Abstract
Uptake of l-[1-14C]ascorbate by intact ascorbate-free spinach (Spinacia oleracea L. cv Vitalr) chloroplasts has been investigated using the technique of silicone oil filtering. Rates greater than 100 micromoles per milligram chlorophyll per hour (external concentration, 10 millimolar) of ascorbate transport were observed. Ascorbate uptake into the sorbitol-impermeable space (stroma) followed the Michaelis-Menten-type characteristic for substrate saturation. A Km of 18 to 40 millimolar was determined. Transport of ascorbate across the chloroplast envelope resulted in an equilibrium of the ascorbate concentrations between stroma and medium. A pH optimum of 7.0 to 7.5 and the lack of alkalization of the medium upon ascorbate uptake suggest that only the monovalent ascorbate anion is able to cross the chloroplast envelope. The activation energy of ascorbate uptake was determined to be 65.8 kilojoules (16 kilocalories) per mole (8 to 20°C). Interference of ascorbate transport with substrates of the phosphate or dicarboxylate translocator could not be detected, but didehydroascorbate was a competitive inhibitor. Preloading of chloroplasts with didehydroascorbate resulted in an increase of Vmax but did not change the Km for ascorbate. Millimolar concentrations of the sulfhydryl reagent p-chloromercuriphenyl sulfonate inhibited ascorbate uptake. The data are interpreted in terms of ascorbate uptake into chloroplasts by the mechanism of facilitated diffusion mediated by a specific translocator.
Full text
PDF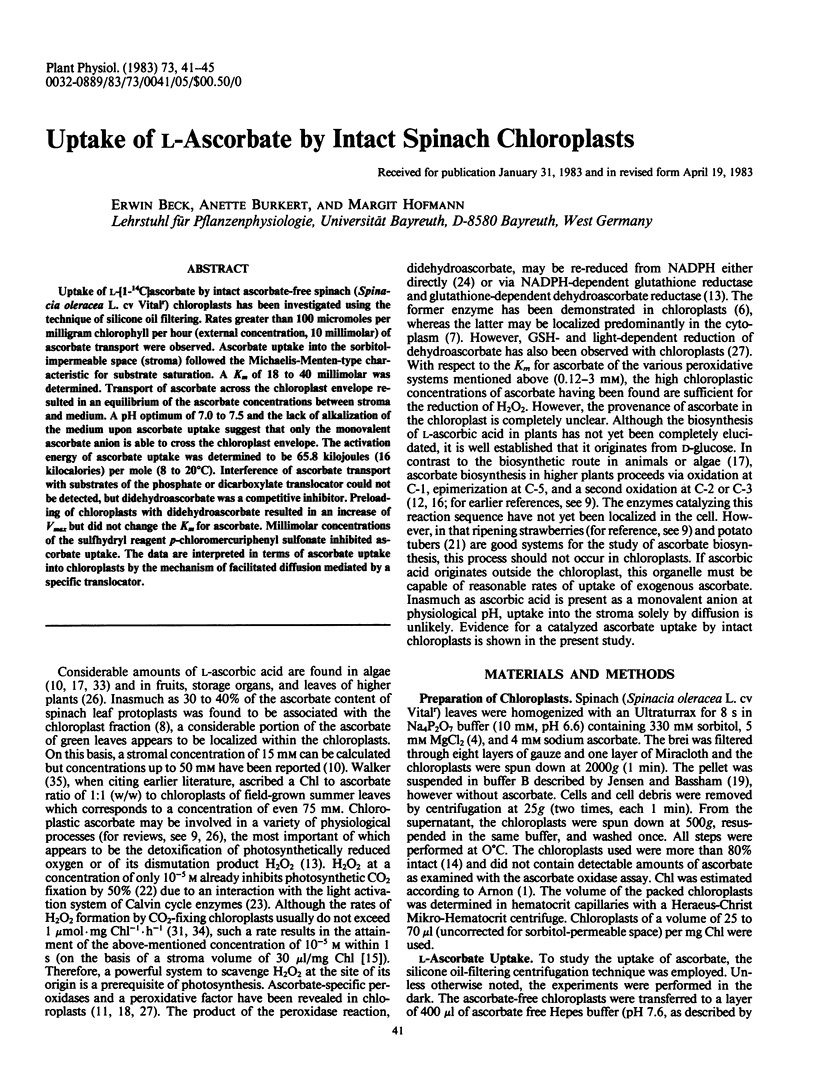


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur H., Heldt H. W. Transport of hexoses across the liver-cell membrane. Eur J Biochem. 1977 Apr 1;74(2):397–403. doi: 10.1111/j.1432-1033.1977.tb11404.x. [DOI] [PubMed] [Google Scholar]
- Cockburn W., Walker D. A., Baldry C. W. Photosynthesis by isolated chloroplasts. Reversal of orthophosphate inhibition by Calvin-cycle intermediates. Biochem J. 1968 Mar;107(1):89–95. doi: 10.1042/bj1070089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliege R., Flügge U. I., Werdan K., Heldt H. W. Specific transport of inorganic phosphate, 3-phosphoglycerate and triosephosphates across the inner membrane of the envelope in spinach chloroplasts. Biochim Biophys Acta. 1978 May 10;502(2):232–247. doi: 10.1016/0005-2728(78)90045-2. [DOI] [PubMed] [Google Scholar]
- Groden D., Beck E. H2O2 destruction by ascorbate-dependent systems from chloroplasts. Biochim Biophys Acta. 1979 Jun 5;546(3):426–435. doi: 10.1016/0005-2728(79)90078-1. [DOI] [PubMed] [Google Scholar]
