c | 2.09 ± 0.10ab | 1.94 ± 0.09bc | <0.0001 |
| Gizzard | 1.59 ± 0.03a | 1.67 ± 0.08a | 1.33 ± 0.03b | 1.40 ± 0.04b | 1.36 ± 0.01b | <0.0001 |
| Proventriculus | 0.34 ± 0.01b | 0.40 ± 0.01a | 0.31 ± 0.01b | 0.32 ± 0.02b | 0.33 ± 0.02b | 0.0031 |
| Heart | 0.43 ± 0.01a | 0.41 ± 0.01a | 0.37 ± 0.01b | 0.41 ± 0.01a | 0.38 ± 0.01b | 0.0014 |
| Abdominal fat | 1.36 ± 0.01a | 1.03 ± 0.04b | 0.97 ± 0.05b | 1.03 ± 0.04b | 1.28 ± 0.04a | <0.0001 |
The data presented as mean ± standard error. Means bearing different superscript letters within the same row are significantly different (P < 0.05). NS: nonsignificant. G1: control, G2: nanomethionine in drinking water, G3: heat stress (HS), G4: nanomethionine+HS, G5: nanomethionine during HS.
Serum Oxidative/Antioxidative Indices
Table 5 shows that in comparison with control values, HS induced a significant (P < 0.0001) elevation in MDA level and a reduction in antioxidant enzymatic activities (GPX and CAT) in HS-treated groups, confirming that exposure to HS induces oxidative stress in broilers and the depletion of antioxidants in this group (G3). Compared to G3, coadministration or treatment with nano-meth in G4 and G5 alleviated the altered serum oxidative/antioxidative indices, which were evidenced by significant (P < 0.0001) reduction in MDA, and GPX and CAT increments, which indicate the antioxidant effect of nano-meth. Additionally, the nano-meth-treated group exhibited high antioxidant activity. However, nano-meth supplementation without HS in G2 caused a reduction in MDA level and elevation in the antioxidant enzymatic activities (GPX and CAT) compared with G1 (control).
Table 5.
Effect of nanomethionine on the lipid peroxidation biomarker (MDA) and antioxidant molecules (GPX, CAT) in the serum of broilers exposed to heat stress.
| Parameter | Group |
P value | ||||
|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | ||
| CAT | 428.86 ± 14.26b | 463.29 ± 4.22a | 379.96 ± 12.23c | 437.76 ± 10.01ab | 433.30 ± 4.21b | <0.0001 |
| GPx | 182.86 ± 8.34b | 219.67 ± 7.30a | 150.48 ± 6.47c | 194.60 ± 1.20b | 188.83 ± 12.50b | <0.0001 |
| MDA | 12.95 ± 0.03b | 9.41 ± 0.55c | 15.34 ± 0.54a | 12.41 ± 0.10b | 12.66 ± 0.11b | <0.0001 |
Data presented as mean ± SE. Means bearing different superscripts letters within the same row are significantly different (P < 0.05). G1: control, G2: Nanomethionine in drinking water, G3: Heat stress (HS), G4: Nanomethionine+HS, G5: Nanomethionine during HS.
Gene Expression
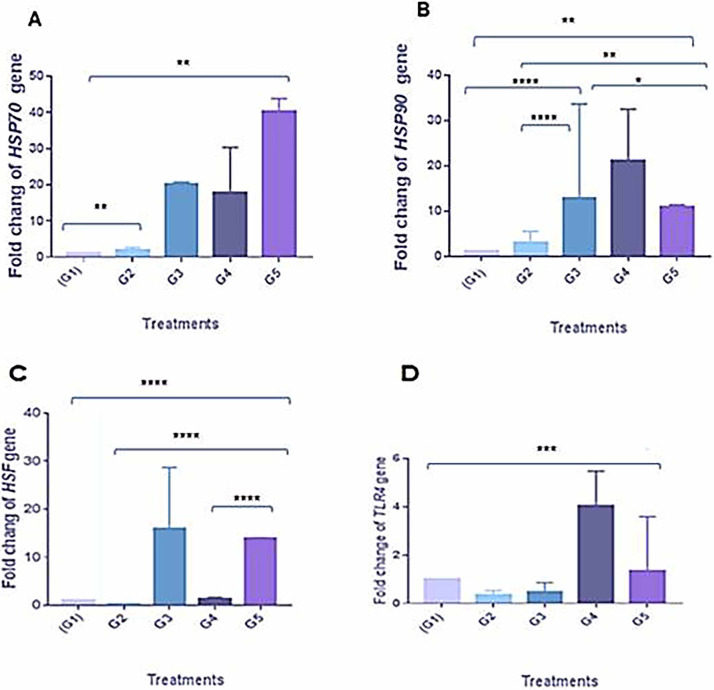
Nano-meth in G2 had a significant upregulation of HS genes HSP70 and HSP90 genes (P < 0.05), increasing 1.8- and 3-fold (Figure 2), and was also associated with a recorded significant downregulation of both HSF1 and TLR4 genes (P < 0.05) to 0.3-fold. In contrast, G3 (exposed to HS without supplementation of nano-meth) showed increased expression of HSP70 and HSF1 by 32.2- and 16-fold, respectively. HSP90 gene expression levels significantly increased (P < 0.05) by 12.7-fold. TLR4 was seen to be downregulated by 0.5-fold (Figure 2).
Figure 2.
Relative expression of some immune response genes HSP70 (A), HSP90 (B), HSF1 (C), and TLR4 (D) in the chicken with different treatment. All values are expressed as mean ± SE. Asterisks on the data bars indicate when P < 0.05 (*), P < 0.005 (**), and P < 0.0005 (***). G1: control, G2: Nanomethionine in drinking water, G3: Heat stress (HS), G4: Nanomethionine+HS, G5: Nanomethionine during HS.
Regarding the effect in G4 (exposed to HS with nano-meth supplementation), upregulation of HSP70, HSP90, and TLR4 to 17.7-, 21.2-, and 4-fold, respectively, was observed. Also, a mildly significant increase of HSF1 (P < 0.05) of 1.8-fold was observed. Supplementation of nano-meth only when HS occurred, as in G5, induced a significant upregulation of HSP70, HSP90, and HSF1 genes (P < 0.05) to 30.2-, 11-, and 14-fold, respectively. Meanwhile, it was associated with a somewhat significantly increased expression of TLR4 (P < 0.05) to 1.3-fold (Figure 2).
