Abstract
Spinal cord injury is a condition in which the parenchyma of the spinal cord is damaged by trauma or various diseases. While rapid progress has been made in regenerative medicine for spinal cord injury that was previously untreatable, most research in this field has focused on the early phase of incomplete injury. However, the majority of patients have chronic severe injuries; therefore, treatments for these situations are of fundamental importance. The reason why the treatment of complete spinal cord injury has not been studied is that, unlike in the early stage of incomplete spinal cord injury, there are various inhibitors of neural regeneration. Thus, we assumed that it is difficult to address all conditions with a single treatment in chronic complete spinal cord injury and that a combination of several treatments is essential to target severe pathologies. First, we established a combination therapy of cell transplantation and drug-releasing scaffolds, which contributes to functional recovery after chronic complete transection spinal cord injury, but we found that functional recovery was limited and still needs further investigation. Here, for the further development of the treatment of chronic complete spinal cord injury, we review the necessary approaches to the different pathologies based on our findings and the many studies that have been accumulated to date and discuss, with reference to the literature, which combination of treatments is most effective in achieving functional recovery.
Keywords: cell transplantation, chronic phase, complete transection, regenerative medicine, spinal cord injury
Introduction
Spinal cord injury (SCI) is a condition in which the spinal cord parenchyma is damaged by trauma or various diseases, resulting in paralysis of the sensory, motor, and autonomic nervous systems below the injured level. Approximately 250,000 to 500,000 people are affected each year worldwide, and it is estimated that 2 to 3 million people live with an SCI-related disability (Quadri et al., 2020). Once damaged, the mammalian central nervous system is known not to regenerate, and there is no absolute cure. However, recent remarkable progress in basic research has gradually revealed that the injured spinal cord can regenerate if the appropriate environment is provided (Nishimura et al., 2013; Hashimoto et al., 2023). The injured spinal cord can be damaged by primary injury due to trauma, followed by secondary injury due to inflammatory responses, leading to loss of function due to further damage, but it has been shown that there is still relatively little neuroplasticity in the early stages of SCI (Keirstead et al., 2005; Kumamaru et al., 2013; Nishimura et al., 2013). Therefore, early therapeutic intervention is an important strategy for functional recovery in the treatment of SCI, and basic and clinical research is being actively performed on the acute and subacute phases of SCI (Ghobrial et al., 2017; Crutis et al., 2018; Fehlings et al., 2018; Honmou et al., 2021). We have conducted a clinical trial of hepatocyte growth factor as a treatment for acute SCI. The safety of this treatment was confirmed in a phase I/II study, and its efficacy is confirmed in a phase III study (Nagoshi et al., 2020). Furthermore, we have started a clinical trial of transplantation of human induced pluripotent stem-derived neural stem/progenitor cell (hiPS-NS/PC) for the treatment of subacute injury and are confirming the safety and efficacy of this therapy (Sugai et al., 2021). However, clinical research is still in its infancy, and important work remains to be done. Most of these studies have focused on early intervention in the acute or subacute phase after injury, and very few have examined the chronic phase. Unlike the early phase of SCI, there are various inhibitors of neural regeneration in chronic SCI (Wanner et al., 2013; Bruda et al., 2014; Dias et al., 2018; Bradbery et al., 2019). Cavitation formed by apoptotic neurons and surrounding scar tissue (glial and fibrotic scars) in the injured area physically inhibits axonal regeneration (Jones and Tuszynski., 2002; Silver et al., 2004). The scar tissues and myelin remnants secrete chemical factors, such as chondroitin sulfate proteoglycans (CSPGs) and myelin-related proteins, which further prevent axon projection. The inflammatory response persists during the chronic phase, and inflammatory cells and cytokines, such as microglia and macrophages, prevent axonal extension, promote scar formation, and accelerate neuronal apoptosis (Nishimura et al., 2014). Due to these changes in the pathophysiology of the spinal cord microenvironment and the emergence of many inhibitory factors as the disease progresses to the chronic phase, research on chronic SCI has been challenging, with few reports worldwide (Keirstead et al., 2005; Kumamaru et al., 2013).
In addition to the pathology of each phase of injury, the severity of the injury as determined by histology is also an important factor in the treatment of SCI (Liu et al., 2019; Yokota et al., 2019). Depending on the presence or absence of neuronal fibers beyond the lesion, the injury can be classified as either a mild incomplete injury or a severe complete injury. This severity is primarily determined by the magnitude of the external force and inflammatory response, but there are cases in which secondary damage after the injury is significant, resulting in a transition from incomplete to complete injury (Tator et al., 1991). In the case of incomplete injuries with residual axonal fibers, it is considered that some functional improvement can be expected from treatment that promotes myelination and the function of neuronal circuits (Barnabe-Heider and Frisen, 2008). However, complete SCI is very challenging because it requires treatment to regenerate severed axons and further promote the reconstruction of new circuits, and there are very few reports of such studies.
As previously described, SCI has various pathologies and treatment targets depending on the phase and severity of the injury, and therefore, effective therapy for chronic complete injury should be tailored to these pathologies (Hashimoto et al., 2023). Based on these findings, we believe that it is difficult to address all conditions with a single treatment in chronic complete SCI, which is the most difficult condition to treat, and that a combination of several treatments is essential for functional recovery (Figure 1). In addition, rather than simply combining them, it is also important to study the regeneration process and determine which treatment should be combined at which stage. Indeed, many SCI patients are in the chronic phase and are severely injured, and it is clear that new treatments need to be developed as soon as possible. Here, based on our findings and the studies accumulated to date, we review the various therapies to the pathologies of chronic complete SCI and discuss, with reference to the literature, what combination of treatments is most effective in enhancing functional recovery (Table 1).
Figure 1.
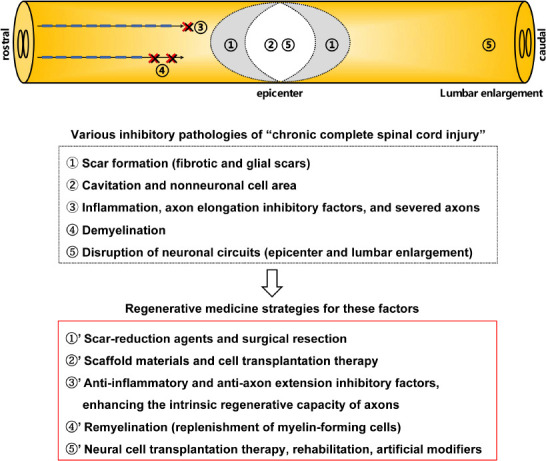
Regenerative medicine strategies for various inhibitory factors of chronic complete spinal cord injury.
Table 1.
