Abstract
Introduction
The kappa-opioid receptor (KOR) has been implicated in mediating the behavioral and biochemical effects associated with nicotine reward and withdrawal; however, its underlying mechanisms remain to be further explored.
Methods
Adult male Sprague-Dawley rats were used to establish a nicotine dependence and withdrawal model by injecting nicotine (3 mg/kg/day, s.c.) or vehicle for 14 days, followed by the termination of nicotine for 7 days. Body weight gain, pain behaviors, and withdrawal scores were assessed in succession. MicroRNA (miRNA) sequencing was performed, and quantitative real-time PCR was used to detect the expression of candidate miRNAs and Oprk1. Western blotting was performed to examine KOR protein expression of KOR. Luciferase assay was conducted to validate the relationship of certain miRNAs/Oprk1.
Results
The behavioral results showed that nicotine dependence and withdrawal induced behavioral changes. Biochemical analyses demonstrated that miR-144-3p expression decreased and Oprk1/KOR expression increased in the prefrontal cortex, nucleus accumben, and hippocampus. Further investigation suggested that miR-144-3p exerted an inhibitory effect on Oprk1 expression in PC12 cells.
Conclusions
This study revealed that miR-144-3p/Oprk1/KOR might be a potential pathway underlying the adverse effects induced by nicotine dependence and withdrawal, and might provide a novel therapeutic target for smoking cessation.
Implications
This study demonstrates an impact of nicotine dependence and nicotine withdrawal on behavioral outcomes and the expressions of miR-144-3p/Oprk1/KOR in male rats. These findings have important translational implications given the continued use of nicotine and the difficulty in smoking cessation worldwide, which can be applied to alleviated the adverse effects induced by nicotine dependence and withdrawal, thus assist smokers to quit smoking.
Introduction
Cigarette smoking is a primary preventable cause of morbidity and mortality worldwide.1–3 Smoking cessation causes adverse withdrawal symptoms, which in turn make it challenging to maintain abstinence and contributes to relapse.4–6
Nicotine withdrawal symptoms are characterized by somatic symptoms (eg, gastrointestinal discomfort, increased appetite, and tremors), affective symptoms (such as depression, anxiety, hyperalgesia, and irritability), attention deficits, and cognitive deficits.7,8 Generally, nicotine withdrawal symptoms peak during the first week of smoking abstinence and may last for up to 4 weeks. Current smoking cessation methods are inadequate, and novel therapeutic targets need to be explored.9,10
The dynorphin/kappa-opioid receptor (KOR) is one of the endogenous opioid receptor systems, which has been implicated in the biological processes of nicotine.11–13 Ugur et al. observed that chronic nicotine treatment modulated DOR and KOR gene expression in the mesocorticolimbic system.14 Ward et al. reported that KOR agonist ±U50,488 H increased the aversive effects of nicotine, whereas the KOR antagonist nor-BNI attenuated them.15 Other studies have also suggested that the KOR antagonist is a potential therapeutic agent for managing withdrawal syndromes in smoking cessation.16–18 In addition, KOR was found to affect the energy balance. Seoane-Collazo et al. reported that nicotine treatment decreased body weight in wild-type mice but not in KOR-null mice. Further investigation revealed that the effect of nicotine on weight was dependent on KOR, specifically in the lateral hypothalamic area.19 Hence, KOR likely plays an important role in nicotine reward and withdrawal in several ways and could be a promising target for smoking cessation.
MicroRNAs (miRNAs) are endogenous short noncoding RNAs (~22 nucleotides long) that usually direct posttranscriptional suppression through translational inhibition or mRNA destabilization by pairing to the 3ʹ untranslated (UTR) region of mRNAs.20 miRNAs are involved in the biological functions of nicotine and are potential biomarkers or therapeutic targets.21–24
We hypothesized that the miRNA-mediated regulations of Oprk1/KOR in different brain regions are one of the mechanisms underlying nicotine administration and withdrawal symptoms. Therefore, we identified and compared miRNAs from different brain regions in nicotine-administered and nicotine-withdrawn rats with those in non-nicotine-administered rats. The expression of the candidate differentially expressed miRNAs (DEmiRNAs) and Oprk1/KOR was examined both in vivo and in vitro. Finally, the relationship between specific DEmiRNAs and Oprk1/KOR was validated using the luciferase reporter assay.
Materials and Methods
Animals and Grouping
A total of 36 male Sprague-Dawley rats (8 weeks old) were acquired from Hunan SJA Laboratory Animal Company (Changsha, Hunan, China). All rats had free access to food and water and were housed under a 12/12 dark/light cycle at 22 ± 1°C. After 7 days of adaptation, the rats were randomly divided into two groups (n = 18): Group NS and Group NI. The animal study protocol was approved by the Animal Care and Use Committee of Hunan Cancer Hospital (Permit Number: 2020-11).
Establishment of Nicotine Dependence Model
(−)-Nicotine hydrogen tartrate salt [(−)-1-methyl-2-(3-pyridyl) pyrrolidine (+)-bitartrate salt] was purchased from Sigma–Aldrich Inc. (St. Louis, MO, USA). Nicotine was dissolved in 0.9% sodium chloride. In Group NI, the rats were subcutaneously injected with nicotine (3 mg/kg/day) twice daily at 08:00–09:00 AM and 16:00–17:00 PM for 14 days. Rats in Group NS were injected with an equal volume of 0.9% saline following the same schedule.