Histopathologic Findings
Liver
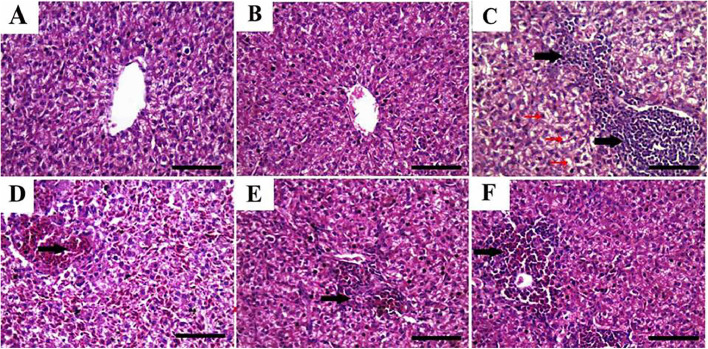
Histomorphologic examination of liver tissues from G1 (control) and nano-meth-treated chicks (G2) revealed normal histopathologic structure of hepatic parenchyma with polygonal hepatocytes, round-shaped nuclei, and normally structured portal veins, hepatic arteries, central veins, and sinusoids (Figure 3A, B). However, hepatic tissues from HS chickens (G3) showed diffuse moderate to severe vacuolization of hepatocytes, mostly of the hydropic type, thickening of portal triads with infiltrated mononuclear inflammatory cells (Figure 3C), multifocal areas of lytic and coagulative necrosis, as well as vascular and sinusoidal congestion and hemorrhage (Figure 3D). In contrast, hepatic tissues from HS chickens treated with nano-meth from d 1 (G4) showed marked improvement of their histologic structures; lesions in the form of mild thickening of portal areas with congested portal veins were noted (Figure 3E). While the chickens subjected to HS and treated with nano-meth at the HS time only (G5) showed moderate improvement of their liver structure; with noted lesions in the form of focal areas of lytic necrosis which were infiltrated with mononuclear inflammatory cells and extravasated red blood cells (Figure 3F).
Figure 3.
Representative photomicrographs (hematoxylin and eosin (H&E) staining) of liver tissues from chicken supplemented with nanomethionine and subjected to heat stress for 28 d. Livers tissues from control (A) and nanomethionine (B) revealed normal histopathologic structure of hepatic tissues. Hepatic tissues from heat stressed chickens showed diffuse moderate to severe vacuolization of hepatocytes mostly of hydropic type (red arrows), thickening of portal triads with the infiltrated mononuclear inflammatory cells (black arrows) (C), vascular and sinusoidal congestion and hemorrhage (arrow) (D). Hepatic tissues from heat stressed chicken treated with nanomethionine from d 1 showed mild thickening of portal areas with the congested portal veins (arrow) (E). Chickens in group subjected to heat stress and treated with nanomethionine at the heat stress time only showed focal areas of lytic necrosis infiltrated with mononuclear inflammatory cells and extravasated red blood cells (arrow) (F). Bar = 50 µm.
Intestines
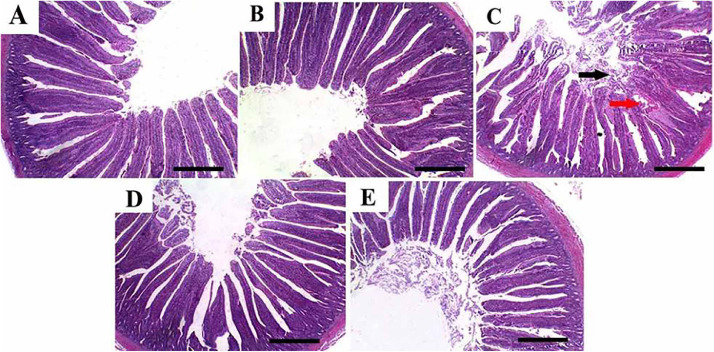
Histologically, chicken's intestinal walls consist of 4 layers: serosa, internal and external muscular layers, mucosa, and submucosa. Figure 4 shows that the intestinal mucosa and submucosa of chickens in G1 (Figure 4A) and G2 (Figure 4B) had normal histomorphologic structures of intestinal villi, associated crypts, and tunica muscularis. Moreover, the submucosal tissues appeared free of inflammatory and/or degenerative changes and the columnar epithelium and goblet cells appeared properly arranged. In contrast, intestinal tissues from chicken subjected to HS (G3) showed blunted and necrotic tips of villi, where the columnar epithelium and goblet cells showed different degrees of necrosis and desquamation within the lumen, congestive capillaries, and mild inflammatory infiltrates were also noted in submucosal tissues (Figure 4C). On the other hand, chickens subjected to HS and treated with nano-meth, either on d 1 (G4) or during HS (G5), showed marked improvement in intestinal morphology compared to HS chickens without nano-meth supplementation (Figure 4D and E, respectively).
Figure 4.
Representative photomicrographs (hematoxylin and eosin (H&E) staining) of intestine from chicken supplemented with nanomethionine and subjected to heat stress for 28 d. Tissues from control (A) and nanomethionine groups (B) revealed normal histomorphologic structure of intestinal villi, associated crypt, and tunica muscularis, in addition to properly arranged columnar epithelium and goblet cells. Tissues from heat stressed chickens showed necrosis of villus epithelium (red arrow) and desquamation within the lumen (black arrow). Intestinal tissues from heat stressed chicken treated with nanomethionine either from d 1 (D) or at the heat stress time only (E) showed marked improvement in the histologic structure of intestine. Bar = 200 µm.
Concerning the histomorphometric analysis of the intestines, results in Table 6 revealed that administration of nano-meth without HS (G2) did not induce a significant change in any of the studied parameters. However, intestinal tissues from chicken subjected to HS (G3) showed a significant (P < 0.05) reduction in villus height and villus width at the tip of villi compared with the control (G1). In contrast, HS chickens in groups supplemented with nano-meth either from d 1 of treatment (G4) or during the period of HS only (G5) showed significant (P < 0.05) improvement in villus height compared with the untreated HS group.