Details of references cited in this review regarding spinal cord treatment research
| Studies | Materials | Phase of intervention | Type of injury | Level of spinal cord | Results of study | Basic or clinical study |
|---|---|---|---|---|---|---|
| Bradbury et al., 2002 | Ch-ABC | Acute | Dorsal hemisection | C4 | Locomotor functional improvement | Basic |
| Chen et al., 2018 | KCC2 agonist | Acute | Double latereal hemisection | T7 and T10 | Locomotor functional improvement | Basic |
| Curtis et al., 2018 | Human NSCs | Chronic | AIS grade A | T2–T12 | Safety and early signs of potential efficacy | Clinical |
| Fehlings et al., 2018 | Rho inhibitor | Acute | AIS grade A or B | Cervical level | Ongoing | Clinical |
| Ghobrial et al., 2017 | Human NSCs | Chronic | AIS grade A or B | Cervical level | Improvement of ISNCSCI score | Clinical |
| Hashimoto et al., 2023 | Human iPS-NS/PCs, scaffold (collagen and gelatin), HGF | Chronic | Complete | T10 | Locomotor and urinary functional improvement | Basic |
| Honmou et al., 2021 | Autologous MSCs | Acute | AIS grade A or B or C | Cervical level | Improvement of ISNCSCI and SCIM-III score | Clinical |
| Ito et al., 2018 | NgR antagonist | Subacute | Incomplete | T10 | Locomotor functional improvement | Basic |
| Iwasaki et al., 2014 | Scaffold (self-assembling artificial proteins) | Acute | Incomplete | C6–C7 | Grip strength recovery | Basic |
| Kamata et al., 2021 | Human iPS-gliogenic NS/PCs | Subacute | Incomplete | T10 | Locomotor functional improvement | Basic |
| Kaneko et al., 2015 | 3D scaffold (hydrogel and collagen) | Acute | Complete | T10 | Locomotor functional improvement | Basic |
| Kaneko et al., 2006 | sema3a inhibitor | Acute | Complete | T10 | Locomotor functional improvement | Basic |
| Kawai et al., 2021 | Human iPS-NS/PCs+DREDD | Subacute | Incomplete | T10 | Locomotor functional improvement | Basic |
| Keirstead et al., 2005 | Human embryonic stem cell-OPCs | Acute or chronic | Incomplete | T10 | Locomotor functional improvement (acute phase) | Basic |
| Koda et al., 2007 | G-CSF | Acute | Incomplete | T8 | Locomotor functional improvement | Basic |
| Koffler et al., 2019 | 3D scaffold (polyethylene glycol-gelatin methacrylate), NSCs | Acute | Complete | T3 | No functional improvement, histological improvement | Basic |
| Kourgiantaki et al., 2020 | Mouse NSCs, scaffold (collagen) | Acute | Incomplete | T10 | Locomotor functional improvement | Basic |
| Kumamaru et al., 2013 | Mouse NSCs | Chronic | Incomplete | T10 | No locomotor function | Basic |
| Liu et al., 2019 | MSCs, scaffold (NeuroRegen), Taxol | Chronic | Complete | T8 | Locomotor functional improvement | Basic |
| Marchini et al., 2019 | NSCs (3D culture) | Acute | Dorsal hemisection | T9–T10 | Locomotor functional improvement | Basic |
| Nagoshi et al., 2020 | HGF | Acute | AIS grade A or B or C | Cervical level | Functional improvement | Clinical |
| Nori et al., 2018 | Human gliogenic NPCs, scaffold (methylcellulose), Ch-ABC | Chronic | Incomplete | T7 | Locomotor functional improvement | Basic |
| Nori et al., 2011 | Human iPS-NS/PCs | Subacute | Incomplete | T10 | Locomotor functional improvement | Basic |
| Okada et al., 2004 | IL-6 receptor monoclonal antibody | Acute | Incomplete | T7 and T10 | Locomotor functional improvement | Basic |
| Okubo et al., 2018 | Human iPS-NS/PCs | Chronic | Incomplete | T10 | Locomotor functional improvement | Basic |
| Osaka et al., 2010 | MSCs | Acute | Incomplete | T9–T10 | Locomotor functional improvement | Basic |
| Rao et al., 2018 | NT-3, scaffold (chitosan) | Acute | Dorsal hemisection | T8 | Locomotor functional improvement | Basic |
| Rosenzweig et al., 2019 | Ch-ABC | Acute | Lateral hemisection | C7 | Hand functional improvement | Basic |
| Ruschel et al., 2015 | Epothilone B | Acute | Dorsal hemisection | Thoracic level | Histological improvement | Basic |
| Shibata et al., 2023 | Human iPS-NS/PCs, rehabilitation | Chronic | Incomplete | T10 | Locomotor functional improvement | Basic |
| Shinozaki et al., 2016 | Ch-ABC, rehabilitation | Chronic | Incomplete | T10 | Locomotor functional improvement | Basic |
| Sugai et al., 2021 | Human iPS-NS/PCs | Subacute | AIS grade A | C3/4–T10 level | On going | Clinical |
| Suzuki et al., 2020 | CPTX | Acute | Dorsal hemisection | T10 | Locomotor functional improvement | Basic |
| Woerly et al., 2000 | Scaffold (NeuroGel) | Acute | Complete | T5 | Locomotor functional improvement | Basic |
| Xu et al., 2022 | 5-FU | Acute | Dorsal hemisection | Thoracic level | Locomotor functional improvement | Basic |
| Yamane et al., 2018 | HGF, scaffild (collagen) | Acute | Incomplete | T9 | Locomotor functional improvement | Basic |
| Zhang et al., 2014 | sema3A inhibitor, rehabilitation | Acute | Complete | T10 | Locomotor functional improvement | Basic |
5-FU: 5-Fluorouracil; AIS: American Spinal Injury Association Impairment Scale; C: cervical; Ch-ABC: chondroitinase ABC; DREDD: designer receptor exclusively activated by designer drug; GCS-F: granulocyte colony stimulating factor; HGF: hepatocyte growth factor; iPS-NS/PCs: induced pluripotent stem-derived neural stem/progenitor cells; ISNCSCI: The International Standards for Neurological Classification of Spinal Cord Injury; KCC2: potassium chloride cotransporter 2; MSCs: mesenchymal stem cells; NgR: Nogo receptor; NSCs: neural stem cells; NT-3: neurotrophin 3; OPCs: oligodendrocyte precursor cells; SCIM-III: Spinal Cord Independence Measure version III; Sema3A: semaphorin 3A; T: thoracic.
Search Strategy and Selection Criteria
Studies cited in this review published until March 2023 were searched on PubMed using the following keywords: spinal cord injury (SCI), chronic, complete SCI, transected SCI, stem cell therapy, transplantation, neuronal regeneration, MSC, OPC, iPS cell, scaffold, inflammation, scar formation, Ch-ABC, scaffold, fibrotic scar, glial scar, autophagy, axon elongation, axonal regrowth, CSPG, rehabilitation, neuronal circuit, and SCI clinical trials. All references cited in this review were screened according to the following criteria and searched until May 20, 2023. Full articles in English; experimental studies in vivo, in vitro and CNS and PNS; acute, subacute, and chronic phase of SCI studies; incomplete and complete SCI studies; basic study and clinical studies.
Strategies for Chronic Complete Spinal Cord Injury
Treatment of scar formation
Scar formation after SCI is triggered by a self-protective response to prevent the spread of secondary injury due to a spillover inflammatory response (Bruda et al., 2014; Bradbery et al., 2019). Scar tissue can be divided into two types: fibrotic and glial scars. Physical injury and the resulting inflammatory response cause neural apoptosis and cavitation, around which a fibrotic scar is formed from the extracellular matrix derived from perivascular pericytes or fibroblasts (Soderblom et al., 2013; Wanner et al., 2013; Dias et al., 2018; Fehlberg et al., 2022). Outside of the fibrotic scar, a glial scar is formed by astrocytic scarring (Silver et al., 2004; Jones et al., 2022). These scars form in layers and physically inhibit axon projection regeneration, and axon re-extension is chemically inhibited because of the secretion of inhibitory factors (e.g., CSPGs) by glial scars (Mckeon et al., 1999; Shen et al., 2009; Lang et al., 2015; Rosenzweig et al., 2021). In addition, glial scars from chondroitin sulfate bind the receptor-type protein tyrosine phosphatase, which inhibits axonal regeneration due to dephosphorylates cortactin-herein and disrupts autophagy flux at the autophagosome-lysosome fusion step which resulting in axonal dystrophic endball formation (Sakamoto et al., 2019). Therapeutic interventions for these scars in chronic injury are reported to be effective in restoring function, and scar-reduction agents and surgical resection are being investigated. Of particular interest is the administration of chondroitinase ABC (Ch-ABC), which has been shown to resolve glial scars.
Ch-ABC is an enzyme that degrades hyaluronic acid, chondroitin sulfate chondroitin, and dermatan sulfate into disaccharides or oligosaccharides (Prabhakar et al., 2005). This enzyme is known to be produced by bacteria such as Proteus vulgaris, and many reports have demonstrated that its administration after SCI contributes to functional improvement by enhancing scar reduction and neuroplasticity. For example, Bradbury et al. (2002) performed intrathecal administration of Ch-ABC to acute SCI in a rat model for the first time. They showed that CSPGs at the lesion were degraded, and neuroprotective proteins were expressed in the injured neurons, promoting the regeneration of ascending sensory projections and descending corticospinal tract axons (Bradbury et al., 2002). Combined treatment with Ch-ABC is also being investigated for chronic SCI. We reported that combination therapy of Ch-ABC and rehabilitation improved locomotor function in a rat model of chronic severe thoracic SCI. Ch-ABC was administered intrathecally for 1 week at 6 weeks after SCI, and treadmill rehabilitation was performed from 6 to 14 weeks after injury. Histology showed an increase in lateral residual tissue area and regeneration of axonal fibers in the caudal region from the injury site, with a significant increase in serotonergic fiber regeneration. These results suggested that Ch-ABC played a beneficial role even in severe chronic SCI when combined with intensive rehabilitation (Shinozaki et al., 2016). In addition, Nori et al. (2018) examined Ch-ABC-based neural stem cell (NSC) transplantation in a rat model of chronic incomplete SCI. The administration of slow-release Ch-ABC-containing methylcellulose at 6 weeks after thoracic SCI and the transplantation of oligodendrocyte-preferentially differentiated NSCs one week later resulted in an improvement in locomotor function. Effective, sustained release of Ch-ABC using biomaterials suppressed CSPGs and enhanced the long-term survival of oligodendrocyte-preferentially differentiated NSCs transplanted around the lesion. Moreover, Ch-ABC promoted oligodendrocyte differentiation, remyelination of preserved axons by transplanted oligodendrocyte-preferentially differentiated NSCs, and enhanced synaptic connections with anterior horn cells, thereby enabling neurobehavioral recovery (Nori et al., 2018). Several treatments for fibrotic scars with medication have been attempted. For example, oral administration of a blood-brain barrier-permeable microtubule-stabilizing drug, epothilone B (epoB), decreased these scars after the rodent SCI model by blocking the migration of scar-forming fibroblasts (Ruschel et al., 2015). The antitumor agent 5-fluorouracil has also been investigated; 5-fluorouracil has been reported to inhibit fibroblast proliferation and promote apoptosis, thereby inhibiting fibrotic scarring and promoting axonal regeneration by intraperitoneal injections (Xu et al., 2022). However, the effect on transplanted cells must be considered when combined with neural stem cell transplantation since 5-fluorouracil has the ability to ablate dividing cells.