Spontaneous Nicotine Withdrawal
After 14 days of consecutive injections, nicotine or saline injections were ceased. On day 1 after nicotine withdrawal, a 15-min observation of somatic signs was observed, including paw and body tremors, head shakes, backing, jumps, curls, and ptosis.7,25 The total number of somatic signs was tallied for each rat, and group averages were plotted. Hyperalgesia was evaluated using the von-Frey fibers and hot plate test immediately following the somatic sign observation period as previously described.26,27 All experiments were performed by an observer blinded to the experimental treatments.
Pain Behavioral Assessments
To assess the effects of nicotine dependence and withdrawal on pain perception, we tested mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL) to monitor the changes in pain. Based on previous reports,28 von-Frey filaments (Touch-Test Sensory Evaluator, Wood Dale, IL, USA) were used for the MWT test. Before the experiment, the rats were allowed to adapt to the test environment for 15–30 min. Each rat was tested at least five times at an interval of 15 s. The 50% threshold for further analysis was calculated using the up- and down-method.
The TWL was measured using a Hargreaves apparatus (Ugo Basile, Italy). During the test, each rat was tested at least three times at an interval of 5 min. The time from the onset of radiant heat stimulation to the withdrawal of the hind paw was recorded.
RNA Isolation, Sequencing, and Target Gene Prediction
On day 14, 24 h after the last injection, the rats were decapitated under deep anesthesia with 4% (v/v) isoflurane inhalation for 3 min. Hippocampi were quickly dissected, and collected on ice. Total RNA was extracted from the hippocampi using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA), and the concentration and purity of the RNA samples were validated using a NanoDrop spectrophotometer. Next, small RNA sequencing was conducted to detect the DEmiRNAs. A cut-off value of 1.5-fold was used to determine the differences in miRNA levels. Finally, a list of target transcripts for miR-144-3p (256 transcripts) was obtained from miRDB.
Western Blot Analyses
Total protein was extracted from the prefrontal cortex (PFC), nucleus accumben (NAc), and hippocampus (Hippo) using radioimmunoprecipitation assay (RIPA) lysis buffer, and protein concentration was determined by BCA Assay kit. Then the samples were loaded onto a 10% SDS-PAGE gel. The primary antibodies used were against KOR (Abcam, Cambridge, MA, USA) and GAPDH (Proteintech, China). Protein bands were visualized using enzyme-catalyzed chemiluminescence (ECL). Images were analyzed by Image J v1.8.o software (Bio-Rad, Hercules, CA, USA).
Quantitative Real-Time PCR
Quantitative real-time PCR (RT-qPCR) analyses were performed to confirm the expressions of miR-144-3p and Oprk1 in the PFC, NAc, Hippo, and PC12 cells. U6 and actin serve as endogenous reference genes (controls). The primers used were as follows: U6 (F: 5ʹ-GCTTCGGCAGCACATATACTAAAAT-3ʹ, R: 5ʹ-CGCTTCACGAATTTGCGTGTCAT-3ʹ), actin (F: 5ʹ-ACATCCGTAAAGACCTCTATGCC-3ʹ, R: 5ʹ-TACTCCTGCTTGCT GATCCAC-3ʹ), miR-144-3p (GSP: 5ʹ-GGGGGGTACAGTATAGATGA-3ʹ, R: 5ʹ-CAGTGCGTGTCGTGGA-3ʹ), and Oprk1(F: 5ʹ-ACTTCTGCATTGCCTTGGGTT-3ʹ, R: 5ʹ-AAGCAGAAGTC CCTAA AACACCG-3ʹ).
Vector Construction, Mutagenesis, and Luciferase Assay
To elucidate the regulatory relationship between Oprk1 and rno-miR-144-3p, the 3ʹ UTR sequences of Oprk1 containing binding sites for rno-miR-144-3p were cloned into pcDNA luciferase vectors (Promega, Madison, WI, USA) to produce vectors for wide-type Oprk1 3ʹ UTRs. Then, site-directed mutagenesis was carried out using a Quick Change II site-directed mutagenesis assay kit (Stratagene, San Diego, CA) to generate mutant 3ʹ UTR sequences of Oprk1 harboring the mutated binding sites for rno-miR-144-3p. Furthermore, the mutant 3ʹ UTR sequences were cloned into pcDNA luciferase vectors to produce vectors for mutant Oprk1 3ʹ UTRs. In the next step, PC12 cells (Zhong Qiao Xin Zhou Biotechnology Company, Shanghai, China) were cotransfected with rno-miR-144-3p in conjunction with wild-type or mutant 3ʹ UTR of Oprk1 using Lipofectamine 3000. At 48 h post-transfection, the luciferase activity of the transfected cells was assayed using a Bright Glo Luciferase kit (Promega, Madison, WI, USA) following the manufacturer’s instructions.
Statistical Analysis
All data were expressed as mean ± SD and analyzed using GraphPad Prism 3.0. Image quantification of western blotting bands was presented as values relative to the control. Differences between the groups were assessed using two-way or one-way analysis of variance (ANOVA), followed by Bonferroni or Dunnett’s post hoc tests. Statistical significance was set at p < .05.