Table 6.
Histomorphometric analysis of intestines from chickens supplemented with nanomethionine and subjected for heat stress for 28 d.
| Measurement (µm) | G1 | G2 | G3 | G4 | G5 | P value |
|---|---|---|---|---|---|---|
| Villus height | 600.00 ± 10.41a | 615.67 ± 6.64a | 498.33 ± 14.24d | 565.00 ± 8.66b | 530.00 ± 5.77c | <0.0001 |
| Villus width at the tip | 51.67 ± 3.76ab | 59.67 ± 1.45a | 37.00 ± 4.04c | 48.33 ± 4.41abc | 45.67 ± 2.96bc | <0.0211 |
| Villus width at the tip crypt/villus junction | 55.00 ± 3.79a | 55.67 ± 2.33a | 42.67 ± 4.81a | 48.00 ± 4.36a | 45.00 ± 3.61a | 0.4388 |
| Crypt depth | 71.00 ± 4.73ab | 79.00 ± 4.73a | 62.67 ± 5.36b | 69.33 ± 3.48ab | 64.33 ± 2.33b | 0.0105 |
| Villus height/crypt depth ratio | 8.51 ± 0.42a | 7.84 ± 0.41a | 8.06 ± 0.69a | 8.20 ± 0.55a | 8.26 ± 0.34a | 0.6370 |
| Tunica muscularis thickness | 222.33 ± 6.74a | 223.33 ± 4.41a | 224.33 ± 5.21a | 218.67 ± 6.77a | 221.33 ± 6.98a | 0.5889 |
Data represent mean ± SE. Values with different letters in the same row indicate significant differences between different test groups at P < 0.05. G1: control, G2: Nanomethionine in drinking water, G3: Heat stress (HS), G4: Nanomethionine+HS, G5: Nanomethionine during HS.
DISCUSSION
It is now well known that HS compromises the health and productivity of chickens. Our study showed that nano-meth supplementation to broiler diets under normal conditions apparently benefits BW, WG, TFI, and FCR. Our results are consistent with others (Marrett and Sunde, 1965; Shen et al., 2015) who reported that young broilers fed L-Meth-supplemented diet showed better growth performance (average daily gain and FCR). The possible mechanism underlying the observed effect of nano-meth on growth is that improved utilization of meth may enhance intestinal development and thereby improve growth performance (Shen et al., 2015).
Our results showed that HS decreased BW, WG, and TFI, while it increased FCR in HS birds in G3 compared to the control (G1). Others (Attia et al., 2011; Ohtsu et al., 2015; Abo-Samaha et al., 2021) have reported similar findings, and this could be attributable to HS-induced oxidative stress, which leads to metabolic changes that subsequently decrease feed intake (Quinteiro-Filho et al., 2010; Akbarian et al., 2016). The poor growth performance observed in broilers under HS may be attributed to higher energy loss via increased respiration, reduced feed intake, and retardation of nutrient digestion (Mello et al., 2015).
Our results showed that dressing percentage and organs relative weights were enhanced by nano-meth supplementation, while abdominal fat was decreased. Similarly, zinc-methionine supplementation decreased the abdominal fat weight of broiler chickens in high ambient temperatures (Saleh et al., 2018). These results may be attributed to the stimulatory effect of methionine on the expression of genes coding for growth-related hormones and myogenic regulatory factors involved in muscular development (Del Vesco et al., 2015). However, our findings indicated that exposure to HS decreased heart, liver, and gizzard yields, and this concurred with Rosa et al. (2007).
HS is a major source of systemic oxidative stress since it decreases the body's antioxidant capacity as well as intestinal immunity (Song et al., 2018). ROS can lead to DNA damage, lipid peroxidation, and protein denaturation (Ray et al., 2012). In the present study, the serum MDA level was increased; in contrast, the GSH and catalase serum concentration was decreased when chicks were exposed to HS, whereas supplementation with nano-meth alleviated the negative effects of HS on serum MDA level, as well as GSH and catalase concentration. This finding concurred with Miao et al. (2021), who revealed that adding 0.45% methionine to the diet decreased GPX in the liver and plasma; and increased the ratio of GSH/GSSG in plasma of high stocking density birds. Moreover, supplementation with methionine decreased the MDA level in the jejunum. Additionally, Magnuson et al. (2020) showed that high methionine levels elevated (P < 0.05) GSH concentration in the thighs of grower and finisher chickens. Furthermore, the extra DL-Meth supplementation led to significant decreases in MDA concentrations in finishers' breast and thigh meat.
Methionine is an essential amino acid and, as a nonenzymatic antioxidant, acts through methionine sulfoxide reductase, thioredoxin reductase, and thioredoxin to prevent oxidative damage to nucleic acids, lipids, and proteins (Luo and Levine, 2009). S-adenosylmethionine is the active form of methionine and serves as the methyl donor and as a glutathione precursor (Kumar et al., 2020). In our study, the addition of methionine in nano-form showed the ability to alleviate the altered serum oxidative/antioxidative indices, which were evidenced by significant (P < 0.0001) reduction in MDA, and increments in GPX and CAT, which indicate the antioxidant effect of nano-meth.
HS proteins are chaperones that allow biological processes to work well in a stressful environment. The usefulness of HSP70 expression to identify HS under field conditions was evaluated (Lund et al., 2006). The present findings confirmed earlier reports (Zulkifli et al., 2002, 2009; Liew et al., 2003) that heat exposure may augment HSP70 expression in chickens. The HSPs are characterized by their ability to be induced by HS and, in molecular terms, by the presence of a functional HS element in their promoter (Sharp et al., 1999). Also, HSPs act as chaperones that bind other proteins following HS damage to preserve their structure, managing the proteins’ migration across membranes or organelles, or keeping the receptor availability or specific enzyme functions under control (Kampinga and Craig, 2010).