Thus, these scar-reduction agents and surgical resection are potent spinal cord environment modifiers that physically and chemically attenuate the inhibitory effects of scar tissues, suggesting that their therapeutic effects could be further enhanced by combination with other treatments, making them essential in treating chronic SCI.
Treatment of spinal cord cavitation and nonneuronal cell area
Cavitation is caused by the apoptosis of neuronal cells in the injured epicenter due to external physical forces during SCI, the persistence of inflammatory reactions, and the dieback of axonal projections (Fitch et al., 1999; Orr et al., 2018). In the chronic phase, the surrounding area is covered by scar tissues, so cell migration and neovascularization, which are scaffolds for neural regeneration, do not occur, and regenerative axons cannot grow there (Bradbery et al., 2019). In the case of complete injury, the cavitation and scar formation could be even greater, and restoration of the lesion becomes even more difficult. Therefore, the formation of regenerative scaffolding by scaffold materials and the replenishment of neuronal cells are being considered as a treatment.
The main purpose of scaffold administration is to create a new site for nerve regeneration by creating support in cavitation and promoting cell migration and vascularization (Ahuja, 2016). The functions of the scaffold are required to promote neuroregeneration, including the ability to promote anti-inflammatory, neuroprotective, and angiogenic effects; the ability to remain for a certain period and attract cells; having affinity without causing biological reactions; and the ability to have a sustained drug-release effect. In particular, it is recognized that the induction of vascularization is necessary for the re-extension of axonal projections (Muramatsu et al., 2012). The action of prostacyclin from blood vessels, oxygenation, and hematopoietic factors can elongate axons along newly formed blood vessels. With regard to the residual period of the scaffold, it is considered important to maintain the structure for at least 4 weeks after administration, and the structure and density of the scaffold are considered to facilitate cell migration and axon elongation (Koffler et al., 2019). Natural or artificial materials made of extracellular matrix and chemicals without antigenic properties have been used as scaffold materials, and there are reports of natural materials such as collagen and gelatin, artificial materials such as self-assembling peptide scaffolds (Woerly et al., 2001; Iwasaki et al., 2014; Kaneko et al., 2015; Marchini et al., 2018; Koffler et al., 2019; Kourgiantaki et al., 2020), and more recently, biofunctionalized electroconducting microfibres which produced better axonal regenerative alignment and promote the beneficial immune responses (Waly et al., 2021), graphene and graphene-based materials which have good histocompatibility, mechanical and adsorption properties and conductivity that can be improved the environment and promote axonal regeneration (Wang et al., 2022), and biological tissue implantation in which tissue is decellularized to form a gel or three-dimensional structure (Xu et al., 2019). Additionally, the effectiveness of scaffolds can be further enhanced by incorporating drugs such as neuroprotective agents and growth factors. For example, physical sustained-release effects, in which the drug is structured to dissolve easily, and chemical sustained-release effects, which focus on chemisorbability and electrical charge, have been studied, and they can be expected to achieve long-term drug activity and promote neuroregeneration through effective drug diffusion in a sustained-release manner (Rao et al., 2018; Hashimoto et al., 2023).
To address the nonneuronal cell area, cell replacement therapy has been investigated for NSC, hiPS-NS/PC, and mesenchymal stem cell (MSC) transplantation, which are the seeds of nerve cells and have neuroprotective effects (Barnabe-Heider and Frisen, 2008; Assinck et al., 2017; Huang et al., 2021; Kawai et al., 2022). We have established hiPS-NS/PC transplantation for subacute incomplete SCI (Nori et al., 2011; Nagoshi et al., 2018; Sugai et al., 2021). The transplanted cells differentiated into three neural lineages and were involved in functional recovery after injury through the formation of new neural circuits by neurons, neuroprotection, and remyelination by oligodendrocytes. In addition, Osaka et al. (2010) reported that intravenous administration of bone marrow-derived MSCs for acute incomplete SCI allowed transplanted cells to accumulate in the lesion and restore motor function through neuroprotective factors such as brain-derived neurotrophic factor, promotion of remyelination and vascularization. Thus, while there have been many reports on the recovery effects of cell transplantation in the acute and subacute phases, there have been only a few reports on the chronic phase. While cell transplantation therapies are found to be effective in the early stages of injury, their effects on chronic injury are insufficient, and the reason for this is assumed to involve differences in the pathophysiology of the spinal cord microenvironment at the site of transplantation (Keirstead et al., 2005; Kumamaru et al., 2013). Even if cell transplantation is performed in an avascular cavitation with various inhibitory factors, it is difficult for the transplanted cells to grow and extend axonal projections, and it is important to improve the spinal cord environment prior to transplantation. Kumamaru et al. (2013) performed NSC transplantation at various times from acute to chronic stages of incomplete SCI mice and reported that they observed improvement in lower limb function only in acute and subacute injury but not in the chronic stage. Transcriptome analysis revealed that grafted cells produced many neurotrophic molecules and differentiated into neurons and oligodendrocytes regardless of the time of transplantation. This means that the reason for the lack of functional improvement in the chronic phase was not mainly due to the lack of therapeutic activity of the neural stem cells but rather to the refractoriness of the spinal cord in the chronic phase (Kumamaru et al., 2013). In addition to focusing on the pathophysiology of chronic spinal cord injury, we have been investigating all possible ways to enhance the efficacy of the transplanted cells themselves. One approach is the administration of drugs to hiPS-NS/PCs prior to transplantation, focusing on γ-secretase inhibitor, which has been reported to promote neuronal differentiation of NSCs by inhibiting Notch signaling. We transplanted γ-secretase inhibitor-treated hiPS-NS/PCs into chronic incomplete SCI mice and found that these cells differentiated predominantly into neurons, and axons extended beyond the glial scar, significantly improving locomotor function (Okubo et al., 2018). Furthermore, several attempts at transplantation therapies for central nervous system disorders are being made to further augment the effects of transplanted cells by performing gene transfer to the transplanted cells (Okano, 2022a, b). For example, Baloh et al. (2022) transplanted human neural progenitor cells transfected with glial cell line-derived neurotrophic factor, a gene encoding a growth factor that has a protective impact on dopamine neurons and spinal motor neurons, into ALS patients and achieved functional recovery.
As described above, cell transplantation alone has proven to be limited in its ability to improve function, especially in complete chronic SCI, it is essential to improve the spinal cord microenvironment with scaffolds, neuroprotective factors, and scar reduction agents prior to cell transplantation, as well as to develop transplanted cells with enhanced efficacy or new developmental treatments.
Furthermore, neural cell tissues induced from human pluripotent stem cells are typically insufficiently mature and cannot imitate the morphological features completely, there are some potentials for tumorigenesis and inadequate efficacy of improvement functions. To address these issues, spinal cord organoids derived from 3D stem cell cultures have recently been reported to have positive implications in the microenvironment of spinal cord injury. These techniques exhibit that the spinal cord organoids can differentiate into major spinal cord neurons and glial cells, and mature synaptic functional activity rather than pluripotent stem cells (Lee et al., 2022). The development of these technologies could also be an important factor in further improving the efficacy and safety of cell transplantation therapy.