Results
Nicotine Dependence and Withdrawal Caused Changes in MWT, TWL, Body Weight Gain, and Withdrawal Scores
To explore the effects of nicotine dependence and withdrawal on the behaviors of rats, we established a rat model by injecting 3 mg/kg/day nicotine s.c. for 14 days followed by the spontaneous withdrawal for 7 days. As shown in Figure 1A and B, the 50% MWT and TWL of rats in Group NI (nicotine administration) were significantly higher than those in Group NS (saline administration) at 1 and 2 weeks postinjection. The 50% MWT and TWL in Group NI declined and were lower than those of Group NS on day 1 of withdrawal. The hyperalgesic state lasted for several days before returning to normal levels. Regarding body weight gain, the rats in Group NI gained less weight than those in Group NS after nicotine administration, whereas after nicotine withdrawal, the weight gain of rats in Group NI (nicotine administration) was higher than those of Group NS (saline administration) (Figure 1C). As shown in Figure 1D, compared with Group NS, the withdrawal scores were significantly higher in Group NI. These tests indicated that 3 mg/kg/day nicotine successfully established a rat nicotine dependence and withdrawal model. Nicotine administration resulted in hypoalgesia and decreased body weight gain, while nicotine withdrawal caused hyperalgesia, increased body weight gain, and higher withdrawal scores.
Figure 1.
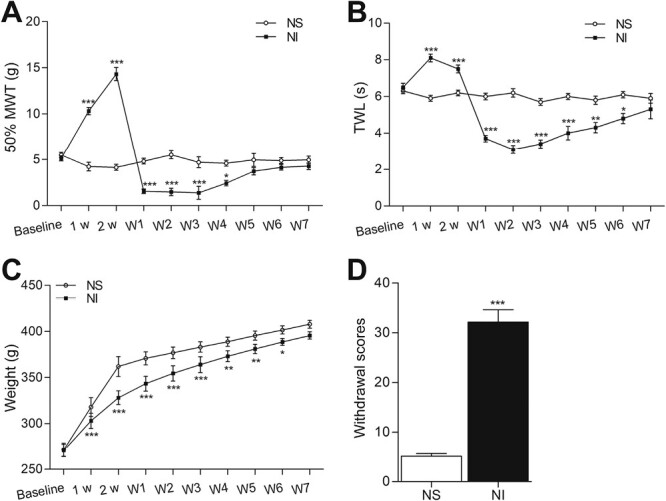
Nicotine dependence and withdrawal cause changes in mechanical withdrawal threshold (MWT), thermal withdrawal latency (TWL), weight gain, and the withdrawal scores in rats. (A) Changes in MWT, (B) changes in TWL, (C) changes in weight gains, and (D) changes in withdrawal scores. Each point represents the mean ± SD (n = 6–12/group). *p < .05, **p < .005, ***p < .001 versus Group NS.
Expression Profile, Validation, and Target Genes Prediction of miRNAs in the Hippocampi of Nicotine Dependence Rats
To determine the effect of nicotine administration on the expression of miRNAs, we tested the expression profile of miRNAs in the hippocampi of rats using microarray. According to the criteria of a fold change > 1.5 and a p-value < .05, a total of 14 DEmiRNAs were identified, including 9 upregulated and 5 downregulated miRNAs (Figure 2A), and the hierarchical clustering heat map for the 14 DEmiRNAs was displayed in Figure 2B. The prediction of target genes suggested that Oprk1 was likely one of the downstream genes of miR-144-3p (Figure 2D). Previous reports have indicated that miR-144-3p is involved in drug abuse. Therefore, we selected miR-144-3p as the candidate for further study. The RT-qPCR results revealed that miR-144-3p was downregulated in Group NIC (day 14 after nicotine administration), compared with Group NS (day 14 after saline administration) (n = 6 in each group, p < .005) (Figure 2C), which was in line with the sequencing data. In summary, the expression of miRNAs was altered in the hippocampi of nicotine-dependent rats, specifically, miR-144-3p was downregulated.
Figure 2.
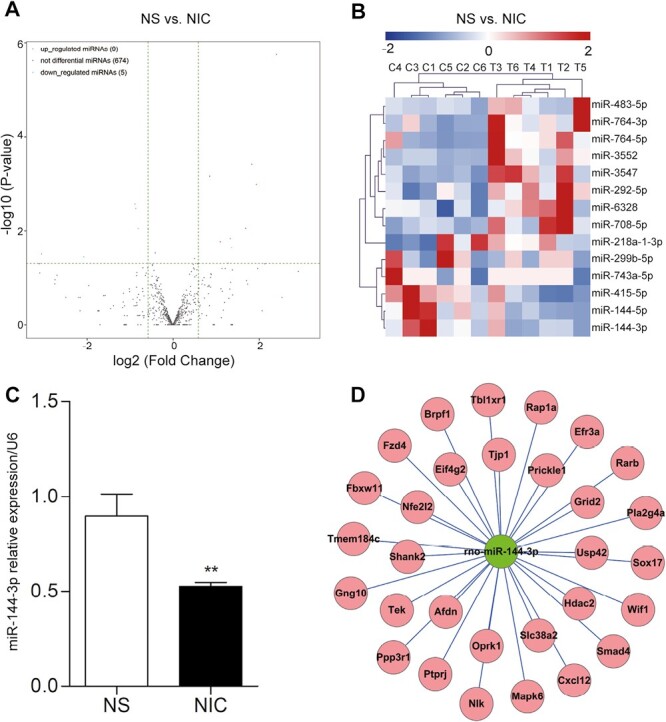
Expression profiles of microRNA (miRNAs) and the miRNA/mRNA network. (A) The volcano plots of DEmiRNAs (n = 6/group). The vertical dotted lines manifested 1.5-fold changes (log2 scaled) up and down, respectively, and the horizontal dotted line showed a p-value of .05 (−log10 scaled). The red and green points denoted the significantly up- and downregulated miRNAs. Gray dots indicated nondifferentially expressed miRNAs. (B) Hierarchical clustering heat map for the 14 DEmiRNAs. The up- and downregulated miRNAs were colored in red and blue, respectively. (C) RT-qPCR verification of miR-144-3p. Compared with Group NS (day 14 after saline administration), miR-144-3p was significantly downregulated in Group NIC (day 14 after nicotine administration). The data were presented as the mean ± SD. **p < .005. (D) The network of miR-144-3p and its putative mRNAs. Nodes in red color were the putative mRNAs, and nodes in green color were the downregulated miR-144-3p.