The liver is one of the most sensitive organs that synthesize HSP70 in response to hyperthermia (King et al., 2002). In this study, HS for 3 d upregulated the expression of HSP70 in G3, and HSF1 also caused a significant increase in HSP90 gene expression levels. In agreement with our findings, Abo-Samaha et al. (2021) reported that HSP70 is upregulated in response to HS in broiler chicken. Similarly, Cedraz et al. (2017) reported that the HSP70 and HSP90 genes were highly expressed in all genetic groups under HS compared with control conditions. Mahmoud et al. (2004) also demonstrated that HS caused induction of HSP90α and HSP90ß in chicken heart, liver, and spleen. Additionally, the quantity of HSP70 was seen to increase when testis cells were exposed to high temperatures (Mezquita et al., 2021). Similarly, a relevant increment in the HSP70 expression in female broiler chicken brains after 4 d of heat treatment was observed (Zulkifli et al., 2003).
The benefits of nano-meth in inducing HSP expression have been reported in our study in both G4 and G5. In G4 the results showed upregulation of HSP70 and HSP90 to 17.7- and 21.2-fold. A mild significant increase of HSF1 (P < 0.05) to 1.8-fold was also seen. The addition of nano-meth when HS started in G5 induced a significant upregulation of HSP70, HSP90, and HSF1 genes (P < 0.05) to 30.2-, 11-, and 14-fold, respectively. Our results suggest that nano-meth supplementation may enhance the induction of HSP70 in poultry under HS conditions. Previous work (Zulkifli et al., 2003, 2009; Al-Aqil and Zulkifli, 2009) suggested that HSP70 plays a profound role in enhancing tolerance to various stressors and resistance to viral infection in broiler chicken. HS proteins are extremely potent molecules that can modify the function and destiny of other proteins and thus play critical roles in numerous physiological and immunological processes (Pockley, 2003). Recently, Han et al. (2017) found that amino acid (AA) levels, including leucine, were significantly decreased due to thermal treatment on embryonic brains and livers. It was also reported that HS could affect sulfur AA (including methionine) metabolism in chicks (Ito et al., 2015), so HS chickens should be supplemented with AA.
Given what has been demonstrated, it is unsurprising that chickens exposed to HS had several pathological lesions in their livers and intestines since HS usually disrupts homeostatic balance. As a result, the stress response involves essential reactions to maintain a constant state, such as increases in heart rate and blood flow to various tissues (Ewing et al., 1999). This could explain the hepatic and intestinal congestion in chickens subjected to HS. It is recognized that when body temperature rises above normal, several different types of cells typically begin to be harmed (Ma et al., 2022). Therefore, increased core body temperature mediated endothelial cell damage during HS.
Also, high blood pressure may result from an autonomic nervous system reaction, leading to circulatory rupture and subsequent organ hemorrhage and congestion, including the liver and intestine. Additionally, fluid, fat, or both accumulation in the cytoplasm (hepatic vacuolization) is a strong diagnostic indicator of liver damage. Hepatocytes with excess fluid and lipids are signs of sublethal damage (Liu et al., 2022).
Additionally, in many animal species, HS harms intestinal architecture and increases intestinal permeability (Hall et al., 2001; Dokladny et al., 2006; Yang et al., 2007; Lambert, 2009). It is widely recognized that intestinal damage, including weakened intestinal integrity and barrier function, is a primary reaction to HS (Quinteiro-Filho et al., 2010; Song et al., 2013; Varasteh et al., 2015). We assessed villus height and crypt depth, as well as the villus height to crypt depth ratio, both of which are helpful indicators of gut health in animals, in order to evaluate intestinal histological damages (Pluske et al., 1996a,b; Xu et al., 2003). HS hens’ shorter intestinal villi suggest the intestinal epithelium was harmed (Fan et al., 1997). In contrast, the histopathologic image of the liver and intestine was significantly improved in chickens treated with nano-meth. This beneficial effect could mostly be ascribed to nano-meth's regulatory function of HSP expression and the resultant downregulation of oxidative stress (Nanto-Hara et al., 2019).
Toll-like receptors (TLR) have been extensively studied in rats, but little is known of their role in broiler chickens undergoing HS (Huang, 2017). As a result of HS, cells can adjust their metabolism to the changing environmental conditions by modifying their gene expression. These alterations offer sufficient flexibility to preserve homeostasis under stress conditions, maintain their viability and functions, as well as adapt to long-term changes (López-Maury et al., 2008). HS triggers the expression of HS-related genes, such as HSP and TLR (Zhang et al., 2015; Huang, 2017). The TLR4-mediated signal pathway is activated in response to stress, particularly to HS (Ju et al., 2014). Our study showed upregulation of TLR4 to 4-fold in G4, and a small significant increase in expression of TLR4 (P < 0.05) to 1.3-fold in G5. Fukata et al. (2005) reported that TLR4 is important for healing injured intestinal epithelium. Hence, the markedly increased TLR4 mRNA expression levels indicate that the expression of HSP70 was elevated and triggered TLR4 overexpression to protect the intestine from injury by HS (Huang, 2017).
CONCLUSIONS
Nanomethionine improved final BW, WG, FCR, and decreased HS's effect on growth performance. Reduction in malondialdehyde and changes in antioxidant enzymes GPX and CAT activity indicated the antioxidant effect of nano-meth. Nano-meth supplementation caused an increase in the expression of HSP70 and HSF1, HSP90, and a downregulation of TLR4 gene expression. Additionally, nano-meth treated groups showed marked improvement of the histological liver structure compared to the control, and nano-meth supplemented groups showed marked improvement in the intestinal morphology when compared with HS groups and also showed significant improvement in villus height compared with the HS-subjected group. Based on these results, adding nano-meth amino acid appears to reduce the negative impacts of HS in chickens.
ACKNOWLEDGMENTS
The authors acknowledge Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R5), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Data Availability Statement: The data presented in this study are available on request from the corresponding author upon reasonable request.
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in the present study.