Axonal regeneration in the presence of inflammation and axon elongation inhibitory factors and re-extension of severed axons
Even in the chronic phase, the inflammatory response persists around the lesion. Axonal regeneration is inhibited by the presence of inflammatory cells and cytokines, axonal guidance molecules such as the ephrin family and semaphorin family, scar tissues, myelin-associated proteins (Nogo, MAG, OMgp), and immunoglobulins (BLyS), which induce demyelination, inhibit axonal re-extension, promote growth cone collapse and cell apoptosis, and even prevent grafted cell engraftment (Silver et al., 2004; Nishimura et al., 2013; Jones et al., 2022). In addition, severed axons are considered to be difficult to regrow in a self-induced manner due to Wallerian degeneration and the formation of a dystrophic endball due to disruption autophagy flux (Silver et al., 2004; Kerschensteiner et al., 2005; Sakamoto et al., 2019). To regenerate axons that have lost their regenerative ability because of these inhibitory factors, many researchers have explored approaches that promote axon regeneration by attenuating the effects of the inhibitory factors and enhancing the intrinsic regenerative capacity of axons. For example, the anti-inflammatory effects of granulocyte colony-stimulating factor and interleukin-6 antibodies, as well as the anti-axon extension inhibitory effects of semaphorin 3A inhibitors, scar suppressive factor, and Nogo receptor antagonists, are reported to enhance axon regrowth (Okada et al., 2004; Kaneko et al., 2005; Koda et al., 2007; Cregg et al., 2013; Ito et al., 2018). Neuroprotective factors and growth factors are supposed to promote anti-axonal re-extension inhibition but also intrinsic self-growth potential (Keefe et al., 2017; Anderson et al., 2018; Yamane et al., 2018; Nagoshi et al., 2020).
We have reported that the administration of an inhibitor against semaphorin3A, one of the major inhibitors of axonal regeneration, to complete acute SCI rats greatly enhanced the regeneration and preservation of injured axons and greatly accelerates functional recovery (Kaneko et al., 2005). Moreover, the combination of a semaphorin3A inhibitor and rehabilitation by treadmill walking further improved locomotor function due to the regeneration of severed axons and the formation and activation of new neuronal circuits and lumbar networks (Zhang et al., 2014). Anderson et al. reported that in an acute complete SCI mouse model, strong expression of insulin-like growth factor 1 and conserved dopamine neurotrophic factor by gene transfer reactivated the growth capacity of descending spinal cord neurons, and induction of scaffolds with fibroblast growth factor-2 and epidermal growth factor resulted in the extension of completely severed axon projections beyond the injured area (Anderson et al., 2018).
Additionally, to increase intrinsic self-axonal regrowth, some researchers have focused on the tumor suppressor phosphatase and tensin homolog, which is a negative regulator of the PI3 kinase/Akt pathway that is important for regulating axon elongation. It has been reported that phosphatase and tensin homolog suppression could enhance axonal progression such as descending serotonergic fibers and corticospinal fibers, resulting in functional recovery in the central nervous system after spinal injury (Ohtake et al., 2015).
Therefore, both inhibiting regenerative inhibitory factors and enhancing intrinsic self-growth aimed at axonal restoration are expected as treatments for SCI. For application to chronic complete injury, considering the presence of various inhibitory factors and the need for regeneration of severed axons, it is suggested that both of these effects are necessary for functional improvement.
Remyelination against demyelination
Demyelination is another cause of functional impairment after SCI, and replenishment of myelin-forming cells is a promising therapeutic strategy (Heidar and Frisen., 2008). We established the induction of hiPS-NS/PCs capable of differentiating into glial cells and neurons, transplanted them into mice with incomplete subacute injury, and reported improvement in locomotor function. Many of the transplanted cells differentiated into oligodendrocytes to promote myelination of host and grafted neurons and differentiated into neurons to create new circuits, suggesting that they were involved in functional recovery (Kamata et al., 2021). In addition, Keirstead et al. (2005) showed that transplantation of human embryonic stem cell-derived oligodendrocyte progenitor cells into an SCI rat model promoted remyelination and improved motor function. Transplantation was performed either 7 days (acute phase) or 10 months (chronic phase) after injury, and in both cases, the transplanted cells survived and differentiated primarily into oligodendrocytes. Transplantation in the acute phase promoted remyelination and greatly improved locomotor performance, while transplantation in the chronic phase did. In other words, these studies demonstrated the therapeutic potential of oligodendrocyte progenitor cell transplantation to enhance myelination in early phases after SCI but suggested that environmental factors can make it less effective in the chronic phase (Keirstead et al., 2005).
Therefore, it is suggested that although a single remyelination treatment using cell transplantation has shown effective functional improvement in the early stage of injury, the effect on the chronic stage is still uncertain, and future studies are expected. Additionally, the regeneration of axons and grafted neurons crossing the lesion is essential for the application of these therapies to complete chronic injury, and their use in combination with other therapies should be considered.
Construction and activation of new neuronal circuits
After the complete injury, spinal cord atrophic changes appear, mainly in the white matter on the rostral side of the injury and in both white and gray matter on the caudal side (Detloff et al., 2008; Min et al., 2012). It has been reported that the expression of neuronal activity markers is decreased, and presynaptic inputs are reduced on the caudal side of the injured area, while the number of motor neurons and the synaptogenic potential of postsynaptic molecules are maintained in the chronic phase (Yokota et al., 2019). Hence, restoration of this reduced presynaptic input has been suggested to improve motor function, and the promotion of effective synaptogenesis is key to treatment. We demonstrated that hiPS-NS/PC transplantation could improve motor function by increasing presynaptic inputs by transplanted and host neurons (Ago et al., 2022; Kitagawa et al., 2022). We also reported that the chemotactic effects of transplanted cells transfected with the DREDD system further enhanced the effects of the transplanted cells by promoting synapse formation between the host and transplant-derived neurons in a subacute incomplete SCI mouse model (Kawai et al., 2021). However, Anderson et al. reported that new neural circuits formed after complete SCI are not expected to acquire function spontaneously and require maturation through rehabilitation to promote integration into the functional network to achieve neuroplasticity (Anderson et al., 2018). In other words, especially for complete injury, the formation of a new neural circuit is not sufficient, and functional activation of its synapses is also an important factor for functional recovery. Many reports exist on how to promote the activation of neural circuits, and activity-based therapies for SCI, such as rehabilitation, epidural stimulation methods, and transcranial magnetic stimulation, are some of the most common treatment methods and have been shown to be involved in improving function after injury (Rando et al., 2018; Tashiro et al., 2021; Shibata et al., 2023). For example, we reported the functional recovery of chronic incomplete SCI in mice by treadmill gait training combined with hiPS-NS/PC transplantation. We demonstrated that the neuroprotective effect of rehabilitation on the host and grafted neurons and the acceleration of synaptogenesis were important for the recovery of motor function (Shibata et al., 2023). Kourgiantaki et al. (2020) also reported that epidural stimulation combined with treadmill gait training after administration of bioscaffolds containing Schwann cells in acute complete SCI rats resulted in an improvement in lower extremity function. They found that functional improvement was obtained not only by regeneration of the injured area but also by activation of new circuits in the lumbar spinal cord, as well as circuits across the injured area (Kourgiantaki et al., 2020).
We propose that activity-dependent therapies, other than rehabilitation, include therapeutic interventions with various artificial modifiers that are also being considered for the activation of newly formed neurocircuits in a broader sense. Suzuki et al. (2020) created a synthetic synaptic organizer protein (CPTX) that combined structural elements of cerebellin-1 and neuronal pentraxin-1 and reported that these interact with presynaptic neurexins and postsynaptic AMPA-type glutamate receptors to induce excitatory synapse formation in vitro and in vivo. The authors also reported that CPTX injected into the injured area of acute incomplete SCI in mice promoted the formation of excitatory neuronal circuits and improved locomotor function (Suzuki et al. 2020). Chen et al. (2018) also discovered that a KCC2 agonist restored hindlimbs stepping abilities after SCI by the mechanism that spinal inhibitory interneurons promoted the integration of descending inputs into relay circuits. They showed that the expression of KCC2 was decreased in the area of SCI, resulting in hyperexcitation of inhibitory spinal interneurons, which inhibited the establishment of local neuronal relay circuits. Therefore, they proved that relay circuits of the injured spinal cord can be established when KCC2 was introduced to suppress the hyperexcitation of local inhibitory neurons (Chen et al., 2018).
In the above, since the promotion of the formation and activation of neural circuits in the lumbar spinal cord and across the injured area is an important factor in achieving functional recovery, we have considered that combined treatment such as rehabilitation, CPTX, and KCC2 agonist is beneficial for complete chronic injury.
