Abstract
The development of thylakoid stacking, accumulation of the light-harvesting chlorophyll a/b protein complex (LHCP), and the changes of circular dichroism (CD) which reflect the organization of chlorophyll molecules in greening thylakoids of bean Phaseolus vulgaris cv Red Kidney leaves were investigated.
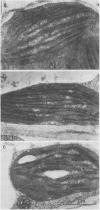
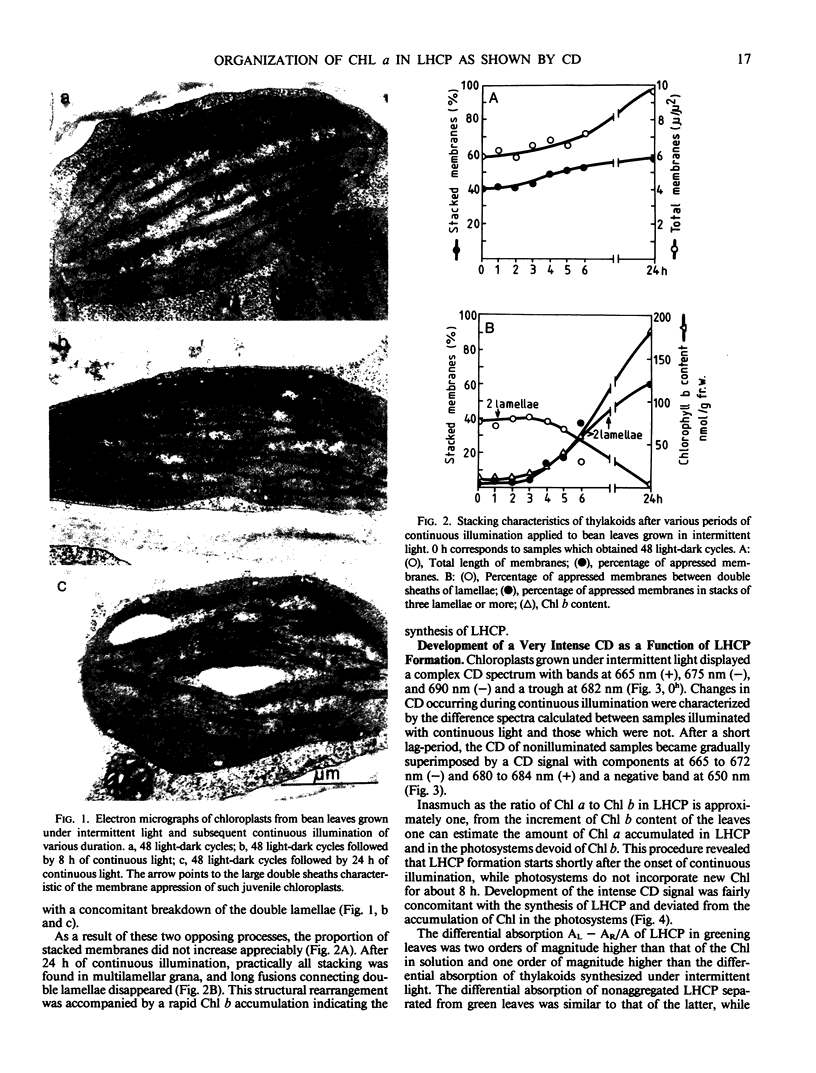
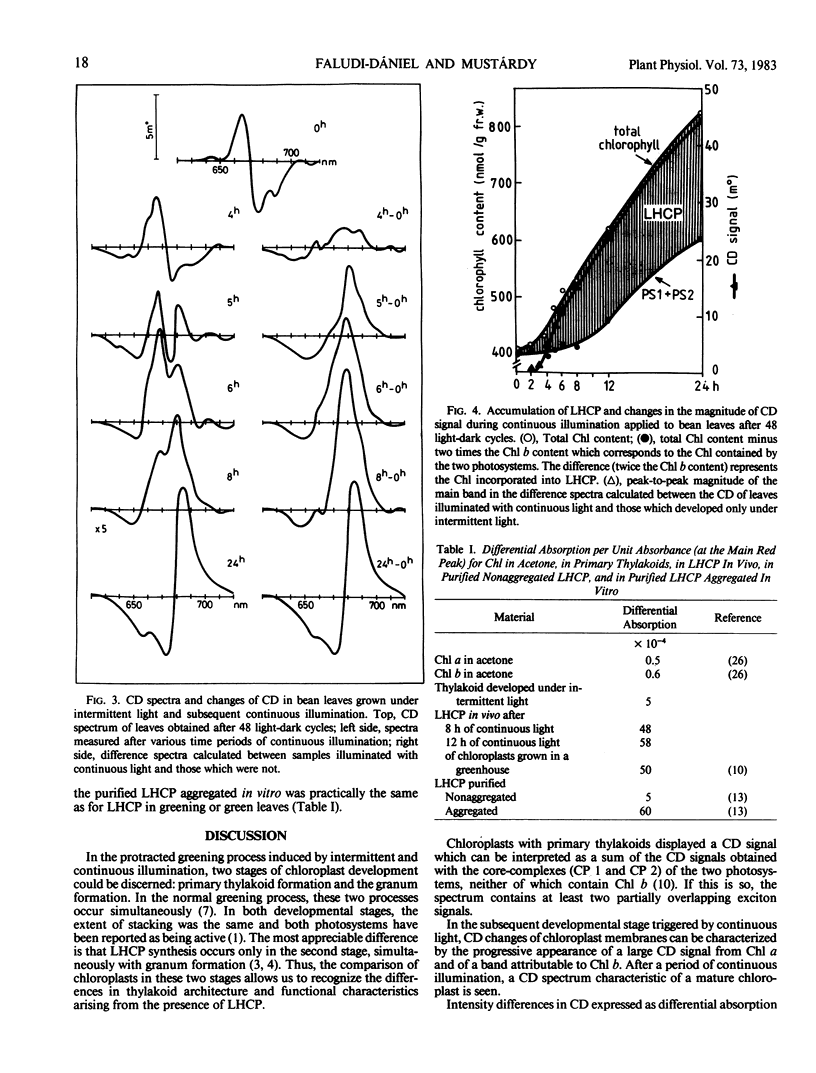
Chloroplasts formed under intermittent light contained large double sheets of membrane with extensive appression in addition to separate lamellae. Thylakoids of such chloroplasts were devoid of LHCP and exhibited a relatively small CD in the chlorophyll absorption region. Upon continuous illumination, the rearrangement of membranes to characteristic grana and the accumulation of the LHCP was accompanied by the gradual appearance of the very intense CD signal with peaks at 682 to 684 (+) and 665 to 672 nanometers (−). The magnitude of differential absorption was approximately 100 times larger than that of the chlorophyll a in solution. This suggests a superhelical liquid crystal-like organization for LHCP, a texture which can be altered by changes of the electric field in the photosynthetic membranes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argyroudi-Akoyunoglou J. H., Akoyunoglou G. Photoinduced changes in the chlorophyll a to chlorophyll B ratio in young bean plants. Plant Physiol. 1970 Aug;46(2):247–249. doi: 10.1104/pp.46.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyroudi-Akoyunoglou J. H., Feleki Z., Akoyunoglou G. Formation of two chlorophyll-protein complexes during greening of etiolated bean leaves. Biochem Biophys Res Commun. 1971 Nov 5;45(3):606–614. doi: 10.1016/0006-291x(71)90460-8. [DOI] [PubMed] [Google Scholar]
- Bennett J. Chloroplast phosphoproteins. The protein kinase of thylakoid membranes is light-dependent. FEBS Lett. 1979 Jul 15;103(2):342–344. doi: 10.1016/0014-5793(79)81358-7. [DOI] [PubMed] [Google Scholar]
- Bennett J., Steinback K. E., Arntzen C. J. Chloroplast phosphoproteins: regulation of excitation energy transfer by phosphorylation of thylakoid membrane polypeptides. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5253–5257. doi: 10.1073/pnas.77.9.