REFERENCES
- Abbasi I.H.R., Abbasi F., A. El-Hack M.E., Abdel-Latif M.A., Soomro R.N., Hayat K., Cao Y. Critical analysis of excessive utilization of crude protein in ruminants ration: impact on environmental ecosystem and opportunities of supplementation of limiting amino acids—a review. Environ. Sci. Pollut. Res. 2018;25:181–190. doi: 10.1007/s11356-017-0555-4. [DOI] [PubMed] [Google Scholar]
- Abdo S.E., El-Kassas S., El-Nahas A.F., Mahmoud S. Modulatory effect of monochromatic blue light on heat stress response in commercial broilers. Oxid. Med. Cell Longev. 2017;2017:1–13. doi: 10.1155/2017/1351945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abioja M.O., Abiona J.A. Pages 275–296 in African Handbook of Climate Change Adaptation (W. Leal Filho et al. (eds.)) Springer International Publishing; Cham, Switzerland: 2021. Impacts of climate change to poultry production in Africa: adaptation options for broiler chickens. [Google Scholar]
- Abo-Samaha M.I., El-Kazaz S.E., Reddy P.G. Effect of dietary ascorbic acid supplementation on performance, behaviour and gene expression in heat-stressed broiler chickens. Eur. Poult. Sci. 2021;85:1–19. [Google Scholar]
- Adedokun S.A., Jaynes P., A. El-Hack M.E., Payne R.L., Applegate T.J. Standardized ileal amino acid digestibility of meat and bone meal and soybean meal in laying hens and broilers. Poult. Sci. 2014;93:420–428. doi: 10.3382/ps.2013-03495. [DOI] [PubMed] [Google Scholar]
- Akbarian A., Michiels J., Degroote J., Majdeddin M., Golian A., De Smet S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016;7:37. doi: 10.1186/s40104-016-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., El-Hack M.E.A., Arif M., Ashour E.A. Individual and combined effects of crude protein, methionine, and probiotic levels on laying hen productive performance and nitrogen pollution in the manure. Environ. Sci. Pollut. Res. 2016;23:22906–22913. doi: 10.1007/s11356-016-7511-6. [DOI] [PubMed] [Google Scholar]
- Alagawany M., El-Hack M.E.A., Laudadio V., Tufarelli V. Effect of low-protein diets with crystalline amino acid supplementation on egg production, blood parameters and nitrogen balance in laying Japanese quails. Avian Biol. Res. 2014;7:235–243. [Google Scholar]
- Al-Aqil A., Zulkifli I. Changes in heat shock protein 70 expression and blood characteristics in transported broiler chickens as affected by housing and early age feed restriction. Poult. Sci. 2009;88:1358–1364. doi: 10.3382/ps.2008-00554. [DOI] [PubMed] [Google Scholar]
- Aledo J.C. Methionine in proteins: the cinderella of the proteinogenic amino acids. Protein Sci. 2019;28:1785–1796. doi: 10.1002/pro.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC . 15th ed. AOAC; Arlington, TX: 1990. Official Methods of Analysis of the Association of Agricultural Chemists. Agricultural Chemicals, Contaminants and Drugs. [Google Scholar]
- Attia Y.A., Hassan R.A., Tag El-Din A.E., Abou-Shehema B.M. Effect of ascorbic acid or increasing metabolizable energy level with or without supplementation of some essential amino acids on productive and physiological traits of slow-growing chicks exposed to chronic heat stress. J. Anim. Physiol. Anim. Nutr. 2011;95:744–755. doi: 10.1111/j.1439-0396.2010.01104.x. [DOI] [PubMed] [Google Scholar]
- Bancroft J. D., and Gamble M. 2013. The hematoxylin and eosin. Pages 179–220 in Theory and Practice of Histological Techniques. Suvarna, S. K., Layton, C., and Bancroft, J.D., eds. 7th ed. Churchill Livingstone, Edinburgh, New York.
- Brugaletta G., Teyssier J.R., Rochell S.J., Dridi S., Sirri F. A review of heat stress in chickens. Part I: insights into physiology and gut health. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.934381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedraz H., Gromboni J.G.G., Garcia A.A.P., Farias Filho R.V., Souza T.M., Oliveira E.R., Oliveira E.B., Nascimento C.S.D., Meneghetti C., Wenceslau A.A. Heat stress induces expression of HSP genes in genetically divergent chickens. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang A.N., Wu H.L., Yeh H.I., Chu C.S., Lin H.C., Lee W.C. Antioxidant effects of black rice extract through the induction of superoxide dismutase and catalase activities. Lipids. 2006;41:797–803. doi: 10.1007/s11745-006-5033-6. [DOI] [PubMed] [Google Scholar]
- Chowdhury V.S., Tomonaga S., Nishimura S., Tabata S., Cockrem J.F., Tsutsui K., Furuse M. Hypothalamic gonadotropin-inhibitory hormone precursor mRNA is increased during depressed food intake in heat-exposed chicks. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012;162:227–233. doi: 10.1016/j.cbpa.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Cobb 700. 2012. Broiler performance and nutrition supplement. Cobb-Vantress. Accessed Jan. 2018. http://www.cobb-vantress.com/docs/default-source/cobb-700-guides/cobb700_broiler_performance_nutrition_supplement_english9294AABB12037B70EE475E39.pdf.