Previous Studies on Complete Chronic Spinal Cord Injury and Their Limitations
Only a few studies have reported functional improvement after complete chronic SCI, but the restorative effects are insufficient and remain a challenge for clinical application. Liu et al. (2019) reported that a combination of Taxol-containing bioscaffolds and MSC transplantation improved locomotor function in beagle dogs with chronic complete thoracic SCI. Notably, in this study, functional improvement was obtained in mammals, and although no motor neurons were observed crossing the injured area, functional improvement was achieved by the construction of new neuronal circuits bridging the lesion. However, the effect of restoring function was limited, and further functional improvement will require axonal regeneration across the lesion and activation of new neurocircuits, so additional treatment is expected to be considered in the future. We reported improvement of locomotor function by transplantation of hiPS-NS/PCs in combination with hepatocyte growth factor-releasing scaffolds in rats with chronic complete thoracic SCI (Hashimoto et al., 2023). Noting that cell transplantation alone does not enhance motor function after complete chronic injury, we attempted to modulate the lesioned microenvironment where neural regeneration was inhibited before transplantation. Administration of hepatocyte growth factor-releasing scaffolds promoted vascularization, had anti-inflammatory effects, and suppressed fibrotic scar formation. After the spinal cord environment was improved, hiPS-NS/PC transplantation increased the survival rate of grafted cells, and recovered locomotor function was observed due to the formation of regenerative scaffolds and synaptic formation between host and grafted cells. However, recovery of function was limited, and not effective enough to allow walking with a load. We considered that one of the reasons for this was that there were few axons beyond the lesion due to inadequate treatment for glial scar formation and insufficient activation of the neuronal networks.
These studies suggest that functional recovery after complete chronic SCI requires both treatments to the injured area and to the reconstructive circuitry and that with current regenerative medicine technology, combined therapeutic intervention is essential to achieve these goals. Specifically, for severed axons to regenerate across the lesion in the chronic phase, a combination of neural cell replenishment and scaffolding for cavitation, intervention for scars, treatment to promote axonal regrowth, and further rehabilitation will be necessary and the subject of future studies.
Clinical Application to Humans
To realize clinical application to humans, basic research should focus on various patterns of combination therapy to enable therapeutic intervention for several pathologies in complete chronic injury and to seek effective combinations of therapies to obtain functional recovery. Safety assurance and efficacy in humans should be considered for clinical application. With regard to the safety aspects of treatment, there are issues related to tumorigenesis of transplanted cells, drug or gene transfer side effects, and there is a need for long-term follow-up. In particular, safety concerns regarding the clinical application of iPS cells cannot be dispelled. Concerns include the particularities of iPS cell culture and storage methods, the possibility of tumorigenesis, genetic abnormalities, and immune rejection. Another issue, not safety, but also in terms of widespread use, is price. iPS cells are extremely expensive, and the issue of versatility is an important issue to consider. As for immune rejection, it is conceivable that it could be prevented by autologous transplantation, but needless to say, it is impractical with today’s technology when considered in terms of price. In addition, there are many unknowns regarding its effectiveness in humans because most basic research has been in rodents. For example, it is known that the neuro-networks involved in motor functions are different between rodents and humans and that neural plasticity differs greatly between them (Alstermark et al., 1999, 2004; Tillakaratne et al., 2010). Therefore, it is also essential to perform experiments using other mammals to examine therapeutic efficacy in humans.
As previously mentioned, SCIs vary in timing and degree of damage, so customized treatment addressing the pathology of each patient is the key to functional improvement. For this reason, accurate diagnosis by imaging, electrophysiology, and biomarkers prior to treatment is crucial, and the development of research to precisely assess the condition of each individual patient is an important element in the effective therapy process. These techniques can also be applied to determine posttreatment efficacy. We have established the evaluation of spinal cord white matter nerve fibers after SCI in humans using diffusion tensor imaging and diffusion tensor tractography in MRI (Fujiyoshi et al., 2013). The white matter of the spinal cord is highly anisotropic due to the diffusion of water in fiber bundles along axonal runs, and these fibers can be tracked and visualized based on this anisotropic information. Using this method, we investigated the conditions for the delineation of residual axonal fibers in a marmoset SCI model, enabling the delineation and regeneration evaluation of various neuronal tracts, including the corticospinal tract, and establishing its application to humans (Konomi et al., 2012, 2021). Furthermore, we devised an imaging technique using pulsed gradient spin-echo MRI to image the histopathological status of the myelin sheath after injury (Fujiyoshi et al., 2016). Focusing on the non-Gaussian diffusion distribution of water molecules trapped in the myelin sheath, we have developed a novel method called the “Myelin map” to visualize myelin in vivo and are currently investigating its clinical application in humans.
Thus, not only the study of SCI treatment but also the development of appropriate diagnostic and treatment-determination tools is important for strategies for clinical application.
Conclusion
The complete chronic injury requires a multifaceted approach due to the presence of a variety of inhibitors of regeneration. In the future, we should investigate which combination therapies provide the greatest functional improvement. For clinical application to humans, the optimal treatment must be tailored to each patient’s condition, taking into account the degree of damage, the degree of paralysis, and the patient’s wishes. To this end, it will be valuable not only to study effective therapies but also to develop diagnostic tools to accurately understand the pathophysiology. Although there are still many issues to be addressed, spinal cord regeneration research is making steady progress little by little, and further development is expected.
Additional file: Open peer review report 1 (79.5KB, pdf) .
Footnotes
Open peer reviewer: Noela Rodriguez-Losada, University of Malaga, Spain.
P-Reviewer: Rodriguez-Losada N; C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
Conflicts of interest: HO declared employment with Keio University School of Medicine, advisory role with SanBio Co., Ltd., and K Pharma, Inc., and research funding from SanBio Co., Ltd., and K Pharma, Inc. All of the other authors declare no potential conflicts of interest.
Data availability statement:
The data are available from the corresponding author on reasonable request.
References
- 1.Ago K, Nagoshi N, Imaizumi K, Kitagawa T, Kawai M, Kajikawa K, Shibata R, Kamata Y, Kojima K, Shinozaki M, Kondo T, Iwano S, Miyawaki A, Ohtsuka M, Bito H, Kobayashi K, Shibata S, Shindo T, Kohyama J, Matsumoto M, Nakamura M, Okano H. A non-invasive system to monitor in vivo neural graft activity after spinal cordinjury. Commun Biol. 2022;5:803. doi: 10.1038/s42003-022-03736-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahuja CS, Fehlings M. Concise review: bridging the gap: novel neuroregenerative and neuroprotective strategies in spinal cord injury. Stem Cells Transl Med. 2016;5:914–924. doi: 10.5966/sctm.2015-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alstermark B, Ogawa J, Isa T. Lack of monosynaptic corticomotoneuronal EPSPs in rats: disynaptic EPSPs mediated via reticulospinal neurons and polysynaptic EPSPs via segmental interneurons. J Neurophysiol. 1999;6:3580–3585. doi: 10.1152/jn.00820.2003. [DOI] [PubMed] [Google Scholar]