Nicotine Dependence and Withdrawal Downregulated miR-144-3p and Upregulated Oprk1 in Rats
Previous studies have implied the role of KOR in nicotine dependence and withdrawal.29 Based on these results, we hypothesized that miR-144-3p participated in the biological processes of nicotine dependence and withdrawal by regulating the expression of Oprk1 (the gene encoding KOR). Moreover, recent studies have correlated brain regions (such as PFC, NAc, Hippo) with nicotine use.3,30 Hence, we detected the expressions of miR-144-3p, Oprk1, and KOR in PFC, NAc, and Hippo on day 14 after nicotine administration (NIC) and day 1 after withdrawal (NW). The results suggested that miR-144-3p and Oprk1 exhibited opposite trends after nicotine administration and withdrawal, such that miR-144-3p decreased and Oprk1 increased at the RNA level (Figure 3A–C). Western blotting indicated that KOR was highly expressed in Group NIC and NW (Figure 3D–F). These data demonstrate that nicotine administration and withdrawal influence the expression of miR-144-3p and Oprk1/KOR in multiple brain regions.
Figure 3.
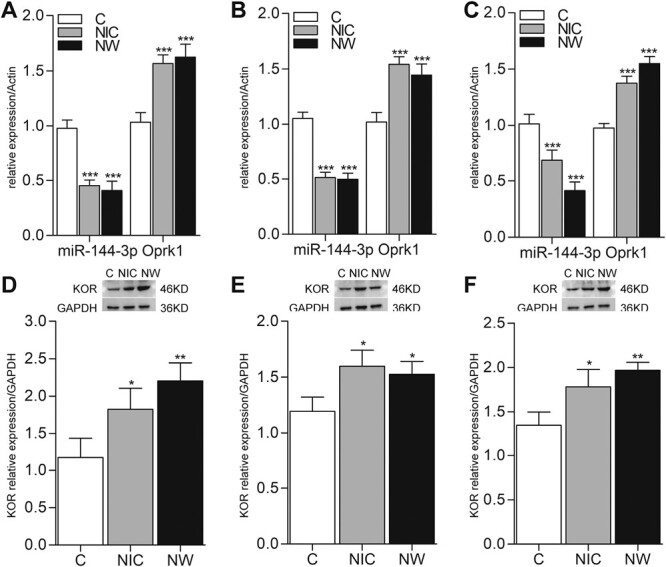
Nicotine dependence and withdrawal change the expressions of miR-144-3p and kappa-opioid receptor (KOR) in rats. (A–C) shown the expressions of miR-144-3p and Oprk1 (the gene of KOR) at RNA level in PFC, hippocampus, and NAc. (D–F) The expressions of KOR at protein level in PFC, hippocampus, and NAc. For RT-qPCR, actin was used as the housekeeping gene, and the relative expression of rno-miR-144-3p, Oprk1 were quantified using the 2−ΔΔCT method. For western blot, GAPDH was used as a loading control. Data were expressed as normalized ratio of protein band density of KOR against GAPDH and presented as the mean ± SD (n = 6). Group NIC represents day 14 after nicotine administration; Group NW represents day 1 after withdrawal. *p < .05, **p < .005, ***p < .001 versus Group C.
Nicotine Exposure Decreased miR-144-3p and Increased Oprk1 in PC12 Cells
To explore the effects of nicotine on the expression of miR-144-3p and Oprk1, we exposed PC12 cells to 10 μM nicotine for 6 h and observed that nicotine exposure could reduce the expression of miR-144-3p and increase the expression of Oprk1 (Figure 4A and C). Transfection of miR-144-3p mimic into PC12 cells could reduce the expression of Oprk1/KOR (Figure 4B and D); however, when we treated the transfected cells with 10 μM nicotine for 6 h, we observed that nicotine exposure could neutralize the effect of miR-144-3p mimic on the expression of Oprk1/KOR (Figure 4E). Similar to the in vivo studies, in vitro, nicotine exposure could modulate the expression of miR-144-3p and Oprk1/KOR.
Figure 4.