- Dai S.F., Gao F., Xu X.L., Zhang W.H., Song S.X., Zhou G.H. Effects of dietary glutamine and gamma-aminobutyric acid on meat colour, pH, composition, and water holding characteristic in broilers under cyclic heat stress. Br. Poult. Sci. 2012;53:471–481. doi: 10.1080/00071668.2012.719148. [DOI] [PubMed] [Google Scholar]
- Del Vesco A.P., Gasparino E. Production of reactive oxygen species, gene expression, and enzymatic activity in quail subjected to acute heat stress. J. Anim. Sci. 2013;91:582–587. doi: 10.2527/jas.2012-5498. [DOI] [PubMed] [Google Scholar]
- Del Vesco A.P., Gasparino E., Grieser Dde O., Zancanela V., Soares M.A., Neto A.R. Effects of methionine supplementation on the expression of oxidative stress-related genes in acute heat stress-exposed broilers. Br. J. Nutr. 2015;113:549–559. doi: 10.1017/S0007114514003535. [DOI] [PubMed] [Google Scholar]
- Dokladny K., Moseley P.L., Ma T.Y. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G204–G212. doi: 10.1152/ajpgi.00401.2005. [DOI] [PubMed] [Google Scholar]
- El-Kassas S., El-Naggar K., Abdo S.E., Abdo W., Kirrella A.A.K., El-Mehaseeb I., Abu El-Magd M. Dietary supplementation with copper oxide nanoparticles ameliorates chronic heat stress in broiler chickens. Anim. Prod. Sci. 2019;60:254–268. [Google Scholar]
- Ewing S.A., Lay D.C., Borell E.V. Farm animal well-being: stress physiology, animal behavior, and environmental design. Prentice Hall; Upper Saddle River, NJ: 1999. [Google Scholar]
- Fan Y.K., Croom J., Christensen V.L., Black B.L., Bird A.R., Daniel L.R., Mcbride B.W., Eisen E.J. Jejunal glucose uptake and oxygen consumption in turkey poults selected for rapid growth. Poult. Sci. 1997;76:1738–1745. doi: 10.1093/ps/76.12.1738. [DOI] [PubMed] [Google Scholar]
- Fukata M., Michelsen K.S., Eri R., Thomas L.S., Hu B., Lukasek K. Toll like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288:423–429. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- Gasparino E., Del Vesco A P., Khatlab A.S., Zancanela V., Grieser D.O., Silva S.C.C. Effects of methionine hydroxy analogue supplementation on the expression of antioxidant-related genes of acute heat stress-exposed broilers. Animal. 2018;12:931–939. doi: 10.1017/S1751731117002439. [DOI] [PubMed] [Google Scholar]
- Hall D.M., Buettner G.R., Oberley L.W., Xu L., Matthes R.D., Gisolfi C.V. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am. J. Physiol. 2001;280:H509–H521. doi: 10.1152/ajpheart.2001.280.2.H509. [DOI] [PubMed] [Google Scholar]
- Han G., Yang H., Bahry M.A., Tran P.V., Do P.H., Ikeda H., Furuse M., Chowdhury V.S. L-Leucine acts as a potential agent in reducing body temperature at hatching and affords thermotolerance in broiler chicks. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2017;204:48–56. doi: 10.1016/j.cbpa.2016.10.013. [DOI] [PubMed] [Google Scholar]
- Hanson S.W.F., Olly J. Application of the Bligh & Dyer method of lipid extraction to tissue homogenates. Biochem. J. 1963;89:101–102. [Google Scholar]
- Hojo K., Hara A., Kitai H., Onishi M., Ichikawa H., Fukumori Y., Kawasaki K. Development of a method for environmentally friendly chemical peptide synthesis in water using water-dispersible amino acid nanoparticles. Chem. Central J. 2011;5:1–10. doi: 10.1186/1752-153X-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R., He Y., Arowolo M.A., Wu S., He J. Polyphenols as potential attenuators of heat stress in poultry production. Antioxidants. 2019;8:67. doi: 10.3390/antiox8030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. Upregulation of TLR4 mRNA expression levels in broiler chickens under acute heat stress. Braz. J. Poult. Sci. 2017;19:87–94. [Google Scholar]
- Iqbal M., Philbin V.J., Smith A.L. Expression patterns of chicken toll-like receptor mRNA in tissues, immune cell subsets and cell lines. Vet. Immunol. Immunopathol. 2005;104:117–127. doi: 10.1016/j.vetimm.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Ito K., Bahry M.A., Hui Y., Furuse M., Chowdhury V.S. Acute heat stress upregulates neuropeptide Y precursor mRNA expression and alters brain and plasma concentrations of free amino acids in chicks. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015;187:13–19. doi: 10.1016/j.cbpa.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Ju X.H., Xu H.J., Yong Y.H., An L.L., Jiao P.R., Liao M. Heat stress upregulation of toll-like receptors 2/4 and acute inflammatory cytokines in peripheral blood mononuclear cell (PBMC) of Bama miniature pigs: an in vivo and in vitro study. Animal. 2014;8:1462–1468. doi: 10.1017/S1751731114001268. [DOI] [PubMed] [Google Scholar]
- Kampinga H.H., Craig E.A. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat. Rev. Mol. 2010;11:579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KFSP . 2nd ed. National Institute of Animal Science; Gyeonggi-do, South Korea: 2012. Korean Feeding Standard for Poultry. [Google Scholar]
- Kim D., Byoung-Ki A., Sungtaek O., Moung-Cheul K., Sang L., Jae-Sang U., Tugay A., Kyung-Woo L. Effects of different methionine sources on growth performance, meat yield and blood characteristics in broiler chickens. J. Appl. Anim. Res. 2019;47:230–235. [Google Scholar]
- Kim G., Weiss S.J., Levine R.L. Methionine oxidation and reduction in proteins. Biochim. Biophys. Acta (BBA)-Gen. Subjects. 2014;1840:901–905. doi: 10.1016/j.bbagen.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King Y.T., Lin C.S., Lin J.H., Lee W.C. Whole-body hyperthermia-induced hermotolerance is associated with the induction of heatshock protein 70 in mice. J. Exp. Biol. 2002;205:273–278. doi: 10.1242/jeb.205.2.273. [DOI] [PubMed] [Google Scholar]
- Kumar A., Pathak R., Palfrey H.A., Stone K.P., Gettys T.W., Murthy S.N. High levels of dietary methionine improves sitagliptin-induced hepatotoxicity by attenuating oxidative stress in hypercholesterolemic rats. Nutr. Metab. 2020;17:2. doi: 10.1186/s12986-019-0422-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert G.P. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J. Anim. Sci. 2009;87:E101–E108. doi: 10.2527/jas.2008-1339. [DOI] [PubMed] [Google Scholar]