- 4.Alstermark B, Isa T, Ohki Y, Saito Y. disynaptic pyramidal excitation in forelimb motoneurons mediated via C3-C4 Propriospinal Neurons in the Macaca fuscata. J Neurophysiol. 2004;6:3580–3585. doi: 10.1152/jn.1999.82.6.3580. [DOI] [PubMed] [Google Scholar]
- 5.Anderson MA, O'Shea TM, Burda JE, Ao Y, Barlatey SL, Bernstein AM, Kim JH, James ND, Rogers A, Kato B, Wollenberg AL, Kawaguchi R, Coppola G, Wang C, Deming TJ, He Z, Courtine G, Sofroniew MV. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature. 2018;561:396–400. doi: 10.1038/s41586-018-0467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W. Cell transplantation therapy for spinal cord injury. Nat Neurosci. 2017;20:637–647. doi: 10.1038/nn.4541. [DOI] [PubMed] [Google Scholar]
- 7.Baloh RH, Johnson JP, Avalos P, Allred P, Svendsen S, Gowing G, Roxas K, Wu A, Donahue B, Osborne S, Lawless G, Shelley B, Wheeler K, Prina C, Fine D, Kendra-Romito T, Stokes H, Manoukian V, Muthukumaran A, Garcia L, et al. Transplantation of human neural progenitor cells secreting GDNF into the spinal cord of patients with ALS: a phase 1/2a trial. Nat Med. 2022;28:1813–1822. doi: 10.1038/s41591-022-01956-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnabe-Heider F, Frisen J. Stem cells for spinal cord repair. Cell Stem Cell. 2008;3:16–24. doi: 10.1016/j.stem.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Bradbury EJ, Lawrence DF, Reena JP, Von RK, Gavin SB, Preena NP, James WF, Stephen BM. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 10.Bradbury EJ, Burnside ER. Moving beyond the glial scar for spinal cord repair. Nat Commun. 2019;10:3879. doi: 10.1038/s41467-019-11707-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81:229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen B, Li Y, Yu B, Zhang Z, Brommer B, Williams PR, Liu Y, Hegarty SV, Zhou S, Zhu J, Guo H, Lu Y, Zhang Y, Gu X, He Z. Reactivation of dormant relay pathways in injured spinal cord by KCC2 manipulations. Cell. 2018;174:521–535. doi: 10.1016/j.cell.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp Neurol. 2014;253:197–207. doi: 10.1016/j.expneurol.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtis E, Martin JR, Gabel B, Sidhu N, Rzesiewicz TK, Mandeville R, Gorp SV, Leerink M, Tadokoro T, Marsala S, Jamieson C, Marsala M, Ciacci JD. A first-in-human, phase I study of neural stem cell transplantation for chronic spinal cord injury. Cell Stem Cell. 2018;22:941–950. doi: 10.1016/j.stem.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Detloff MR, Fisher LC, McGaughy V, Longbrake EE, Popovich PG, Basso DM. Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp Neurol. 2008;212:337–347. doi: 10.1016/j.expneurol.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dias DO, Kim H, Holl D, Solnestam BW, Lundeberg J, Carlen M, Goritz C, Frisen J. Reducing pericyte-derived scarring promotes recovery after spinal cord injury. Cell. 2018;173:153–165. doi: 10.1016/j.cell.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Waly B, Escarrat V, Perez-Sanchez J, Kaur J, Pelletier F, Collazos-Castro JE, Debarbieux F. Intravital assessment of cells responses to conducting polymer-coated carbon microfibres for bridging spinal cord injury. Cells. 2021;10:73. doi: 10.3390/cells10010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fehlberg CR, Lee JK. Fibrosis in the central nervous system: from the meninges to the vasculature. Cell Tissue Res. 2022;387:351–360. doi: 10.1007/s00441-021-03491-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fehlings MG, Kim KD, Aarabi B, Rizzo M, Bond LM, McKerracher L, Vaccaro AR, Okonkwo DO. Rho inhibitor VX-210 in acute traumatic subaxial cervical spinal cord injury: design of the spinal cord injury rho inhibition investigation (SPRING) clinical trial. J Neurotrauma. 2018;35:1049–1056. doi: 10.1089/neu.2017.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitch MT, Doller C, Combs CK, Landreth GE, Silver J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J neurosci. 1999;19:8182–8198. doi: 10.1523/JNEUROSCI.19-19-08182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujiyoshi K, Konomi T, Yamada M, Hikishima K, Tsuji O, Komaki Y, Momoshima S, Toyama Y, Nakamura M, Okano H. Diffusion tensor imaging and tractography of the spinal cord: from experimental studies to clinical application. Exp Neurol. 2013;242:74–82. doi: 10.1016/j.expneurol.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Fujiyoshi K, Hikishima K, Nakahara J, Tsuji O, Hata J, Konomi T, Nagai T, Shibata S, Kaneko S, Iwanami A, Momoshima S, Takahashi S, Jinzaki M, Suzuki N, Toyama Y, Nakamura M, Okano H. Application of q-space diffusion MRI for the visualization of white matter. J Neurosci. 2016;36:2796–2808. doi: 10.1523/JNEUROSCI.1770-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghobrial G, Anderson K, Dididze M, Martinez-Barrizonte J, Sunn G, Gant K, Allan D. Human neural stem cell transplantation in chronic cervical spinal cord injury: functional outcomes at 12 Months in a phase II clinical trial. Neurosurgery. 2017;64:87–91. doi: 10.1093/neuros/nyx242. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto S, Nagoshi N, Shinozaki M, Nakanishi K, Suematsu Y, Shibata T, Kawai M, Kitagawa T, Ago K, Kamata Y, Yasutake K, Koya I, Ando Y, Minoda A, Shindo T, Shibata S, Matsumoto M, Nakamura M, Okano H. Microenvironmental modulation in tandem with human stem cell transplantation enhances functional recovery after chronic complete spinal cord injury. Biomaterials. 2023;295:122002. doi: 10.1016/j.biomaterials.2023.122002. [DOI] [PubMed] [Google Scholar]
- 25.Honmou O, Yamashita T, Morita T, Oshigiri T, Hirota R, Iyama S, Kato J, Sasaki Y, Ishiai S, Ito YM, Namioka A, Namioka T, Nakazaki M, Sasaki YK, Onodera R, Oka S, Sasaki M, Waxman SG, Kocsis JD. Intravenous infusion of auto serum-expanded autologous mesenchymal stem cells in spinal cord injury patients: 13 case series. Clin Neurol Neurosurg. 2021;203:106565. doi: 10.1016/j.clineuro.2021.106565. [DOI] [PubMed] [Google Scholar]
- 26.Huang SG, Fu LC, Xiong F, He C, Wei Q. Stem cell therapy for spinal cord injury. Cell Transplant. 2021;30:963689721989266. doi: 10.1177/0963689721989266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito S, Nagoshi N, Tsuji O, Shibata S, Shinozaki M, Kawabata S, Kojima K, Yasutake K, Hirokawa K, Matsumoto M, Takei K, Nakamura M, Okano H. LOTUS inhibits neuronal apoptosis and promotes tract regeneration in contusive spinal cord injury model mice. eNeuro. 2018;5 doi: 10.1523/ENEURO.0303-18.2018. ENEURO.0303-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwasaki M, Wilcox JT, Nishimura Y, Zweckberger K, Suzuki H, Wang J, Liu Y, Karadimas SK, Fehlings MG. Synergistic effects of self-assembling peptide and neural stem/progenitor cells to promote tissue repair and forelimb functional recovery in cervical spinal cord injury. Biomaterials. 2014;35:2617–2629. doi: 10.1016/j.biomaterials.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Jones L, Tuszynski MH. Spinal cord injury elicits expression of keratan sulfate proteoglycans by macrophages, reactive, microglia and oligodendrocyte progenitors. J Neurosci. 2022;22:4611–4624. doi: 10.1523/JNEUROSCI.22-11-04611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamata Y, Isoda M, Sanosaka T, Shibata R, Ito S, Okubo T, Shinozaki M, Inoue M, Koya I, Shibata S, Shindo T, Matsumoto M, Nakamura M, Okano H, Nagoshi N, Kohyama J. A robust culture system to generate neural progenitors with gliogenic competence from clinically relevant induced pluripotent stem cells for treatment of spinal cord injury. Stem Cells Transl Med. 2021;10:398–413. doi: 10.1002/sctm.20-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaneko A, Matsushita A, Sankai Y. A 3D nanofibrous hydrogel and collagen sponge scaffold promotes locomotor functional recovery, spinal, repair and neuronal regeneration after complete transection of the spinal cord in adult rats. Biomed Mater. 2015;10:015008. doi: 10.1088/1748-6041/10/1/015008. [DOI] [PubMed] [Google Scholar]
- 32.Kaneko S, Iwanami A, Nakamura M, Kishino A, Kikuchi K, Shibata S, Okano HJ, Ikegami T, Moriya A, Konishi O, Nakayama C, Kumagai K, Kimura T, Sato Y, Goshima Y, Taniguchi M, Ito M, He Z, Toyama Y, Okano H. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med. 2006;12:1380–1389. doi: 10.1038/nm1505. [DOI] [PubMed] [Google Scholar]