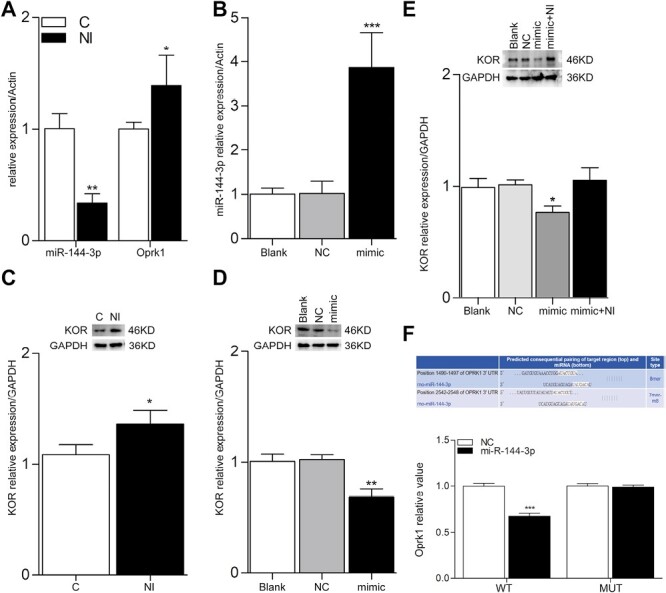
Nicotine exposure regulates the expressions of miR-144-3p and KOR in PC12 cells. (A) and (B) The RT-qPCR results of nicotine exposure and the transfection of miR-144-3p mimic, respectively. Actin was used as the housekeeping gene. (C) and (D) The protein bands and its quantification analysis of western blot of nicotine exposure and the transfection of miR-144-3p mimic, respectively. GAPDH was used as a loading control. (E) The protein bands and its quantification analysis of western blot of the transfection of miR-144-3p mimic combined with exposure to nicotine. (F) The luciferase reporter. Rno-miR-144-3p inhibited the luciferase activity of wild-type Oprk1 through binding to its 3ʹ UTR. Data were presented as the mean ± SD. *p < .05, **p < .005, ***p < .001.
miR-144-3p Inhibited the Luciferase Activity of Wild-Type Oprk1 Through Binding to its 3’ UTR
To further elucidate the relationship between miR-144-3p and Oprk1, luciferase vectors containing wild-type and mutant Oprk1 3ʹ UTRs were established and cotransfected with miR-144-3p into PC12 cells. miR-144-3p suppressed the luciferase activity of wild-type Oprk1 but had no inhibitory effect on mutant Oprk1(Figure 4F). These results indicate that miR-144-3p directly targets Oprk1.
Discussion
In this study, we explored the role of miR-144-3p/Oprk1/KOR in the adverse behaviors induced by nicotine administration and withdrawal for the first time. The results revealed that nicotine administration and withdrawal altered the expressions of miRNA-144-3p, Oprk1, and KOR in multiple brain regions, indicating that miRNA-144-3p negatively modulates the expression of Oprk1, which might be the potential pathway underlying nicotine-induced adverse effects.
Nicotine use and withdrawal can cause neuroadaptive changes in the brain and, in turn, alter the behaviors. In rats, repeated subcutaneous nicotine injection can induce dependence, and termination of nicotine injections can lead to spontaneous withdrawal symptoms such as hyperalgesia and somatic withdrawal signs.31 Body weight gain in rats can also be affected. Specifically, nicotine exposure reduced body weight gain, whereas increased the body weight gain for a short time after cessation of nicotine injections and finally returned to baseline levels. Based on previous reports,7,32,33 we successfully established a nicotine dependence and spontaneous withdrawal model in rats via subcutaneous injection of 3 mg/kg/day nicotine for 14 days, followed by spontaneous termination of injections for 7 days. Behavioral tests revealed that the MTW and TWL increased during nicotine administration, but decreased during nicotine withdrawal in rats. The rats in the Group NI gained less weight than in Group NS, whereas those in the Group NI gained more weight than in the NS group after spontaneous withdrawal. In addition, compared to the rats in Group NS, rats in Group NI exhibited higher withdrawal scores. All of the above results are consistent with those of previous studies.
Alterations in gene expressions are one of the underlying mechanisms of nicotine-associated behavioral changes. Recent research has demonstrated that smoking or nicotine consumption can alter the expression of miRNAs in various cell lines, organisms, and tissues and that miRNAs regulate addiction-related neuroplasticity in the brain.34–37 Kameshwar et al. reported that exosomal miRNAs were differentially expressed in cigarette smokers and might be the novel circulating biomarkers for smokers.22 Rauthan et al. reported that miR-238 mediated nicotine withdrawal response in Caenorhabditis elegans following chronic nicotine exposure by suppressing nAChR ACR-19 expression.38 This alteration in miRNA has also been reported in other studies.39,40 Besides, the hippocampus, PFC, and NAc are well-studied areas in nicotine research.3 Therefore, in this study, we detected 14 DEmiRNAs, including 9 upregulated and 5 downregulated miRNAs, in the hippocampi of nicotine-dependent rats. The results of previous studies suggested that the downregulated DEmiRNA miRNA-144-3p was involved in many biological processes, such as cell proliferation, carbohydrate metabolism, and the pathophysiology of depression.41–43 Predictions of target genes of miRNA-144-3p indicated Oprk1 as one of its target genes. Hence, we considered miRNA-144-3p as the most promising candidate. We validated the expression of miRNA-144-3p in several crucial brain regions (Hippo, PFC, and NAc) and observed that the expression of miRNA-144-3p was downregulated in these regions.