- Lan X., Hsieh J.C., Schmidt C.J., Zhu Q., Lamont S.J. Liver transcriptome response to hyperthermic stress in three distinct chicken lines. BMC Genom. 2016;17:1–11. doi: 10.1186/s12864-016-3291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew P.K., Zulkifli I., Hair-Bejo M., Omar A.R., Israf D.A. Effects of early age feed restriction and heat conditioning on heat shock protein 70 expression, resistance to infectious bursal disease, and growth in male broiler chickens subjected to heat stress. Poult. Sci. 2003;82:1879–1885. doi: 10.1093/ps/82.12.1879. [DOI] [PubMed] [Google Scholar]
- Liu L., Ren M., Ren K., Jin Y., Yan M. Heat stress impacts on broiler performance: a systematic review and meta-analysis. Poult. Sci. 2020;99:6205–6211. doi: 10.1016/j.psj.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu E., Zhao X., Li C., Wang Y., Li L., Zhu H., Ling Q. Effects of acute heat stress on liver damage, apoptosis and inflammation of pikeperch (Sander lucioperca) J. Therm. Biol. 2022;106 doi: 10.1016/j.jtherbio.2022.103251. [DOI] [PubMed] [Google Scholar]
- López-Maury L., Marguerat S., Bähler J. Tuning gene expression to changing environments: from rapid responses to evolutionary adaptation. Nat. Rev. Genet. 2008;9:583–593. doi: 10.1038/nrg2398. [DOI] [PubMed] [Google Scholar]
- Lund S.G., Ruberté M.R., Hofmann G.E. Turning up the heat: the effects of thermal acclimation on the kinetics of hsp70 gene expression in the eurythermal goby, Gillichthys mirabilis. Compar. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2006;143:435–446. doi: 10.1016/j.cbpa.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Luo S., Levine R.L. Methionine in proteins defends against oxidative stress. FASEB J. 2009;23:464–472. doi: 10.1096/fj.08-118414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B., Xing T., Li J., Zhang L., Jiang Y., Gao F. Chronic heat stress causes liver damage via endoplasmic reticulum stress-induced apoptosis in broilers. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson A.D., Liu G., Sun T., Tolba S.A., Xi L., Whelan R., Lei X.G. Supplemental methionine and stocking density affect antioxidant status, fatty acid profiles, and growth performance of broiler chickens. J. Anim. Sci. 2020;98 doi: 10.1093/jas/skaa092. skaa092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud K.Z., Edens F.W., Eisen E.J., Havenstein G.B. The effect of dietary phosphorus on heat shock protein mRNAs during acute heat stress in male broiler chickens (Gallus gallus) Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004;137:11–18. doi: 10.1016/j.cca.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Marrett L.E., Sunde M.L. The effect of other D-amino acids on the utilization of the isomers of methionine and its hydroxy analogue. Poult. Sci. 1965;44:957–964. doi: 10.3382/ps.0440957. [DOI] [PubMed] [Google Scholar]
- Mello J.L., Boiago M.M., Giampietro-Ganeco A., Berton M.P., Vieira L.D., Souza R.A., Ferrari F.B., Borba H. Periods of heat stress during the growing affects negatively the performance and carcass yield of broilers. Arch. Zootec. 2015;64:248. [Google Scholar]
- Mezquita B., Mezquita C., Mezquita J. Marked differences between avian and mammalian testicular cells in the heat shock induction and polyadenylation of Hsp70 and ubiquitin transcripts. FEBS Lett. 2021;436:382–386. doi: 10.1016/s0014-5793(98)01172-7. Animals. 11: 46 18 of 19. [DOI] [PubMed] [Google Scholar]
- Miao Z.Q., Dong Y.Y., Qin X., Yuan J.M., Han M.M., Zhang K.K., Li H. Dietary supplementation of methionine mitigates oxidative stress in broilers under high stocking density. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaei M., Bouyeh M., Zahedi A., Seidavi A., Khan R.U., Tufarelli V., Laudadio V., Abd El-Hack M.E., Ragni M., Taha A.E., Swelum A.A. Influence of dietary L-carnitine and lysine–methionine levels on reproductive performance and blood metabolic constituents of breeder ducks. Reprod. Domestic Anim. 2022;57:253–261. doi: 10.1111/rda.14047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanto-Hara F., Kikusato M., Ohwada S., Toyomizu M. Heat stress directly affects intestinal integrity in broiler chickens. J. Poult. Sci. 2019 doi: 10.2141/jpsa.0190004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (NRC) 9th revised ed. National Academic Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Ohtsu H., Yamazaki M., Abe H., Murakami H., Toyomizu M. Heat stress modulates cytokine gene expression in the spleen of broiler chickens. J. Poult. Sci. 2015;52:282–287. [Google Scholar]
- Paglia D.E., Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967;70:158–169. [PubMed] [Google Scholar]
- Pluske J.R., Williams I.H., Aherne F.X. Maintenance of villus height and crypt depth in piglets by providing continuous nutrition after weaning. Anim. Sci. 1996;62:131–144. [Google Scholar]
- Pluske J.R., Williams I.H., Aherne F.X. Villus height and crypt depth in piglets in response to increases in the intake of cows' milk after weaning. Anim. Sci. 1996;62:145–1581. [Google Scholar]
- Pockley A.G. Heat shock proteins as regulators of the immune response. Lancet. 2003;9:469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M.L., Sakai M., Sá L.R., Ferreira A.J., Palermo-Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010;89:1905–1914. doi: 10.3382/ps.2010-00812. [DOI] [PubMed] [Google Scholar]
- Randhir S., Pradhan K. Printox, Dhawan Printing Works; New Dalhi, India: 1981. Forage Evaluation. First Published. [Google Scholar]
- Rao X., Huang X., Zhou Z., Lin X. An improvement of the 2ˆ(-delta delta ct) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013;3:71–85. [PMC free article] [PubMed] [Google Scholar]
- Ray P.D., Huang B., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman A.U., Arif M., Husnain M.M., Alagawany M., El-Hack A.M.E., Taha A.E., Allam A.A. Growth performance of broilers as influenced by different levels and sources of methionine plus cysteine. Animals. 2019;9:1056. doi: 10.3390/ani9121056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa P.S., Faria Filho D.E., Dahlke F., Vieira B.S., Macari M., Furlan R.L. Performance and carcass characteristics of broiler chickens with different growth potential and submitted to heat stress. Braz. J. Poult. Sci. 2007;9:181–186. [Google Scholar]
- Ross 308. 2014. Ross 308 broiler: nutrition specifications. Aviagen. Accessed Jan. 2018. http://en.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross308BroilerNutritionSpecs2014-EN.pdf.