- 33.Kawai M, Imaizumi K, Ishikawa K, Shibata S, Shinozaki M, Shibata T, Hashimoto S, Kitagawa T, Ago K, Kajikawa K, Shibata R, Kamata Y, Ushiba J, Koga K, Furue H, Matsumoto M, Nakamura M, Nagoshi N, Okano H. Long-term selective stimulation of transplanted neural stem/progenitor cells for spinal cord injury improves locomotor function. Cell Rep. 2021;37:110019. doi: 10.1016/j.celrep.2021.110019. [DOI] [PubMed] [Google Scholar]
- 34.Kawai M, Nagoshi N, Okano H, Nakamura M. A review of regenerative therapy for spinal cord injury using human iPS cells. N Am Spine Soc J. 2023;13:100184. doi: 10.1016/j.xnsj.2022.100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keefe KM, Sheikh IS, Smith GM. Targeting neurotrophins to specific populations of neurons: NGF, BDNF and NT-3 and their relevance for treatment of spinal cord injury. Int J Mol Sci. 2017;18:548. doi: 10.3390/ijms18030548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerschensteiner M, Scwab ME, Lichtman JW, Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nature Med. 2005;5:572–577. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- 38.Kitagawa T, Nagoshi N, Kamata Y, Kawai M, Ago K, Kajikawa K, Shibata R, Sato Y, Imaizumi K, Shindo T, Shinozaki M, Kohyama J, Shibata S, Matsumoto M, Nakamura M, Okano H. Modulation by DREADD reveals the therapeutic effect of human iPSC-derived neuronal activity on functional recovery after spinal cord injury. Stem Cell Reports. 2022;17:127–142. doi: 10.1016/j.stemcr.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koda M, Nishio Y, Kamada T, Someya Y, Okawa A, Mori C, Yoshinaga K, Okada S, Moriya H, Yamazaki M. Granulocyte colony-stimulating factor (G-CSF) mobilizes bone marrow-derived cells into injured spinal cord and promotes functional recovery after compression-induced spinal cord injury in mice. Brain Res. 2007;1149:223–231. doi: 10.1016/j.brainres.2007.02.058. [DOI] [PubMed] [Google Scholar]
- 40.Koffler J, Zhu Q, Qu X, Platoshyn O, Dulin JN, Brock J, Graham L, Lu P, Sakamoto J, Marsala M, Chen S, Tuszynski MH. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat Med. 2019;25:263–269. doi: 10.1038/s41591-018-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konomi T, Fujiyoshi K, Hikishima K, Komaki Y, Tsuji O, Okano HJ, Toyama Y, Okano H, Nakamura M. Conditions for quantitative evaluation of injured spinal cord by in vivo diffusion tensor imaging and tractography: preclinical longitudinal study in common marmosets. Neuroimage. 2012;63:1841–1853. doi: 10.1016/j.neuroimage.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 42.Konomi T, Suda K, Ozaki M, Harmon SM, Komatsu M, Iimoto S, Tsuji O, Minami A, Takahata M, Iwasaki N, Matsumoto M, Nakamura M. Predictive factors for irreversible motor paralysis following cervical spinal cord injury. Spinal cord. 2021;59:554–562. doi: 10.1038/s41393-020-0513-8. [DOI] [PubMed] [Google Scholar]
- 43.Kourgiantaki A, Tzeranis DS, Karali K, Georgelou K, Bampoula E, Psilodimitrakopoulos S, Yannas IV, Stratakis E, Sidiropoulou K, Charalampopoulos I, Gravanis A. Neural stem cell delivery via porous collagen scaffolds promotes neuronal differentiation and locomotion recovery in spinal cord injury. NPJ Regen Med. 2020;5:12. doi: 10.1038/s41536-020-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumamaru H, Saiwai H, Kubota K, Kobayakawa K, Yokota K, Ohkawa Y, Shiba K, Iwamoto Y, Okada S. Therapeutic activities of engrafted neural stem/precursor cells are not dormant in the chronically injured spinal cord. Stem Cells. 2013;31:1535–1547. doi: 10.1002/stem.1404. [DOI] [PubMed] [Google Scholar]
- 45.Lang BT, Cregg JM, DePaul MA, Tran AP, Xu K, Dyck SM, Madalena KM, Brown BP, Weng YL, Li S, Karimi-Abdolrezaee S, Busch SA, Shen Y, Silver J. Modulation of the proteoglycan receptor PTPsigma promotes recovery after spinal cord injury. Nature. 2015;518:404–408. doi: 10.1038/nature13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JH, Shin H, Shaker MR, Kim HJ, Park SH, Kim JH, Lee N, Kang M, Cho S, Kwak TH, Kim JW, Song MR, Kwon SH, Han DW, Lee S, Choi SY, Rhyu IJ, Kim H, Geum D, Cho IJ, Sun W. Production of human spinal-cord organoids recapitulating neural-tube morphogenesis. Nat Biomed Eng. 2022;6:435–448. doi: 10.1038/s41551-022-00868-4. [DOI] [PubMed] [Google Scholar]
- 47.Li X, Liu D, Xiao Z, Zhao Y, Han S, Chen B, Dai J. Scaffold-facilitated locomotor improvement post complete spinal cord injury: motor axon regeneration versus endogenous neuronal relay formation. Biomaterials. 2019;197:20–31. doi: 10.1016/j.biomaterials.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 48.Liu D, Li X, Xiao Z, Yin W, Zhao Y, Tan J, Chen B, Jiang X, Dai J. Different functional bio-scaffolds share similar neurological mechanism to promote locomotor recovery of canines with complete spinal cord injury. Biomaterials. 2019;214:119230. doi: 10.1016/j.biomaterials.2019.119230. [DOI] [PubMed] [Google Scholar]
- 49.Marchini A, Raspa A, Pugliese R, ElMalek MA, Pastori V, Lecchi M, Vescovi AL, Gelain F. Multifunctionalized hydrogels foster hNSC maturation in 3D cultures and neural regeneration in spinal cord injuries. Proc Natl Acad Sci U S A. 2019;116:7483–7492. doi: 10.1073/pnas.1818392116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKeon RJ, Jurynec MJ, Buck CR. The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J Neurosci. 1999;19:10778–10788. doi: 10.1523/JNEUROSCI.19-24-10778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Min KJ, Jeong HK, Kim B, Hwang DH, Shin HY, Nguyen AT, Kim JH, Jou I, Kim BG, Joe EH. Spatial and temporal correlation in progressive degeneration of neurons and astrocytes in contusion-induced spinal cord injury. J Neuroimfla. 2012;9:100. doi: 10.1186/1742-2094-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muramatsu R, Takahashi C, Miyake S, Fujimura H, Mochizuki H, Yamashita T. Angiogenesis induced by CNS inflammation promotes neuronal remodeling through vessel-derived prostacyclin. Nat Med. 2012;18:1658–1664. doi: 10.1038/nm.2943. [DOI] [PubMed] [Google Scholar]
- 53.Nagoshi N, Okano H. iPSC-derived neural precursor cells: potential for cell transplantation therapy in spinal cord injury. Cell Mol Life Sci. 2018;75:989–1000. doi: 10.1007/s00018-017-2676-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagoshi N, Tsuji O, Kitamura K, Suda K, Maeda T, Yato T, Abe T, Hayata D, Matsumoto M, Okano H, Nakamura M. Phase I/II study of intrathecal administration of recombinant human hepatocyte growth factor in patients with acute spinal cord injury: a double-blind, randomized, clinical trial of safety and efficacy. J Neurotrauma. 2020;37:1752–1758. doi: 10.1089/neu.2019.6854. [DOI] [PubMed] [Google Scholar]
- 55.Nishimura S, Yasuda A, Iwai H, Takano M, Kobayashi Y, Nori S, Tsuji O, Fujiyoshi K, Ebise H, Toyama Y, Okano H, Nakamura M. Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol Brain. 2013;6:3. doi: 10.1186/1756-6606-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nishimura S, Sasaki T, Shimizu A, Yoshida K, Iwai H, Koya I, Kobayashi Y, Itakura G, Shibata S, Ebise H, Horiuchi K, Kudoh J, Toyama Y, Anderson AJ, Okano H, Nakamura M. Global gene expression analysis following spinal cord injury in non-human primates. Exp Neurol. 2014;261:171–179. doi: 10.1016/j.expneurol.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 57.Nori S, Okada Y, Yasuda A, Tsuji O, Takahashi Y, Kobayashi Y, Fujiyoshi K, Koike M, Uchiyama Y, Ikeda E, Toyama Y, Yamanaka S, Nakamura M, Okano H. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci U S A. 2011;108:16825–16830. doi: 10.1073/pnas.1108077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nori S, M Khazaei CS, Ahuja K, Yokota JE, Ahlfors Y, Liu J, Wang S, Shibata S, Chio J, Hettiaratchi MH, Fuhrmann T, Shoichet MS, Fehlings MG. Human oligodendrogenic neural progenitor cells delivered with chondroitinase ABC facilitate functional repair of chronic spinal cord injury. Stem Cell Reports. 2018;11:1433–1448. doi: 10.1016/j.stemcr.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohtake Y, Hayat U, Li S. PTEN inhibition and axon regeneration and neural repair. Neural Regen Res. 2015;10:1363–1368. doi: 10.4103/1673-5374.165496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okada S, Nakamura M, Mikami Y, Shimazaki T, Mihara M, Ohsugi Y, Iwamoto Y, Yoshizaki K, Kishimoto T, Toyama Y, Okano H. Blockade of interleukin-6 receptor suppresses reactive astrogliosis and ameliorates functional recovery in experimental spinal cord injury. J Neurosci Res. 2004;76:265–276. doi: 10.1002/jnr.20044. [DOI] [PubMed] [Google Scholar]