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeter S., Mustardy L., Machowicz E. The development of the intense circular-dichroic signal during granum formation in greening etiolated maize. Biochem J. 1976 May 15;156(2):469–472. doi: 10.1042/bj1560469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faludi-Dániel A., Bialek G. E., Horváth G., Sz-Rózsa Z., Gregory R. P. Differential light-scattering of granal and agranal chloroplasts and their fragments. Biochem J. 1978 Aug 15;174(2):647–651. doi: 10.1042/bj1740647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faludi-Dániel A., Demeter S., Garay A. S. Circular dichroism spectra of granal and agranal chloroplasts of maize. Plant Physiol. 1973 Jul;52(1):54–56. doi: 10.1104/pp.52.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory R. P., Borbély G., Demeter S., Faludi-Dániel A. Chiroptical properties of chlorophyll-protein complexes separated on Deriphat/polyacrylamide gel. Biochem J. 1982 Jan 15;202(1):25–29. doi: 10.1042/bj2020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory R. P., Demeter S., Faludi-Dániel A. Macromolecular organization of chlorophyll a in aggregated chlorophyll a/b protein complex as shown by circular dichroism at room and cryogenic temperatures. Biochim Biophys Acta. 1980 Jul 8;591(2):356–360. doi: 10.1016/0005-2728(80)90166-8. [DOI] [PubMed] [Google Scholar]
- Gregory R. P., Raps S. The differential scattering of circularly polarized light by chloroplasts and evaluation of their true circular dichroism. Biochem J. 1974 Aug;142(2):193–201. doi: 10.1042/bj1420193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Hollingshead C. Formation of crystalline arrays of chlorophyll a/b - light-harvesting protein by membrane reconstitution. Biophys J. 1982 Jan;37(1):363–370. doi: 10.1016/S0006-3495(82)84684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J. M., Ke B., Thompson K. H. Exciton interaction among chlorophyll molecules in bacteriochlorophyllaproteins and bacteriochlorophyllareaction center complexes from green bacteria. Biochim Biophys Acta. 1976 Jun 8;430(3):524–537. doi: 10.1016/0005-2728(76)90028-1. [DOI] [PubMed] [Google Scholar]
- Pearlstein R. M., Davis R. C., Ditson S. L. Giant circular dichroism of high molecular weight chlorophyllide-apomyoglobin complexes. Proc Natl Acad Sci U S A. 1982 Jan;79(2):400–402. doi: 10.1073/pnas.79.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson K. D., Sauer K. Light-scattering effects on the circular dichroism of chloroplasts. Biochemistry. 1973 Aug 28;12(18):3454–3458. doi: 10.1021/bi00742a015. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B., Gregory R. P. Properties of protein-chlorophyll complexes from pea (Pisum sativum L.) leaves. The organization of chlorophyll. Biochem J. 1975 Aug;149(2):341–347. doi: 10.1042/bj1490341. [DOI] [PMC free article] [PubMed] [Google Scholar]