Studies have indicated that opioid receptors are involved in the development of nicotine dependence and abstinence-induced withdrawal syndromes.12,13,44 However, recent studies have mainly focused on µ-opioid receptor (MOR) and δ opioid receptor (DOR). Studies on the involvement of KOR are limited. Banks et al. outlined that KOR was altered during drug abuse, and functioned as a promising therapeutic target.29 In the present study, we found that both nicotine administration and withdrawal upregulated the expression of Oprk1/KOR in the Hippo, PFC, and NAc. This trend was confirmed in an in vitro study. The expression of miRNA-144-3p was downregulated in the same brain tissues and the cell model. Further bioinformatic analysis indicated that Oprk1 was likely a putative downstream gene of miRNA-144-3p. Hence, we hypothesized that miRNA-144-3p negatively regulated KOR expression. To date, several KOR-selective antagonists, including nor-BNI, JDTic, and LY2456302, as well as their agonists (such as U50,488 and ICI 204,448) have been shown to modulate nicotine administration and nicotine withdrawal-induced adverse effects.17,18,45 The efficacy of KOR antagonists and agonists in human trials has been unsatisfactory. Therefore, it is necessary to explore novel molecules that target KOR. Our investigation identified miRNA-144-3p as the most suitable potential target.
To further clarify the relationship between miR-144-3p and Oprk1, we performed in vitro experiments in PC12 cells. The results revealed that nicotine exposure reduced the expression of miR-144-3p and increased that of Oprk1/KOR, similar to in vivo results. Overexpression of miR-144-3p by the transfection with a miR-144-3p mimic markedly decreased Oprk1/KOR expression in PC12 cells. Further investigation revealed that nicotine exposure neutralized the effect of the miR-144-3p mimic on the expression of Oprk1/KOR. Through in silico analyses, we observed that Oprk1 3ʹ UTR region contains a matching site for miR-144-3p. Finally, dual-luciferase activity assay validated that miR-144-3p directly targeted Oprk1, which indicated that miR-144-3p probably have a negative regulatory effect on the expression of Oprk1/KOR. Further investigation is need in vivo.
This study has several limitations. First, although we tested the relationship between miR-144-3p/Oprk1/KOR in PC12 cells, we did not validate it in a rat model. Second, the behavioral assessments of nicotine-induced withdrawal symptoms were inadequate, we did not observe affective symptoms (such as depression and anxiety), attention deficits, and cognitive deficits. Third, only male rats, one drug, and one dose were included in this study. In addition, the expressions of KOR and miR-144-3p should be detected dynamically over time. In future studies, we will try to construct the lentivirus vector, or the agomir and antagomir for miR-144-3p to perform in vivo study and further verify this specific modulatory mechanism. Meanwhile, we will explore the effects of different concentrations of nicotine on behavioral and molecular changes, repeat this experimental protocol in female rats, and detect these molecules at multiple time points, to better elucidate the specific modulatory mechanisms.
Conclusions
KOR has been shown to be involved in nicotine-induced adverse effects, and further investigation indicated that KOR agonists aggravated the adverse effects caused by nicotine administration and withdrawal, and KOR antagonists alleviated these adverse effects in animal models. However, these effects were not observed in smokers, and KOR antagonists failed to relieve withdrawal symptoms. We found that in several brain regions (PFC, NAc, and Hippo) of rats, the adverse effects induced by nicotine dependence and withdrawal were associated with the downregulated expression of miR-144-3p and the upregulated expression of Oprk1/KOR. It is crucial to clarify the specific regulatory effects on adverse reactions and verify their roles in future human trials. In summary, our findings indicated that miR-144-3p/Oprk1/KOR is a potential pathway underlying nicotine-induced adverse effects, thereby providing a novel target for smoking cessation.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Contributor Information
Jiali Shao, Department of Anesthesiology, Hunan Cancer Hospital, School of Xiangya Medicine, Central South University, Hunan, China.
Yanxia Fei, Department of Anesthesiology, Women’s Hospital, School of Medicine Zhejiang University, Zhejiang, China.
Ji Xiao, Department of Anesthesiology, Hunan Cancer Hospital, School of Xiangya Medicine, Central South University, Hunan, China.
Lijuan Wang, Department of Anesthesiology, Hunan Cancer Hospital, School of Xiangya Medicine, Central South University, Hunan, China.
Shuangfa Zou, Department of Anesthesiology, Hunan Cancer Hospital, School of Xiangya Medicine, Central South University, Hunan, China.
Jinfeng Yang, Department of Anesthesiology, Hunan Cancer Hospital, School of Xiangya Medicine, Central South University, Hunan, China.
Funding
This research was funded by the Science and Technology Innovation Program of Hunan Province (2021SK4014) and the Hunan Cancer Hospital Climb Plan.
Declaration of Interests
All authors declare that they have no competing interests.
Author Contributions
Jiali Shao (Formal analysis [Equal], Investigation [Equal], Methodology [Equal], Software [Equal], Visualization [Equal], Writing – original draft [Equal]), Yanxia Fei (Data curation [Equal], Formal analysis [Equal], Investigation [Equal], Validation [Equal]), Ji Xiao (Data curation [Equal], Methodology [Equal], Validation [Equal], Writing – review & editing [Equal]), Lijuan Wang (Investigation [Equal], Software [Equal], Validation [Equal], Visualization [Equal]), Shuangfa Zou (Conceptualization [Equal], Data curation [Equal], Methodology [Equal], Project administration [Equal], Writing – review & editing [Equal]), and Jinfeng Yang (Conceptualization [Equal], Funding acquisition [Equal], Methodology [Equal], Project administration [Equal], Resources [Equal], Supervision [Equal], Writing – review & editing [Equal])
Data Availability Statement
The data that support the findings of this study will be openly available at OSF (https://osf.io/ek6wx), upon manuscript acceptance.