- 61.Okano H. Transplantation of neural progenitor cells into the human CNS. Trends Mol Med. 2022a;28:897–899. doi: 10.1016/j.molmed.2022.09.009. [DOI] [PubMed] [Google Scholar]
- 62.Okano H. A combined stem-cell-gene therapy strategy for ALS. Nat Med. 2022b;28:1751–1752. doi: 10.1038/s41591-022-01983-0. [DOI] [PubMed] [Google Scholar]
- 63.Okubo T, Nagoshi N, Kohyama J, Tsuji O, Shinozaki M, Shibata S, Kase Y, Matsumoto M, Nakamura M, Okano H. Treatment with a gamma-secretase inhibitor promotes functional recovery in human iPSC-derived transplants for chronic spinal cord injury. Stem Cell Reports. 2018;11:1416–1432. doi: 10.1016/j.stemcr.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Orr MB, Gensel JC. Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics. 2018;15:541–553. doi: 10.1007/s13311-018-0631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Osaka M, Honmou O, Murakami T, Nonaka T, Houkin K, Hamada H, Kocsis JD. Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res. 2010;1343:226–235. doi: 10.1016/j.brainres.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 66.Prabhakar V, Capila I, Bosques KJ, Pojasek K, Sasieharan M. Chondroitinase ABC I from Proteus vulgaris: cloning, recombinant expression and active site identification. Biochem J. 2005;386:103–112. doi: 10.1042/BJ20041222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quadri SA, Farooqui M, Ikram A, Zafar A, Khan MA, Suriya SS, Claus CF, Fiani B, Rahman M, Ramachandran A, Armstrong IIT, Taqi MA, Mortazavi MM. Recent update on basic mechanisms of spinal cord injury. Neurosurg Rev. 2020;43:425–441. doi: 10.1007/s10143-018-1008-3. [DOI] [PubMed] [Google Scholar]
- 68.Rando TA, Ambrosio F. Regenerative rehabilitation: applied biophysics meets stem cell therapeutics. Cell Stem Cell. 2018;22:306–309. doi: 10.1016/j.stem.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rao JS, Zhao C, Zhang A, Duan H, Hao P, Wei RH, Shang J, Zhao W, Liu Z, Yu J, Fan KS, Tian Z, He Q, Song W, Yang Z, Sun YE, Li X. NT3-chitosan enables de novo regeneration and functional recovery in monkeys after spinal cord injury. Proc Natl Acad Sci U S A. 2018;115:E5595–5604. doi: 10.1073/pnas.1804735115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robert JM, Michael JJ, Charles RB. The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J Neurosci. 1999;19:10778–10788. doi: 10.1523/JNEUROSCI.19-24-10778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosenzweig ES, Salegio EA, Liang JJ, Weber JL, Weinholtz CA, Brock JH, Moseanko R, Hawbecker S, Pender R, Cruzen CL, Iaci JF, Caggiano AO, Blight AR, Haenzi B, Huie JR, Havton LA, Nout-Lomas YS, Fawcett JW, Ferguson AR, Beattie MS, et al. Chondroitinase improves anatomical and functional outcomes after primate spinal cord injury. Nat Neurosci. 2019;22:1269–1275. doi: 10.1038/s41593-019-0424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ruschel J, Hellal F, Flynn KC, Dupraz S, Elliott DA, Tedeschi A, Bates M, Sliwinski C, Brook G, Dobrindt K, Peitz M, Brüstle O, Norenberg MD, Blesch A, Weidner N, Bunge MB, Bixby JL, Bradke F. Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science. 2015;348:347–352. doi: 10.1126/science.aaa2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sakamoto K, Ozaki T, Ko YC, Tsai CF, Gong Y, Morozumi M, Ishikawa Y, Uchimura K, Nadanaka S, Kitagawa H, Zulueta MML, Bandaru A, Tamura JI, Hung SC, Kadomatsu K. Glycan sulfation patterns define autophagy flux at axon tip via PTPRsigma-cortactin axis. Nat Chem Biol. 2019;15:699–709. doi: 10.1038/s41589-019-0274-x. [DOI] [PubMed] [Google Scholar]
- 74.Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shibata T, Tashiro S, Shibata S, Shinozaki M, Shindo T, Hashimoto S, Kawai M, Kitagawa T, Ago K, Matsumoto M, Nakamura M, Okano H, Nagoshi N. Rehabilitative training enhances therapeutic effect of human-iPSC-derived neural stem/progenitor cells transplantation in chronic spinal cord injury. Stem Cells Transl Med. 2023;12:83–96. doi: 10.1093/stcltm/szac089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shinozaki M, Iwanami A, Fujiyoshi K, Tashiro S, Kitamura K, Shibata S, Fujita H, Nakamura M, Okano H. Combined treatment with chondroitinase ABC and treadmill rehabilitation for chronic severe spinal cord injury in adult rats. Neurosci Res. 2016;113:37–47. doi: 10.1016/j.neures.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 77.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 78.Soderblom C, Luo X, Blumenthal E, Bray E, Lyapichev K, Ramos J, Krishnan V, Lai-Hsu C, Park KK, Tsoulfas P, Lee JK. Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J Neurosci. 2013;33:13882–13887. doi: 10.1523/JNEUROSCI.2524-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sugai K, Sumida M, Shofuda T, Yamaguchi R, Tamura T, Kohzuki T, Abe T, Shibata R, Kamata Y, Ito S, Okubo T, Tsuji O, Nori S, Nagoshi N, Yamanaka S, Kawamata S, Kanemura Y, Nakamura M, Okano H. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: study protocol. Regen Ther. 2021;18:321–333. doi: 10.1016/j.reth.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tashiro S, Tsuji O, Shinozaki M, Shibata T, Yoshida T, Tomioka Y, Unai K, Kondo T, Itakura G, Kobayashi Y, Yasuda A, Nori S, Fujiyoshi K, Nagoshi N, Kawakami M, Uemura O, Yamada S, Tsuji T, Okano H, Nakamura M. Current progress of rehabilitative strategies in stem cell therapy for spinal cord injury: a review. NPJ Regen Med. 2021;6:81. doi: 10.1038/s41536-021-00191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suzuki K, Elegheert J, Song I, Sasakura H, Senkov O, Matsuda K, Kakegawa W, Clayton AJ, Chang VT, Ferrer-Ferrer M, Miura E, Kaushik R, Ikeno M, Morioka Y, Takeuchi Y, Shimada T, Otsuka S, Stoyanov S, Watanabe M, Takeuchi K, et al. A synthetic synaptic organizer protein restores glutamatergic neuronal circuits. Science. 2020;369:eabb4853. doi: 10.1126/science.abb4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15–26. doi: 10.3171/jns.1991.75.1.0015. [DOI] [PubMed] [Google Scholar]
- 83.Tillakaratne NJ, Guu JJ, Leon RD, Bigbee AJ, London NJ, Zhong H, Ziegler MD, Joynes RL, Roy RR, Edgerton VR. Functional recovery of stepping in rats after a complete neonatal spinal cord transection is not due to regrowth across the lesion site. Neuroscience. 2010;166:23–33. doi: 10.1016/j.neuroscience.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang SX, Lu YB, Wang XX, Wang Y, Song YJ, Wang X, Nyamgerelt M. Graphene and graphene-based materials in axonal repair of spinal cord injury. Neural Regen Res. 2022;17:2117–2125. doi: 10.4103/1673-5374.335822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z, Ao Y, Sofroniew MV. Glial scar borders are formed by newly proliferated, elongated, astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci. 2013;33:12870–12886. doi: 10.1523/JNEUROSCI.2121-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Woerly S, Prnet E, Robertis L, Diep Van, Bousmina M. Spinal cord repair with PHPMA hydrogel containing RGD peptides (NeuroGelTM) Biomaterials. 2000;22:1095–1111. doi: 10.1016/s0142-9612(00)00354-9. [DOI] [PubMed] [Google Scholar]
- 87.Xu Y, He X, Wang Y, Jian J, Peng X, Zhou L, Kang Y, Wang T. 5-Fluorouracil reduces the fibrotic scar via inhibiting matrix metalloproteinase 9 and stabilizing microtubules after spinal cord injury. CNS Neurosci Ther. 2022;28:2011–2023. doi: 10.1111/cns.13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamane K, Mazaki T, Shiozaki Y, Yoshida A, Shinohara K, Nakamura M, Yoshida Y, Zhou D, Kitajima T, Tanaka M, Ito Y, Ozaki T, Matsukawa A. Collagen-binding hepatocyte growth factor (HGF) alone or with a gelatin-furfurylamine hydrogel enhances functional recovery in mice after spinal cord injury. Sci Rep. 2018;8:917. doi: 10.1038/s41598-018-19316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yokota K, Kubota K, Kobayakawa K, Saito T, Hara M, Kijima K, Maeda T, Katoh H, Ohkawa Y, Nakashima Y, Okada S. Pathological changes of distal motor neurons after complete spinal cord injury. Mol Brain. 2019;12:4. doi: 10.1186/s13041-018-0422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang L, Kaneko S, Kikuchi K, Sano A, Maeda M, Kishino A, Shibata S, Mukaino Y, Toyama Y, Liu M, Kimura T, Okano, Nakamura M. Rewiring of regenerated axons by combining treadmill training with semaphorin3A inhibition. Molecular Brain. 2014;7:14. doi: 10.1186/1756-6606-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available from the corresponding author on reasonable request.