- Grün M., Renstrøm B., Loewus F. A. Loss of Hydrogen from Carbon 5 of d-Glucose during Conversion of d-[5-H,6-C]Glucose to l-Ascorbic Acid in Pelargonium crispum (L.) L'Hér. Plant Physiol. 1982 Nov;70(5):1233–1235. doi: 10.1104/pp.70.5.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Heldt W. H., Werdan K., Milovancev M., Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973 Aug 31;314(2):224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- Helsper J. P., Kagan L., Hilby C. L., Maynard T. M., Loewus F. A. l-Ascorbic Acid Biosynthesis in Ochromonas danica. Plant Physiol. 1982 Feb;69(2):465–468. doi: 10.1104/pp.69.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingebretsen O. C., Normann P. T. Transport of ascorbate into guinea pig liver mitochondria. Biochim Biophys Acta. 1982 Jan 4;684(1):21–26. doi: 10.1016/0005-2736(82)90044-x. [DOI] [PubMed] [Google Scholar]
- Jablonski P. P., Anderson J. W. Light-dependent reduction of hydrogen peroxide by ruptured pea chloroplasts. Plant Physiol. 1982 Jun;69(6):1407–1413. doi: 10.1104/pp.69.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W. The effect of hydrogen peroxide on CO2 fixation of isolated intact chloroplasts. Biochim Biophys Acta. 1976 Sep 13;440(3):476–482. doi: 10.1016/0005-2728(76)90035-9. [DOI] [PubMed] [Google Scholar]
- Lehner K., Heldt H. W. Dicarboxylate transport across the inner membrane of the chloroplast envelope. Biochim Biophys Acta. 1978 Mar 13;501(3):531–544. doi: 10.1016/0005-2728(78)90119-6. [DOI] [PubMed] [Google Scholar]
- Plaut Z., Gibbs M. Glycolate formation in intact spinach chloroplasts. Plant Physiol. 1970 Apr;45(4):470–474. doi: 10.1104/pp.45.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmer R., Kok B., Ollinger O. Kinetics and Apparent K(m) of Oxygen Cycle under Conditions of Limiting Carbon Dioxide Fixation. Plant Physiol. 1978 Jun;61(6):915–917. doi: 10.1104/pp.61.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Gibbs M. Hydrogen peroxide synthesis in isolated spinach chloroplast lamellae : an analysis of the mehler reaction in the presence of NADP reduction and ATP formation. Plant Physiol. 1982 Nov;70(5):1249–1254. doi: 10.1104/pp.70.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P. Transport of Glycerate across the Envelope Membrane of Isolated Spinach Chloroplasts. Plant Physiol. 1982 Oct;70(4):1032–1038. doi: 10.1104/pp.70.4.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer G., Heber U. Glucose transport into spinach chloroplasts. Plant Physiol. 1977 Aug;60(2):286–289. doi: 10.1104/pp.60.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W. Accumulation of bicarbonate in intact chloroplasts following a pH gradient. Biochim Biophys Acta. 1972 Dec 14;283(3):430–441. doi: 10.1016/0005-2728(72)90260-5. [DOI] [PubMed] [Google Scholar]