References
- 1. Surgeon General’s Report. The Health Consequences of Smoking—50 Years of Progress. Rockville, MD; 2014. https://www.surgeongeneral.gov/library/reports/50-years-of-progress/full-report.pdf. Accessed December 3, 2017. [Google Scholar]
- 2. Reitsma MB, Kendrick PJ, Ababneh E, et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet. 2021;397(10292):2337–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Le Foll B, Piper ME, Fowler CD, et al. Tobacco and nicotine use. Nat Rev Dis Primers. 2022;8(1):1–19. [DOI] [PubMed] [Google Scholar]
- 4. Ditre JW, Zale EL, LaRowe LR, Kosiba JD, De Vita MJ.. Nicotine deprivation increases pain intensity, neurogenic inflammation, and mechanical hyperalgesia among daily tobacco smokers. J Abnorm Psychol. 2018;127(6):578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bruijnzeel AW. Neuropeptide systems and new treatments for nicotine addiction. Psychopharmacology (Berl). 2017;234(9–10):1419–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jackson KJ, Muldoon PP, De Biasi M, Damaj MI.. New mechanisms and perspectives in nicotine withdrawal. Neuropharmacology. 2015;96(Pt B):223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chellian R, Behnood-Rod A, Bruijnzeel DM, et al. Rodent models for nicotine withdrawal. J Psychopharmacol. 2021;35(0):1169–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. LaRowe LR, Ditre JW.. Pain, nicotine, and tobacco smoking: current state of the science. Pain. 2020;161(2020):1688–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. NIDA. Introduction. National Institute on Drug Abuse website. https://nida.nih.gov/publications/research-reports/tobacco-nicotine-e-cigarettes/introduction. August 3, 2021 Accessed July 18, 2023. [Google Scholar]
- 10. Prochaska JJ, Benowitz NL.. The past, present, and future of nicotine addiction therapy. Annu Rev of Med. 2016;67:467–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berrendero F, Robledo P, Trigo JM, Martin-Garcia E, Maldonado R.. Neurobiological mechanisms involved in nicotine dependence and reward: participation of the endogenous opioid system. Neurosci Biobehav R. 2010;35(2):220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Domi A, Barbier E, Adermark L, Domi E.. Targeting the opioid receptors: a promising therapeutic avenue for treatment in “Heavy Drinking Smokers.” Alcohol Alcoholism. 2021;56(2):127–138. [DOI] [PubMed] [Google Scholar]
- 13. Kishioka S, Kiguchi N, Kobayashi Y, Saika F.. Nicotine effects and the endogenous opioid system. J Pharmacol Sci. 2014;125(2):117–124. [DOI] [PubMed] [Google Scholar]
- 14. Ugur M, Kaya E, Gozen O, et al. Chronic nicotine-induced changes in gene expression of delta and kappa-opioid receptors and their endogenous ligands in the mesocorticolimbic system of the rat. Synapse. 2017;71(9):e21985. [DOI] [PubMed] [Google Scholar]
- 15. Ward M, Norman H, D’Souza MS.. Effects of pharmacological manipulation of the kappa opioid receptors on the aversive effects of nicotine. Behav Brain Res. 2018;338:56–65. [DOI] [PubMed] [Google Scholar]
- 16. Jackson KJ, Carroll FI, Negus SS, Damaj MI.. Effect of the selective kappa-opioid receptor antagonist JDTic on nicotine antinociception, reward, and withdrawal in the mouse. Psychopharmacology (Berl). 2010;210(2):285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jackson KJ, McLaughlin JP, Carroll FI, Damaj MI.. Effects of the kappa opioid receptor antagonist, norbinaltorphimine, on stress and drug-induced reinstatement of nicotine-conditioned place preference in mice. Psychopharmacology (Berl). 2013;226(4):763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jackson KJ, Jackson A, Carroll FI, Damaj MI.. Effects of orally-bioavailable short-acting kappa opioid receptor-selective antagonist LY2456302 on nicotine withdrawal in mice. Neuropharmacology. 2015;97:270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seoane-Collazo P, Linares-Pose L, Rial-Pensado E, et al. Central nicotine induces browning through hypothalamic kappa opioid receptor. Nat Commun. 2019;10(1):4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Banerjee A, Waters D, Camacho OM, Minet E.. Quantification of plasma microRNAs in a group of healthy smokers, ex-smokers and non-smokers and correlation to biomarkers of tobacco exposure. Biomarkers. 2015;20(2):123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singh KP, Maremanda KP, Dongmei L, Rahman I.. Exosomal microRNAs are novel circulating biomarkers in cigarette, waterpipe smokers, E-cigarette users and dual smokers. BMC Med Genomics. 2020;13(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zdenka Navratilova SL, Pavla P, Katerina S, Alzbeta C, Martin P.. The effect of tobacco smoking and smoking cessation on urinal miRNAs in a pilot study. Life-Basel. 2020;10(9):191. doi: 10.3390/life-10090191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thiago Arzua CJ, Yasheng Y, Xiaowen B.. The importance of non-coding RNAs in environmental stress-related developmental brain disorders: a systematic review of evidence associated with exposure to alcohol, anesthetic drugs, nicotine, and viral infections. Neurosci Biobehav R. 2021;128:633–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Malin DH, Lake JR, Maultsby PN, et al. Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Be. 1992;43(3):779–784. [DOI] [PubMed] [Google Scholar]