- Saleh A.A., Ragab M.M., Ahmed E.A., Abudabos A.M., Ebeid T.A. Effect of dietary zinc-methionine supplementation on growth performance, nutrient utilization, antioxidative properties and immune response in broiler chickens under high ambient temperature. J. Appl. Anim. Res. 2018;46:820–827. [Google Scholar]
- SAS Institute Inc. SAS Institute Inc; Cary, NC: 2014. SAS Proprietary Software Release 9.3. [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejian V., Bhatta R., Gaughan J.B., Dunshea F.R., Lacetera N. Adaptation of animals to heat stress. Animal. 2018;12:s431–s444. doi: 10.1017/S1751731118001945. [DOI] [PubMed] [Google Scholar]
- Sharp F.R., Massa S.M., Swanson R.A. Heat-shock protein protection. Trends Neurosci. 1999;22:97–99. doi: 10.1016/s0166-2236(98)01392-7. [DOI] [PubMed] [Google Scholar]
- Shen Y.B., Ferket P., Park I., Malheiros R.D., Kim S.W. Effects of feed grade-methionine on intestinal redox status, intestinal development, and growth performance of young chickens compared with conventional-methionine. J. Anim. Sci. 2015;93:2977–2986. doi: 10.2527/jas.2015-8898. [DOI] [PubMed] [Google Scholar]
- Song Z.H., Cheng K., Zheng X.C., Ahmad H., Zhang L.L., Wang T. Effects of dietary supplementation with enzymatically treated Artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult. Sci. 2018;97:430–437. doi: 10.3382/ps/pex312. [DOI] [PubMed] [Google Scholar]
- Song J., Xiao K., Ke Y.L., Jiao L.F., Hu C.H., Diao Q.Y., Zou X.T. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 2013;93:581–588. doi: 10.3382/ps.2013-03455. [DOI] [PubMed] [Google Scholar]
- Soomro R.N., Hu R., Qiao Y., El-Hack A.M.E., Abbasi I.H.R., Mohamed M.A.E.…Kuldeep D. Effect of dietary protein sources and amino acid balances on performance, intestinal permeability and morphology in broiler chickens. Int. J. Pharmacol. 2017;13:378–387. [Google Scholar]
- Soomro R.N., Yao J., El-Hack A.M.E., Abbasi I.H.R., Saeed M., Tufarelli V. Significance of endogenous amino acid losses in the nutrition of some poultry species: a review. JAPS: J. Anim. Plant Sci. 2018;28:1547–1557. [Google Scholar]
- Souza M.G., Oliveira R.F.M., Donzele J.L., Assis Maia A.P., Balbino E.M., Oliveira W.P. Utilizac¸a∼o das vitaminas C e E em rac¸o∼es para frangos de corte mantidos em ambiente de alta temperatura. R. Bras. Zootec. 2011;40:2192–2198. [Google Scholar]
- Surai P.F., Kochish I.I., Fisinin V.I., Kidd M.T. Antioxidant defence systems and oxidative stress in poultry biology: an update. Antioxidants. 2019;8:235. doi: 10.3390/antiox8070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamanna N., Kroeker K., Braun K., Banh S., Treberg J.R. The effect of short-term methionine restriction on glutathione synthetic capacity and antioxidant responses at the whole tissue and mitochondrial level in the rat liver. Exp. Gerontol. 2019;127 doi: 10.1016/j.exger.2019.110712. [DOI] [PubMed] [Google Scholar]
- Uyanga V.A., Oke E.O., Amevor F.K., Zhao J., Wang X., Jiao H., Onagbesan O.M., Lin H. Functional roles of taurine, L-theanine, L-citrulline, and betaine during heat stress in poultry. J. Anim. Sci. Biotechnol. 2022;13:1–20. doi: 10.1186/s40104-022-00675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varasteh S., Braber S., Akbari P., Garssen J., Fink-Gremmels J. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed R., El Asely A.M., Bakery H., El-Shawarby R., Abuo-Salem M., Abdel-Aleem N., Malhat F., Khafaga A.F., Abdeen A. Thermal stress accelerates mercury chloride toxicity in Oreochromis niloticus via up-regulation of mercury bioaccumulation and HSP70 mRNA expression. Sci. Total Environ. 2020;718:137326. doi: 10.1016/j.scitotenv.2020.137326. [DOI] [PubMed] [Google Scholar]
- Willemsen H., Swennen Q., Everaert N., Geraert P.A., Mercier Y., Stinckens A., Decuypere E., Buyse J. Effects of dietary supplementation of methionine and its hydroxy analog DL-2-hydroxy-4- methylthiobutanoic acid on growth performance, plasma hormone levels, and the redox status of broiler chickens exposed to high temperatures. Poult. Sci. 2011;90:2311–2320. doi: 10.3382/ps.2011-01353. [DOI] [PubMed] [Google Scholar]
- Xu Z.R., Hu C.H., Xia M.S., Zhan X.A., Wang M.Q. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003;82:1030–1036. doi: 10.1093/ps/82.6.1030. [DOI] [PubMed] [Google Scholar]
- Yang P.C., He S.H., Zheng P.Y. Investigation into the signal transduction pathway via which heat stress impairs intestinal epithelial barrier function. J. Gastroenterol. Hepatol. 2007;22:1823–1831. doi: 10.1111/j.1440-1746.2006.04710.x. [DOI] [PubMed] [Google Scholar]
- Zhang L.J., Wang K.F., Jing Y.P., Zhuang H.M., Wu G. Identification of heat shock protein genes HSP70s and HSP70 and their associated mRNA expression under heat stress in insecticide-resistant and susceptible diamondback moth, plutella xylostella (Lepidoptera: Plutellidae) Eur. J. Entomol. 2015;112:215–226. [Google Scholar]
- Zulkifli I., Al-Aqil A., Omar A.R., Sazili A.Q., Rajion M.A. Crating and heat stress influence blood parameters and heat shock protein 70 expression in broiler chickens showing short or long tonic immobility reactions. Poult. Sci. 2009;88:471–476. doi: 10.3382/ps.2008-00287. [DOI] [PubMed] [Google Scholar]
- Zulkifli I., Che Norma M.T., Israf D.A., Omar A.R. The effect of early-age food restriction on heat shock protein response in heat-stressed female broilers chickens. Br. Poult. Sci. 2002;43:141–145. doi: 10.1080/00071660120109953. [DOI] [PubMed] [Google Scholar]
- Zulkifli I., Liew P., Israf D., Omar A., Hair-Bejo M. Effects of early age feed restriction and heat conditioning on heterophil/lymphocyte ratios, heat shock protein 70 expression and body temperature of heat-stressed broiler chickens. J. Therm. Biol. 2003;28:217–222. [Google Scholar]