- 26. Kallupi M, de Guglielmo G, Larrosa E, et al. Exposure to passive nicotine vapor in male adolescent rats produces a withdrawal-like state and facilitates nicotine self-administration during adulthood. Eur Neuropsychopharm. 2019;29(11):1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith LC, Tieu L, Suhandynata RT, et al. Cannabidiol reduces withdrawal symptoms in nicotine-dependent rats. Psychopharmacology (Berl). 2021;238(8):2201–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang Y, Yang J, Sevilla A, et al. The mechanism of chronic nicotine exposure and nicotine withdrawal on pain perception in an animal model. Neurosci Lett. 2020;715(715):134627. [DOI] [PubMed] [Google Scholar]
- 29. Banks ML. The rise and fall of kappa-opioid receptors in drug abuse research. Handb Exp Pharmacol. 2020;258:147–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Djemil S, Chen X, Zhang Z, et al. Activation of nicotinic acetylcholine receptors induces potentiation and synchronization within in vitro hippocampal networks. J Neurochem. 2020;153(4):468–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morud J, Strandberg J, Andren A, et al. Progressive modulation of accumbal neurotransmission and anxiety-like behavior following protracted nicotine withdrawal. Neuropharmacology. 2018;128:86–95. [DOI] [PubMed] [Google Scholar]
- 32. Akimoto H, Oshima S, Michiyama Y, et al. Metabolic profiling of the hippocampus of rats experiencing nicotine-withdrawal symptoms. Biol Pharm Bull. 2018;41(12):1879–1884. [DOI] [PubMed] [Google Scholar]
- 33. Liu Z, Liu XW, Lu SF, Yu AL, Zhang ZW.. Effect of nicotine withdrawal on pain sensitivity in rats tomechanical stimulation and thermal stimulation. Eur Rev Med Pharmaco. 2014;18(18):2759–2765. [PubMed] [Google Scholar]
- 34. Kenny PJ. Epigenetics, microRNA, and addiction. Dialogues Clin Neuro. 2014;16(16):335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Casserly AP, Tsuji J, Zhao-Shea R, et al. Integrated miRNA-/mRNA-Seq of the habenulo-interpeduncular circuit during acute nicotine withdrawal. Sci Rep. 2020;10(813):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pinson MR, Miranda RC.. Noncoding RNAs in development and teratology, with focus on effects of cannabis, cocaine, nicotine, and ethanol. Birth Defects Res. 2019;111(17):1308–1319. [DOI] [PubMed] [Google Scholar]
- 37. Taki FA, Xiaoping P, Lee M-H, Zhang B.. Nicotine exposure and transgenerational impact: a prospective study on small regulatory microRNAs. Sci Rep. 2014;4:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rauthan M, Gong J, Liu J, et al. MicroRNA regulation of nAChR expression and nicotine-dependent behavior in C. elegans. Cell Rep. 2017;21(6):1434–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Takahashi K, Yokota S, Tatsumi N, et al. Cigarette smoking substantially alters plasma microRNA profiles in healthy subjects. Toxicol Appl Pharm. 2013;272(1):154–160. [DOI] [PubMed] [Google Scholar]
- 40. Pittenger ST, Schaal VL, Moore D, et al. MicroRNA cluster miR199a/214 are differentially expressed in female and male rats following nicotine self-administration. Sci Rep. 2018;8(1):17464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ma K, Zhang H, Wang S, et al. The molecular mechanism underlying GABAergic dysfunction in nucleus accumbens of depression-like behaviours in mice. J Cell Mol Med. 2019;23(10):7021–7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kang JY, Kim H, Mun D, Yun N, Joung B.. Co-delivery of curcumin and miRNA-144-3p using heart-targeted extracellular vesicles enhances the therapeutic efficacy for myocardial infarction. J Control Release. 2021;331:62–73. [DOI] [PubMed] [Google Scholar]
- 43. Wang B, Xu L, Zhang J, et al. LncRNA NORAD accelerates the progression and doxorubicin resistance of neuroblastoma through up-regulating HDAC8 via sponging miR-144-3p. Biomed Pharmacother. 2020;129:110268. [DOI] [PubMed] [Google Scholar]
- 44. Raffa RB, Baron S, Bhandal JS, et al. Opioid receptor types involved in the development of nicotine physical dependence in an invertebrate (Planaria) model. Pharmacol Biochem Behav. 2013;112:9–14. [DOI] [PubMed] [Google Scholar]
- 45. Sudakov SK, Nazarova GA, Alekseeva EV, Kolpakov AA.. Effect of activated peripheral k-opioid receptors on the action of nicotine and its withdrawal in nicotine-dependent rats. Bull Exp Biol and Med. 2014;156(5):609–611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study will be openly available at OSF (https://osf.io/ek6wx), upon manuscript acceptance.


